DNA-induced chirality in water-soluble poly(cobaltoceniumethylene)†
Huibin
Qiu
,
Joe B.
Gilroy
and
Ian
Manners
*
School of Chemistry, University of Bristol, Bristol, BS8 1TS, UK. E-mail: ian.manners@bristol.ac.uk
First published on 5th November 2012
Abstract
Poly(cobaltoceniumethylene), a water-soluble cationic metal-containing polyelectrolyte, adopts a chiral structure when bound electrostatically to DNA.
Metal-containing polymers are emerging as advanced functional materials and have attracted much attention due to the useful physical and catalytic properties that arise from the presence of metal centers.1 For example, metallopolymers with reversible redox properties are of interest for applications in electrocatalysis2 and sensing,3 as responsive surfaces4 and capsules,5 and as the active components of photonic crystal displays.6 Although the formation of helical organic and main group polymers has been observed,7 to the best of our knowledge, the introduction of chirality in a metallopolymer has not been achieved to date.
Polyelectrolytes are a class of macromolecules which possess cationic or anionic charges along the polymer chain and are of considerable industrial and commercial importance.8 Despite extensive studies on organic polyelectrolytes, examples of inorganic polyelectrolytes are rare,9,10 especially for transition metal-containing polymers.11 Recently, the development of the ring-opening polymerization (ROP) of sila[1]ferrocenophanes has enabled the synthesis of water-soluble polyferrocenylsilane polyelectrolytes with a variety of anionic or cationic pendant groups attached at the silicon centres.12 More recently, we have synthesized high-molecular-weight (Mw ∼ 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, DPw ∼ 198) water-soluble poly(cobaltoceniumethylene) nitrate PCE-NO3 through thermal ROP of dicarba[2]cobaltocenophane 1, followed by oxidation in the presence of NH4NO3 (Scheme 1).13 One of the unique characteristics of this polymer is the location of the positive charge on the repeating unit of the polymer backbone. The centrally located metal-centered cation may enhance the structural replication of an anionic chiral template (e.g., DNA) compared to polyelectrolytes with pendant charges. Furthermore, the rotational freedom of the cobaltocenium units and the ethylene linkage may enable a facile conformational change to give a helical structure. Herein we describe experiments designed to induce chirality in PCE through complexation to chiral DNA templates.14
000, DPw ∼ 198) water-soluble poly(cobaltoceniumethylene) nitrate PCE-NO3 through thermal ROP of dicarba[2]cobaltocenophane 1, followed by oxidation in the presence of NH4NO3 (Scheme 1).13 One of the unique characteristics of this polymer is the location of the positive charge on the repeating unit of the polymer backbone. The centrally located metal-centered cation may enhance the structural replication of an anionic chiral template (e.g., DNA) compared to polyelectrolytes with pendant charges. Furthermore, the rotational freedom of the cobaltocenium units and the ethylene linkage may enable a facile conformational change to give a helical structure. Herein we describe experiments designed to induce chirality in PCE through complexation to chiral DNA templates.14
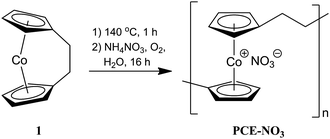 | ||
| Scheme 1 Synthesis of PCE-NO3.13 | ||
The previously synthesized PCE-NO313b and a commercially available native double-stranded DNA (purified from salmon testes, in its sodium salt form) containing ca. 2000 base pairs were used in this work. PCE-NO3 and DNA were dissolved in deionized water separately and combined at different mass ratios in order to induce complex formation. The light olivine solution became cloudy immediately, indicating a fast complexation of PCE and DNA, presumably as a result of the strong electrostatic interactions between the cobaltocenium cations and the anionic phosphodiester groups of the DNA backbone (Fig. 1). The resultant colloidal solution was found to be stable for several days.
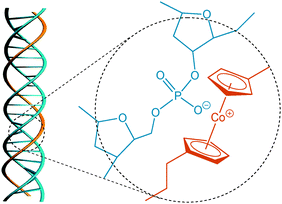 | ||
| Fig. 1 A speculated helical structure of the DNA/PCE complex and the electrostatic interaction between the cobaltocenium cation and the anionic phosphodiester group of the DNA backbone. | ||
Cryogenic transmission electron microscopy (cryo-TEM) image (Fig. 2a) revealed that the colloidal solution that comprised a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass ratio of DNA and PCE-NO3 contained spherical nanoparticles with diameters of 15–40 nm. A large portion of the nanoparticles were fused together and formed small irregular aggregates. Dynamic light scatting (DLS) studies showed a narrow distribution of apparent hydrodynamic radius (RH,app = 81 nm, Fig. 2b), which is consistent with the formation of aggregates in solution. Atomic force microscopy (AFM) height images confirmed that the complex retained spherical morphology after drying (Fig. 2c). However, owing to drying effects, large particles were observed on the silicon substrate and the size distribution of the particles became much broader (Fig. 2c and d, also see Fig. S2 (ESI†), for drop-cast TEM images).
1 mass ratio of DNA and PCE-NO3 contained spherical nanoparticles with diameters of 15–40 nm. A large portion of the nanoparticles were fused together and formed small irregular aggregates. Dynamic light scatting (DLS) studies showed a narrow distribution of apparent hydrodynamic radius (RH,app = 81 nm, Fig. 2b), which is consistent with the formation of aggregates in solution. Atomic force microscopy (AFM) height images confirmed that the complex retained spherical morphology after drying (Fig. 2c). However, owing to drying effects, large particles were observed on the silicon substrate and the size distribution of the particles became much broader (Fig. 2c and d, also see Fig. S2 (ESI†), for drop-cast TEM images).
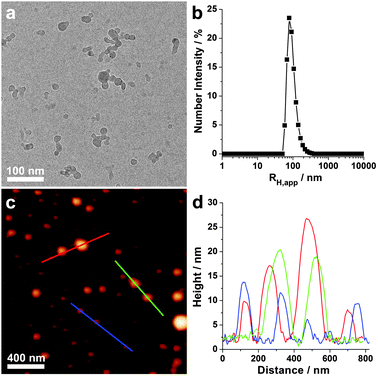 | ||
Fig. 2 (a) Cryo-TEM image, (b) DLS plot, (c) AFM height image and (d) height profiles of the DNA/PCE complex. The mass ratio of DNA to PCE-NO3 was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
To evaluate the chiral properties of the complexes, circular dichroism (CD) experiments were carried out on the colloidal solutions. The native double-stranded DNA exhibited a positive long wavelength band between 260–280 nm, a negative band around 250 nm, and a positive band around 220 nm (Fig. 3a, red line), implying a right-handed B-form helical conformation.15 After binding with PCE (DNA/PCE-NO3 mass ratio >1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), the intensity of the short wavelength bands gradually decreased, probably due to the decreasing proportion of DNA in the complex, while their position and shape remained very similar. These results indicated that DNA retained its helical secondary structure in these complexes in spite of the tight combination with PCE. However, such chirality vanished when the DNA/PCE-NO3 mass ratio dropped to below 1
2), the intensity of the short wavelength bands gradually decreased, probably due to the decreasing proportion of DNA in the complex, while their position and shape remained very similar. These results indicated that DNA retained its helical secondary structure in these complexes in spite of the tight combination with PCE. However, such chirality vanished when the DNA/PCE-NO3 mass ratio dropped to below 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Fig. 3a, green line), implying the collapse of the helical structure of DNA.
2 (Fig. 3a, green line), implying the collapse of the helical structure of DNA.
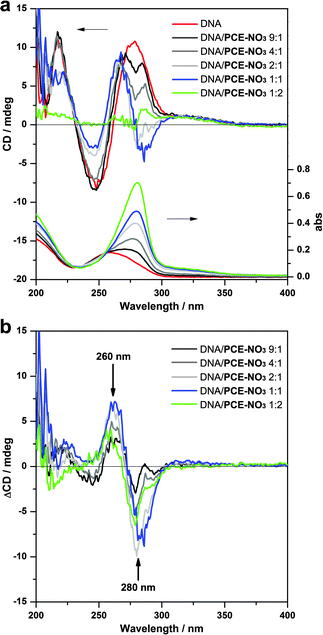 | ||
| Fig. 3 (a) CD (top) and UV-vis (bottom) spectra of the DNA/PCE complexes formed with different DNA to PCE-NO3 mass ratios. (b) CD difference between the DNA/PCE complexes and the native DNA. ΔCD = CDcomplex − χCDDNA, where χ is mass proportion of DNA in the DNA/PCE complex. | ||
The change of the CD spectrum mainly occurred in the long wavelength region. At a lower proportion of PCE (Fig. 3a, dark gray and gray lines), the CD band split into two asymmetric peaks in the region of 250–300 nm, leaving a valley around 280 nm. The valley further grew into a negative band for the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes (Fig. 3a, light gray and blue lines). The evolution of the CD signal appears to indicate the emergence of a new band corresponding to PCE, which showed an intense UV-vis absorption band around 280 nm (see Fig. S3, ESI†). To efficiently elucidate the subtle changes observed, the “background” CD contribution of DNA was proportionally subtracted from the CD spectrum of the complex (Fig. 3b). The CD difference (ΔCD) patterns revealed a negative band around 280 nm and a positive band around 260 nm with intensity gradually increasing with an increase in PCE content in the complex (until the mass ratio of DNA
1 complexes (Fig. 3a, light gray and blue lines). The evolution of the CD signal appears to indicate the emergence of a new band corresponding to PCE, which showed an intense UV-vis absorption band around 280 nm (see Fig. S3, ESI†). To efficiently elucidate the subtle changes observed, the “background” CD contribution of DNA was proportionally subtracted from the CD spectrum of the complex (Fig. 3b). The CD difference (ΔCD) patterns revealed a negative band around 280 nm and a positive band around 260 nm with intensity gradually increasing with an increase in PCE content in the complex (until the mass ratio of DNA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCE-NO3 = 1
PCE-NO3 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The former band fits perfectly with the adsorption band of PCE, strongly suggesting that PCE has adopted a chiral structure within the complex as a result of templating by DNA. On the other hand, the second band may suggest a slight structural change of DNA induced by the electrostatic binding with PCE.
1). The former band fits perfectly with the adsorption band of PCE, strongly suggesting that PCE has adopted a chiral structure within the complex as a result of templating by DNA. On the other hand, the second band may suggest a slight structural change of DNA induced by the electrostatic binding with PCE.
Two possibilities would exist for the origin of induced chirality of PCE. One is similar to that of 1,1′-bi-2-naphthol,16 where the chirality originates from an axially chiral structure of the cobaltoceniumethylene units. As shown in Fig. S4 (ESI†), when binding to DNA, the cobaltoceniumethylene units may orient along the long axis of DNA and adopt a chiral conformation to fit the right-handed double-helices of DNA. However, such orientation would result in a serious mismatch between the positions of positive (cobaltocenium cation) and negative (phosphodiester anion) binding sites, which is thermodynamically unfavourable. Another possibility involves the cobaltocenium units possessing no specific orientation, but rather the main chain of PCE taking on a helical conformation along the DNA helices, most possibly embedded in the major or minor groove of DNA (Fig. 1), to favour electrostatic interactions and to minimize interaction with the solvent. The chromophoric groups (cobaltocenium centres) would then be aligned in a helical fashion and would eventually generate the CD signal. Raman spectra were conducted on freeze-dried complexes (see Fig. S5, ESI†). Unfortunately, structural deformation of DNA occurred during drying and structural analysis was complicated by the absence of a PCE reference due to the strong fluorescent background.
Two shorter samples of DNA (ca. 700 bp and<50 bp, see ESI† for details), showed typical B-type helical structures and were also complexed to PCE. The DNA/PCE complexes had similar CD evolution profiles to those described above (Fig. S6 and S7, ESI†). However, the complexes formed with the short DNA (<50 bp) exhibited relatively weaker induced CD in the region of 250–300 nm, especially for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex. In the long DNA-based complexes, a single DNA fiber has ample space for several PCE chains to complex to and replicate its helical structure. In contrast, in the short DNA-based complexes, a single DNA strand is unable to provide sufficient negative binding sites (<100 phosphodiester groups) to neutralize the positive charges of a single PCE chain (DPw ∼ 198) and thus a single PCE chain may interact simultaneously with two or more DNA strands. This behavior may result in the appearance of template-free randomly oriented PCE in the gap between two DNA strands thus reducing the induced CD intensity.
1 complex. In the long DNA-based complexes, a single DNA fiber has ample space for several PCE chains to complex to and replicate its helical structure. In contrast, in the short DNA-based complexes, a single DNA strand is unable to provide sufficient negative binding sites (<100 phosphodiester groups) to neutralize the positive charges of a single PCE chain (DPw ∼ 198) and thus a single PCE chain may interact simultaneously with two or more DNA strands. This behavior may result in the appearance of template-free randomly oriented PCE in the gap between two DNA strands thus reducing the induced CD intensity.
In conclusion, the template induced chiral structure of a water-soluble metal-containing polymer, PCE, has been achieved for the first time using DNA as an anionic template. The induction of chirality was thought to be facilitated by the location of the positive charges and the structural flexibility of PCE. In principle, the electrostatic templating demonstrated in this study may be applied to other metallopolymers, e.g., polyferrocenylsilanes, to induce chirality. We are currently investigating the use of chiral molecules as templates and the cooperative interaction of chiral templates with self-assembled block copolymers.17
We thank Dr Paul Verkade and Judith Mantell (Wolfson Bioimaging Facility, University of Bristol) for the cryo-TEM imaging and Professor Shunai Che (Shanghai Jiao Tong University) for her help with the Raman experiments and Prof. Derek N. Woolfson (University of Bristol) for the use of his CD equipment. We thank the EU Marie Curie program (H.Q. and J.B.G.) and the NSERC of Canada (J.B.G.) for postdoctoral fellowships. I.M. also thanks the EU for an ERC Advanced Investigator Grant.
Notes and references
- (a) K. A. Williams, A. J. Boydston and C. W. Bielawski, Chem. Soc. Rev., 2007, 36, 729 RSC; (b) G. R. Whittell and I. Manners, Adv. Mater., 2007, 19, 3439 CrossRef CAS; (c) V. Marin, E. Holder, R. Hoogenboom and U. S. Schubert, Chem. Soc. Rev., 2007, 36, 618 RSC; (d) W.-Y. Wong and P. D. Harvey, Macromol. Rapid Commun., 2010, 31, 671 CrossRef CAS; (e) A. O. Moughton and R. K. O'Reilly, Macromol. Rapid Commun., 2010, 31, 37 CrossRef CAS; (f) X. Wang and R. McHale, Macromol. Rapid Commun., 2010, 31, 331 CrossRef CAS; (g) G. R. Whittell, M. D. Hager, U. S. Schubert and I. Manners, Nat. Mater., 2011, 10, 176 CrossRef; (h) J. M. Stanley and B. J. Holliday, Coord. Chem. Rev., 2012, 256, 1520 Search PubMed.
- (a) D. J. Caruana and A. Heller, J. Am. Chem. Soc., 1999, 121, 769 CrossRef CAS; (b) N. Gajovic, G. Binyamin, A. Warsinke, F. W. Scheller and A. Heller, Anal. Chem., 2000, 72, 2963 CrossRef CAS; (c) X. Sui, X. Feng, J. Song, M. A. Hempenius and G. J. Vancso, J. Mater. Chem., 2012, 22, 11261 RSC.
- (a) C. Valério, J.-L. Fillaut, J. Ruiz, J. Guittard, J.-C. Blais and D. Astruc, J. Am. Chem. Soc., 1997, 119, 2588 CrossRef CAS; (b) M.-C. Daniel, J. Ruiz and D. Astruc, J. Am. Chem. Soc., 2003, 125, 1150 CrossRef CAS; (c) R. Djeda, A. Rapakousiou, L. Liang, N. Guidolin, J. Ruiz and D. Astruc, Angew. Chem., Int. Ed., 2010, 49, 8152 CrossRef CAS.
- (a) M. Péter, R. G. H. Lammertink, M. A. Hempenius and G. J. Vancso, Langmuir, 2005, 21, 5115 CrossRef CAS; (b) K. Kulbaba, A. Cheng, A. Bartole, S. Greenberg, R. Resendes, N. Coombs, A. Safa-Sefat, J. E. Greedan, H. D. H. Stöver, G. A. Ozin and I. Manners, J. Am. Chem. Soc., 2002, 124, 12522 CrossRef CAS.
- Y. Ma, W.-F. Dong, M. A. Hempenius, H. Möhwald and G. J. Vancso, Nat. Mater., 2006, 5, 724 CrossRef CAS.
- D. P. Puzzo, A. C. Arsenault, I. Manners and G. A. Ozin, Angew. Chem., Int. Ed., 2009, 48, 943 CrossRef CAS.
- For recent reviews on helical polymers, see: (a) E. Yashima, K. Maeda and Y. Furusho, Acc. Chem. Res., 2008, 41, 1166 CrossRef CAS; (b) E. Yashima, K. Maeda, H. Iida, Y. Furusho and K. Nagai, Chem. Rev., 2009, 109, 6102 CrossRef CAS.
- (a) Synthetic Water-Soluble Polymers, in Handbook of Thermo-plastics, ed. O. Olabisi, Plastics Engineering Series 41, Marcel Dekker, New York, 1997 Search PubMed; (b) Water-Soluble Polymers: Synthesis, Solution Properties and Applications, ed. S. W. Shalaby, G. B. Butler and C. L. McCormick, ACS Symposium Series 467, American Chemical Society, Washington, DC, 1991 Search PubMed.
- For water-soluble polyphosphazenes, see, for example: (a) Inorganic and Organometallic Polymers II: Advanced Materials and Intermediates, ed. P. Wisian-Neilson, H. R. Allcock and K. J. Wynne, ACS Symposium Series 572, American Chemical Society, Washington, DC, 1994 Search PubMed; (b) H. R. Allcock and J. Y. Chang, Macromolecules, 1991, 24, 993 CrossRef CAS; (c) H. R. Allcock, S. R. Pucher, M. L. Turner and R. J. Fitzpatrick, Macromolecules, 1992, 25, 5573 CrossRef CAS.
- For water-soluble polysilanes and polysiloxanes, see, for example: (a) C. A. van Walree, T. J. Cleij, J. W. Zwikker and L. W. Jenneskens, Macromolecules, 1995, 28, 8696 CrossRef CAS; (b) T. Seki, A. Tohnai, T. Tamaki and A. Kaito, Macromolecules, 1996, 29, 4813 CrossRef CAS; (c) T. J. Cleiy, L. W. Jenneskens and S. G. J. M. Kluijtmans, Adv. Mater., 1997, 9, 961 CrossRef; (d) D. Terunuma, K. Nagumo, N. Kamata, K. Matsuoka and H. Kuzuhara, Chem. Lett., 1998, 681 Search PubMed; (e) R. Hooper, L. J. Lyons, M. K. Mapes, D. Schumacher, D. A. Moline and R. West, Macromolecules, 2001, 34, 931 CrossRef CAS.
- For metal-containing polyeletrolytes, see, for example: (a) R. Knapp, A. Schott and M. Rehahn, Macromolecules, 1996, 29, 478 CrossRef CAS; (b) E. W. Neuse and F. B. D. Khan, Macromolecules, 1986, 19, 269 Search PubMed; (c) D. G. Kurth and R. Osterhout, Langmuir, 1999, 15, 4842 CrossRef CAS; for side-chain cobaltocenium polymers, see: (d) L. Ren, C. G. Hardy and C. Tang, J. Am. Chem. Soc., 2010, 132, 8874 CrossRef CAS; (e) L. Ren, C. G. Hardy, S. Tang, D. B. Doxie, N. Hamidi and C. Tang, Macromolecules, 2010, 43, 9304 CrossRef CAS; (f) P. Chadha and P. J. Ragogna, Chem. Commun., 2011, 47, 5301 RSC.
- For water-soluble polyferrocenylsiliane polyelectrolytes, see, for example: (a) K. N. Power-Billard and I. Manners, Macromolecules, 2000, 33, 26 CrossRef CAS; (b) M. A. Hempenius, N. S. Robins, R. G. H. Lammertink and G. J. Vancso, Macromol. Rapid Commun., 2001, 22, 30 CrossRef CAS; (c) M. A. Hempenius and G. J. Vancso, Macromolecules, 2002, 35, 2445 CrossRef CAS; (d) Z. Wang, A. Lough and I. Manners, Macromolecules, 2002, 35, 7669 CrossRef CAS; (e) M. A. Hempenius, F. F. Brito and G. J. Vancso, Macromolecules, 2003, 36, 6683 CrossRef CAS.
- (a) U. F. J. Mayer, J. P. H. Charmant, J. Rae and I. Manners, Organometallics, 2008, 27, 1524 CrossRef CAS; (b) U. F. J. Mayer, J. B. Gilroy, D. O'Hare and I. Manners, J. Am. Chem. Soc., 2009, 131, 10382 CrossRef CAS.
- Previous reports on novel DNA/metallopolymer complexes have not focused on the issue of chirality, see: (a) Z. Y. Zhong, C. Lin, Y. Ma, M. A. Hempenius, M. C. Lok, M. M. Fretz, J. F. J. Engbersen, G. J. Vancso, W. E. Hennink and J. Feijen, J. Controlled Release, 2006, 116, e81 CrossRef CAS; (b) Y. Ma, W.-F. Dong, M. A. Hempenius, H. Möhwald and G. J. Vancso, Angew. Chem., Int. Ed., 2007, 46, 1702 CrossRef CAS.
- (a) W. A. Baase and W. C. Johnson Jr., Nucleic Acids Res., 1979, 6, 797; (b) J. Kypr, I. Kejnovská, D. Renčiuk and M. Vorlíčková, Nucleic Acids Res., 2009, 37, 1713 CrossRef CAS.
- J. Brussee and A. C. A. Jansen, Tetrahedron Lett., 1983, 24, 3261 CrossRef CAS.
- J. B. Gilroy, S. K. Patra, J. M. Mitchels, M. A. Winnik and I. Manners, Angew. Chem., Int. Ed., 2011, 50, 5851 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and additional results. See DOI: 10.1039/c2cc37026c |
| This journal is © The Royal Society of Chemistry 2013 |
