Sensing applications of synthetic transport systems
Toshihide
Takeuchi†
and
Stefan
Matile
*
Department of Organic Chemistry, University of Geneva, Geneva, Switzerland. E-mail: stefan.matile@unige.ch; Web: www.unige.ch/sciences/chiorg/matile/ Fax: +41 22 379 5123; Tel: +41 22 379 6523
First published on 16th November 2012
Abstract
This feature article offers a comprehensive account of a decade of research devoted to the combination of the grand sensing principles with synthetic transport systems that act in lipid bilayers. Differential sensing, that is pattern generation and pattern recognition, is exemplified with an artificial nose. The aptamer version of immunosensing is realized with sticky-end polymers of DNA double helices for both signal generation and signal transduction. Biosensing, that is the use of enzymes for signal generation, is exemplified first with an artificial tongue and then expanded to analytes such as cholesterol, phytate or polyphenols. Enjoyable also for the general reader, we hope that this account will inspire supramolecular organic as well as analytical, physical and biological chemists.
Introduction
This account summarizes the development of sensors that operate with synthetic transport systems comprehensively, covering 18 original papers published over one decade.1–18 Since the beginning, more than three decades ago, the field of synthetic transport systems, particularly ion channels and pores that act in lipid bilayer membranes, has reached remarkable maturity. Most classical motifs of supramolecular chemistry can be found in many elegant variations, including cyclodextrins, calixarenes, calixpyrroles, crown ethers, G quartets, peptidomimetics, steroids, particularly cholates, porphyrins, organometallic architectures, and so on.19–25 Key contributions from this group include rigid rods,26 artificial β-barrels,27 cation– and anion–π slides,28 polyion–counterion complexes, folate quartets, calixarenes and peptide/urea nanotubes.29 After initial “can-do” curiosity, attention shifted to transport systems that respond to chemical and physical stimulation. Progress in this direction was essential to start thinking about applications toward medicine, biology as well as the materials sciences. More recently, synthetic transport systems have also emerged as analytical tools to explore the functional relevance of less recognized interactions such as anion–π interactions,28 halogen bonds30 and anion–macrodipole interactions.29The use of synthetic transport systems as sensors was inviting because our gustatory and olfactory systems operate with receptors in lipid bilayer membranes that respond to chemical stimulation.31–33 Moreover, applications of bioengineered ion channels and pores have attracted attention with regard to immunosensing as well as the stochastic sensing of single analytes.34 Most visible are ongoing efforts to sequence single genes with bioengineered pores.35 However, sensing applications with synthetic transport systems remained as rare as the exploration of sensing principles such as biosensing, differential sensing or immunosensing with aptamers. We felt that we could contribute to changing this situation. In the following, the development of sensors that operate with synthetic transport systems, an adventure covering one decade of intense research, is summarized comprehensively.
Differential sensing
Compared to biosensing or immunosensing with aptamers, differential sensing was the most difficult method to realize with synthetic transport systems. This was surprising for two reasons. Firstly, differential sensing has been adapted with great success to almost any other existing chemosensing system.36,37 Secondly, our own nose operates by differential sensing in lipid bilayers.31 There is no way the ∼350 olfactory receptors could differentiate between tens of thousands of odorants other than by pattern generation in the nose and pattern recognition in the brain (Fig. 1).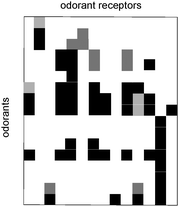 | ||
| Fig. 1 Pattern generated by selected odorant receptors. Adapted from ref. 31. | ||
The problem for differential sensing with synthetic transport systems was twofold. The first problem was to detect hydrophobic analytes, as they tend to disappear in the lipid bilayer rather than interacting with the transport system. The second, more specific problem was to generate patterns. Both problems were solved with the synthesis of reactive cations that can covalently capture aldehyde- or ketone-containing hydrophobic analytes (Fig. 2).1 Many odorants, including analytes 1–22, belong to this class (Fig. 3). Initially, ammonium (A1) and guanidinium (G1) cations with two (H2) or three (H3) reactive hydrazides were prepared. Covalent capture of analytes such as octanal 1 produces four different cationic amphiphiles with hydrazone bridges between heads and tails, i.e., A1H2-1, A1H3-1, G1H2-1 and G1H3-1. These four amphiphiles can all activate DNA as a cation transporter in lipid bilayer membranes. This activation is detectable by many different methods, using fluorogenic, chromogenic or even chirogenic probes in vesicles, conductance experiments in planar or supported membranes, and so on (see below). The different ability of the four amphiphiles to activate DNA is then used to generate patterns. Each amphiphile produces three characteristic values per analyte (EC50, YMAX and n, i.e., the effective concentration needed to reach 50% activity, the maximal activity and the Hill coefficient). With four amphiphiles per analyte, this results in 12-dimensional patterns for any collection of analytes (Fig. 3a).
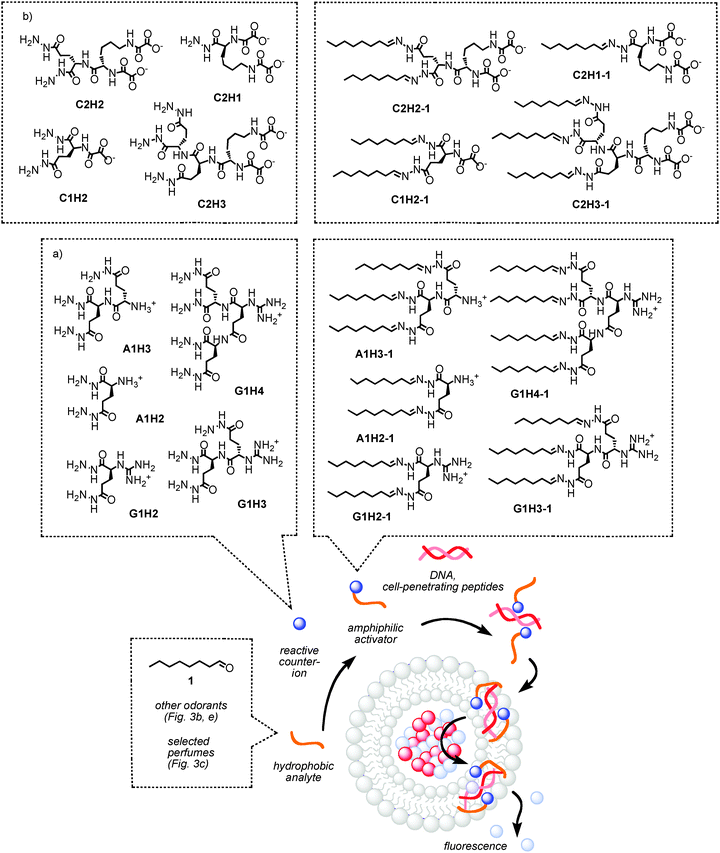 | ||
| Fig. 2 Pattern generation for differential sensing with synthetic transport systems. Reactive hydrazide cations (a) or anions (b) are incubated with 1 or other odorants or perfumes. The obtained amphiphiles activate DNA (a) or polyarginine (b) as transporters in fluorogenic vesicles. The different ability of these fragrant amphiphiles to activate polyionic transporters is generating the desired pattern (e.g., Fig. 3a). | ||
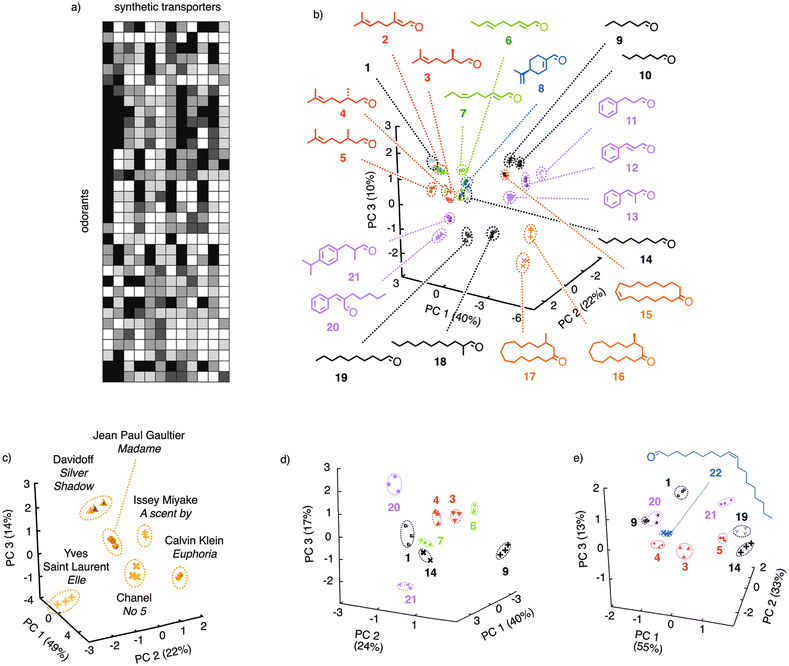 | ||
| Fig. 3 Pattern generation and pattern recognition with synthetic transport systems. (a) Generation of a 12-dimensional pattern from the different ability of amphiphiles containing G1H2, G1H3, A1H2 and A1H3 as heads and odorants 1–21 or perfumes as tails to activate calf-thymus DNA as cation transporter in fluorogenic vesicles (see Fig. 2). (b, c) Pattern recognition by principal component (PC) analysis of odorants (b) and perfumes (c) present in pattern (a); adapted from ref. 1, reproduced by permission of The Royal Society of Chemistry. (d) Same for a pattern generated with C2H2, C2H1, C1H2 and C2H3 as heads, the stated odorants as tails, and polyarginine as transporter; adapted from ref. 2 with permission, © Wiley 2011). (e) Same for a pattern generated with G1H2, G1H3 and G1H4 as heads and the stated odorants as tails, measured in fluorogenic polymersomes; adapted from ref. 3, reproduced by permission of The Royal Society of Chemistry. | ||
The pattern obtained for odorants 1–21 and selected perfumes corresponds roughly to the patterns generated in our nose (Fig. 3avs.Fig. 1). The pattern recognition taking place in our brain can be achieved by routine methods such as hierarchical cluster analysis or principal component analysis (PCA). In PCA, the information available in the n-dimensional pattern is bundled into fully virtual principal component space. Most discrimination power is present in the first axis (PC1, 40% in Fig. 3b). The more the information left in PC2 and, if needed, PC3, the better the sensing system. PCA for odorant sensing with synthetic transport systems gave 22% in PC2 and 10% in PC3. This suggested that many different interactions contribute to the discrimination of the analytes. In other words, the differential sensor is very good. Indeed, closely related odorants appeared well separated in the PC space. Apparent clusters in global PC plots (Fig. 3b) originate from projection into 2D and could be readily dissolved either in different projections or more focused PC plots. Single carbon homologs in the alkyl series, reaching from hexanal 9 to 2-methylundecanal 18, were no problem at all (Fig. 3b, black). Shorter linear and branched homologs down to acetone were accessible by inverse detection. The cinnamaldehyde family 11–13 was well resolved, extensions to cyclamen aldehyde 21 and jasmine aldehyde 20 proved detectable as well. The discrimination of cis–trans isomers was demonstrated with cucumber aldehyde 7 and its trans-isomer 6. Stereoisomers were addressed in the citronellal family 2–5. The two enantiomers of citronellal 3 and 4 as well as their racemic mixture 5 appeared well resolved in the PC space (compare also focused PC plots).1 The same could be said for racemic muscone 17 and enantioenriched muscone 16, appearing far apart and far from the macrocyclic homolog civetone 15.
To prove compatibility with samples from the supermarket, differential sensing was applied to selected perfumes. After incubation with reactive cations A1H2, A1H3, G1H2 and G1H3, their ability to activate calf-thymus DNA as a cation transporter in fluorogenic vesicles was characterized, and the obtained pattern was subjected to PCA. In the PC space, all tested perfumes were clearly identified (Fig. 3c). In this example, the differential sensors obtained from synthetic transport systems work really like a nose, differentiating analyte mixtures without information needed or revealed concerning their molecular composition.
Differential sensing was expanded next to include reactive anions. Incubation of odorants such as 1 with C1H2, C2H1, C2H2 and C2H3 now gives anionic amphiphiles with hydrazone bridges between heads and tails (Fig. 2b).2 Their different ability to activate cell-penetrating peptides such as polyarginine as anion transporters in fluorogenic vesicles was then used to generate patterns, which were subjected to PCA. In the PC space, all tested odorants were clearly recognized (Fig. 3d). Cationic amphiphiles were found to also activate DNA as cation transporters in polymersomes, that is “plastic” vesicles composed not of phospholipids but of amphiphilic poly(dimethylsiloxane)-b-poly(2-methyloxazoline)s.3 Under these conditions, a focused collection of analytes including the elongated aldehyde 22 captured by cations G1H2, G1H3 and G1H4 was clearly recognized in the PC space (Fig. 3e).
Today, a rich collection of reactive cations and anions is available, covering hydrazone, oxime and disulfide bridges with up to six tails.38 This expanded collection is of interest for differential sensing applications but also to provide rapid access to large libraries of amphiphiles. These libraries are currently screened for the cellular uptake of siRNA. Preliminary results with a GFP knockdown assay in HeLa cells contain hits as powerful as the best commercial agents known today.
Taken together, differential sensing with synthetic transport systems was a mixed experience. Whereas all analytes of interest could be identified without problems and compatibility with complex matrices was straightforward, the sensing systems needed much training to work. This was not necessary with aptamero- and biosensors and made work with these methods overall more pleasant.
Before moving on, a comment on the detection of activation or inactivation of synthetic transport systems might be helpful. In a routine assay, the transport of anionic molecules by, e.g., counterion-activated cell-penetrating peptides is detected in large unilamellar vesicles (LUVs) composed of egg yolk phosphatidylcholine (EYPC LUVs). These vesicles are loaded with carboxyfluorescein (CF) at concentrations high enough to assure self-quenching. Reduction of the local concentration upon CF release is then reported as fluorescence recovery (Fig. 4a).
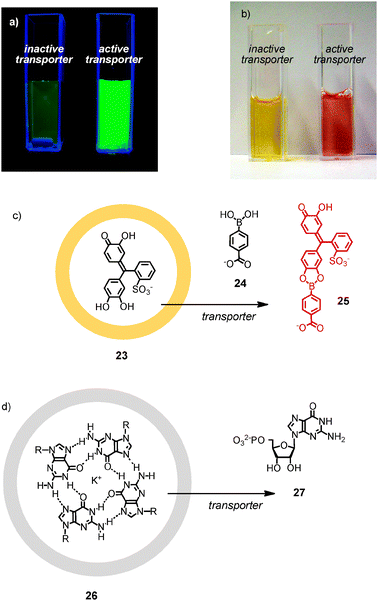 | ||
| Fig. 4 Detection of active transporters (a) as fluorescence recovery with CF-loaded vesicles (adapted from ref. 8 with permission, © 2005 American Chemical Society) or (b) as color change from yellow to red (adapted from ref. 39 with permission, reproduced by permission of The Royal Society of Chemistry). (c) Molecular basis of colorimetric detection in (b). (d) Chirogenic detection of active transporters with entrapped G-quartets. | ||
The transport of cationic molecules by, e.g., counterion-activated DNA is recorded with a similar assay. EYPC LUVs are loaded with the anionic fluorophore HPTS and the cationic quencher DPX. Export of the quencher (or the fluorophore, or both) then results in fluorescence recovery. Colorimetric probes have been developed for sensing with the “naked eye” (Fig. 4b).39 In these assays, vesicles are loaded with the yellow pyrocatechol violet 23, and the anionic carboxyphenylboronic acid 24 is added externally (Fig. 4c). Upon release of the chromophore, the red boronic ester 25 is formed. In practice, this assay can be calibrated to raise a red flag once a critical concentration of an analyte of medicinal relevance is reached, for example glucose or cholesterol (see below). Even assays for the detection of transport by circular dichroism (CD) spectroscopy have been conceived.40 Here, CD active G quartets 26 are loaded into vesicles (Fig. 4d). Local dilution upon efflux causes the disassembly into the CD silent GMP 27. This assay is also useful to determine the ion selectivity of ion channels, as G quartets form selectively with potassium.
Immunosensing, aptamer version
The development of immunosensors with synthetic transport systems was redundant because several elegant combinations of bioengineered pores with antibodies already exist in the literature.34 However, sensing with aptamers in lipid bilayer membranes has not been realized so far and was attractive because aptamers have emerged as the more accessible “DNA version” of antibodies that can in principle be selected for any analyte of choice.41,42To adapt aptamer technology to sensing with synthetic transport systems, the original ATP aptamer, a short single-stranded DNA that folds into G quartets upon ATP binding, was selected as an example (Fig. 5).41,42 Direct activation with amphiphilic cations such as 28 results in insufficient activity because the single-stranded DNA is too short to transport cations efficiently across lipid bilayer membranes.4 Formation of a double-stranded helix 29 with the complementary anti-aptamer was considered first to increase the number of negative charges in the active transport system. With double-stranded aptamer/anti-aptamer transporter 29, the maximal detectable activity increased to YMAX = 75%. Since binding occurs to single-stranded aptamers, ATP causes the disassembly of duplex 29 and was thus detectable as decrease in activity. With duplex 29, inactivation by ATP occurred with an IC50 = 2.1 mM (Fig. 5). Reduction of duplex stability with truncated anti-aptamers in 30 and mismatches in 31 improved the sensitivity toward ATP to IC50 = 1.4 mM and IC50 = 970 μM, respectively (Fig. 5).
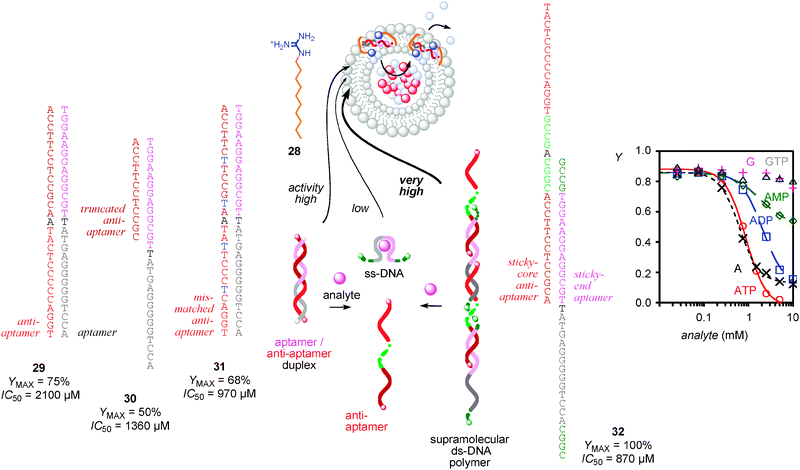 | ||
| Fig. 5 Aptamer version of immunosensing with synthetic transport systems. The activity of DNA duplexes 29–32 as counterion-activated (28) cation transport systems in fluorogenic vesicles disappears as response to their disassembly into ss-DNA upon analyte binding; YMAX refers to maximal activity accessible with transporters 29–32, IC50 to inactivation with ATP. The ds-DNA monomers 29 have lower YMAX than ds-DNA polymers 32, increasing responsiveness to ATP (decreasing IC50) with truncated (30) or mismatched (31) anti-aptamers coincides with decreasing YMAX. Dose response curves showing activity Y of 32 as a function of analyte concentration demonstrate that selectivity and sensitivity of the aptamer is as in the literature (adapted from ref. 4 with permission, © 2009 American Chemical Society). | ||
To further increase the activity of the transport systems, the aptamers were equipped with sticky ends, and sticky cores were inserted into the anti-aptamers. The resulting supramolecular polymer 32 showed perfect YMAX = 100%. Analyte binding disassembled the entire supramolecular architecture into inactive single-stranded DNA, providing the contrast needed for convenient sensing applications. The selectivity found with synthetic transport systems was as reported in the literature.40,41 Namely, adenosine and ATP were recognized best, guanosine and GTP were not recognized (Fig. 5). The relatively poor sensitivity around 1 mM was also consistent with other reports in the literature.40,41 Attempts to improve sensitivity were not made because this is a topic of aptamer chemistry that is unrelated to transport in lipid bilayers. Despite this open question, the aptamer version of immunosensing with synthetic transport systems is very promising and deserves further attention. Firstly, the method is universal, adaptable, in principle, to any analyte of free choice. Moreover, signal generation and signal transduction are unified in one and the same molecule. This differs clearly from the situation with biosensing summarized in the following.
Biosensing
Biosensors have been realized first with synthetic transport systems. As a result, the field is most developed, with many variations of the theme available to illustrate the power of the approach.The term biosensing stands for the use of enzymes for signal generation, that is to selectively pick an analyte out of a sample from the supermarket or the hospital.43 The combination of enzymes with synthetic transport systems has been introduced ten years ago.5 A l′époque, it was shown that the activity of glycosyltransferases can be detected with synthetic pores. Among all involved partners, UDP inactivated the pore most efficiently (Fig. 6, Table 1, entry 2). To follow the transformation of GlcNAc 33 into disaccharide 34, aliquots were taken from the reaction mixture and changes in their ability to interfere with the synthetic transport system were recorded as a function of reaction time. Inactivation of the synthetic transport system with increasing concentrations of the co-product UDP was observed.
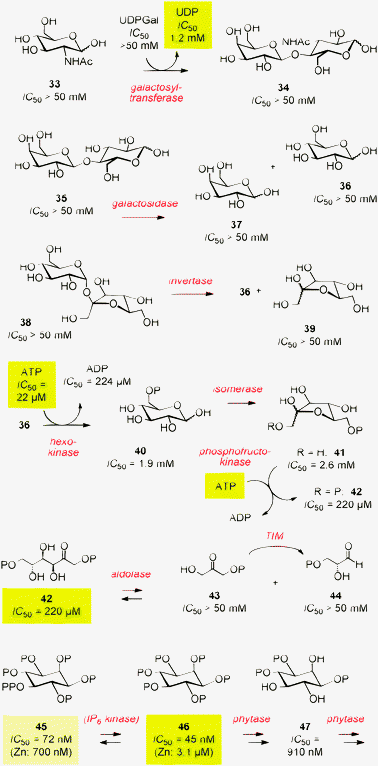 | ||
| Fig. 6 Some enzymatic reactions available for biosensing with synthetic transport systems (compare Table 1). UDPGal = uridine-5′-diphosphate galactose, UDP = uridine-5′-diphosphate, ATP = adenosine-5′-triphosphate, ADP = adenosine-5′-diphosphate, TIM = triosephosphate isomerase, OP = phosphate, OPP = pyrophosphate. | ||
| Entry | Enzymea | In-/Activatorb | IC50/EC50c | Analyted | Samplee | Contentf | Ref.g |
|---|---|---|---|---|---|---|---|
| a Enzymes confirmed as operational signal generators. b Substrate, product or derivative used to inactivate or activate the synthetic transport system as signal transducer (compare Fig. 6–8 for selected structures). c Inhibitory or effective concentration needed to inactivate or activate the synthetic transporter to 50%, respectively. d Analyte detected in complex matrices with synthetic transport systems. e Complex matrices confirmed for compatibility with biosensing with synthetic transport systems. f Analyte content detected in complex matrices. g References. h Triosephosphate isomerase. i Pronase, papain, ficin, elastase and subtilisin. j Also, Red Bull: 113 mM expected, 118 mM found; Fanta Orange: 101 mM expected, 98 mM found; Nestea Lemon: 76 mM expected, 78 mM found. k CD studies revealed an EC50 = 5 μM for the covalent capture of the divalent epigallocatechin gallate (EGCG) by 57.49 l Also, lentils: 2.3 mg g−1 expected, 1.5 mg g−1 found; soybeans: 10–28 mg g−1 expected, 9.5 mg g−1 found. m Also, caviar: 3.0–3.5 mg g−1 expected, 3.5 mg g−1 found. | |||||||
| 1 | Aldolase + TIMh | 1,6-FDP (42) | 220 μM | — | — | — | 5 |
| 2 | Galactosyltransferase | UDP | 1 mM | — | — | — | 5,6 |
| 3 | Alkaline phosphatase | UDP | 1 mM | — | — | — | 5 |
| 4 | Apyrase | ATP | 2 μM | — | — | — | 5 |
| 5 | DNA exonuclease III | [Poly(dA,dT)]2 | 190 pM | — | — | — | 6 |
| 6 | DNA polymerase I | [Poly(dA,dT)]2 | 190 pM | — | — | — | 6 |
| 7 | RNase A | Poly(U) | 210 pM | — | — | — | 6 |
| 8 | Proteasesi | Poly(E) | 18 nM | — | — | — | 6,7,11 |
| 9 | Heparinase I | Heparin | 47 nM | — | — | — | 6 |
| 10 | Hyaluronidase | Hyaluronan | 160 pM | — | — | — | 6,9 |
| 280 nM | |||||||
| 11 | Hexokinase (HK) | ATP | 82 μM | — | — | — | 7,8 |
| 12 | Phosphofructokinase | ATP | 82 μM | — | — | — | 7 |
| 13 | Invertase + HK | ATP | 22 μM | Sucrose | Coca-Colaj | 325 mM | 8,10 |
| Cola Light | 0 mM | ||||||
| 14 | β-Galactosidase + HK | ATP | 22 μM | Lactose | Milk | 122 mM | 10 |
| 15 | Acetate kinase | ATP | 22 μM | Acetate | Rice vinegar | 757 mM | 10 |
| 16 | Lactate oxidase | Pyruvate (49) | 38 mM | Lactate | Sour milk | 54–72 mM | 10,15,16 |
| Pyruvate (49) + 50 | 3 μM | ||||||
| 17 | Citrate lyase | Pyruvate (49) + 50 | 3 μM | Citrate | Orange juice | 46 mM | 10 |
| 18 | Tyrosinase | (+)-Catechin (59) + 57 | 204 μM | Polyphenols | Green tea bags | 49 mg g−1 | 13 |
| EGCG + 57 | 5 μMk | Shincha leaves | 111 mg g−1 | ||||
| 19 | Tyrosinase | (+)-Catechin (49) + 55 | 77 μM | — | — | — | 14 |
| Resveratrol (61) + 55 | 8 μM | ||||||
| 20 | Phytase | Phytate (IP6, 49) | 45 nM | Phytate | Almondsl | 19 mg g−1 | 12,18 |
| 21 | Cholesterol oxidase | Cholestenone + 50 | 600 nM | Cholesterol | Egg yolk | 10.2 mg g−1 | 17 |
| Blood serumm | 1.2 mg g−1 | ||||||
The same strategy was successful for the detection of enzymes involving substrates and products 35–47 (Fig. 6). The activity of alkaline phosphatase and apyrase was recorded following the consumption of UDP and ATP as the most powerful inactivators involved (Table 1, entries 3 and 4). Enzymes catalyzing the formation or degradation of biopolymers were ideal to detect because the latter inactivate synthetic transport systems most efficiently.6,44 This was true for the synthesis and hydrolysis of DNA with polymerase I and exonuclease III, respectively (Table 1, entries 5 and 6). RNase A was as readily accessible as a series of proteases (Table 1, entries 7 and 8).6,7 Heparin, an anionic polysaccharide of medicinal importance, was detectable with high sensitivity as activation of transport in response to incubation with heparinase (Table 1, entry 9). The detection of hyaluronidase, realized with artificial β-barrel pores6 as well as with polyion–counterion complexes,9 was attractive to screen for inhibitors needed for several reasons, including cosmetics (Table 1, entry 10).
The enzymes catalyzing the early steps of glycolysis were all detectable with synthetic transport systems. The phosphorylation of glucose 36 to glucose 6-phosphate 40 with hexokinase was monitored following the consumption of ATP, a powerful inactivator of certain β-barrel pores7,8 and counterion-activated CPPs,9 up to 10-times more potent than the ADP product (Fig. 6, Table 1, entry 11). The isomerase converting glucose 6-phosphate 40 into fructose 6-phosphate 41 was not directly detectable because substrate and product were similarly poor inactivators of synthetic transport systems (Fig. 6). However, this reaction could be coupled to the next step, in which another ATP-dependent phosphorylation leads to 1,6-FDP 42 (Fig. 6, Table 1, entry 12).7 The activity of aldolases, driven toward the retro-aldol products with TIM, was monitored following the consumption of the appreciable inactivator D-fructose 1,6-diphosphate 42 (Fig. 6, Table 1, entry 1).5 The detection of pyruvate near the end of glycolysis will be described later on (Table 1, entries 16 and 17).
These examples provide extensive experimental evidence that synthetic transport systems are ideal for the development of universal, fluorometric or colorimetric enzyme assays. The first application in biosensing focused on sucrose 36 (Fig. 6, Table 1, entry 13).8 Glycoside hydrolysis with invertase to give glucose 36 and fructose 37 was not detectable because no strong activator and inactivator of synthetic transport systems were involved. However, subsequent phosphorylation with hexokinase could be monitored with appreciable sensitivity following the consumption of the inactivator ATP.
For sugar sensing in Coca Cola, the soft drink was incubated with invertase, hexokinase and ATP, the consumption of ATP was detected as activation of the synthetic transporter and calibrated for the concentration of sucrose (Table 1, entry 13). As a negative control, Coca Cola Light failed to activate the transport system. The sucrose content in other soft drinks including Red Bull, Fanta Orange or Nestea Lemon was sensed correctly. The same system obviously works also as a glucose sensor.
To sense lactose 35 in milk serum, the enzymatic signal generator simply had to be changed from invertase to β-galactosidase (Table 1, entry 14).10 An ATP-dependent kinase was used to directly sense acetate, the sour component in vinegar (Table 1, entry 15). The situation became more challenging when we considered to build a biosensor for lactate 48, the sour component in milk. Lactate oxidase was available for signal generation (Fig. 7, Table 1, entry 16). However, neither substrate 48 nor product 49 could interfere significantly with the activity of synthetic transport systems.
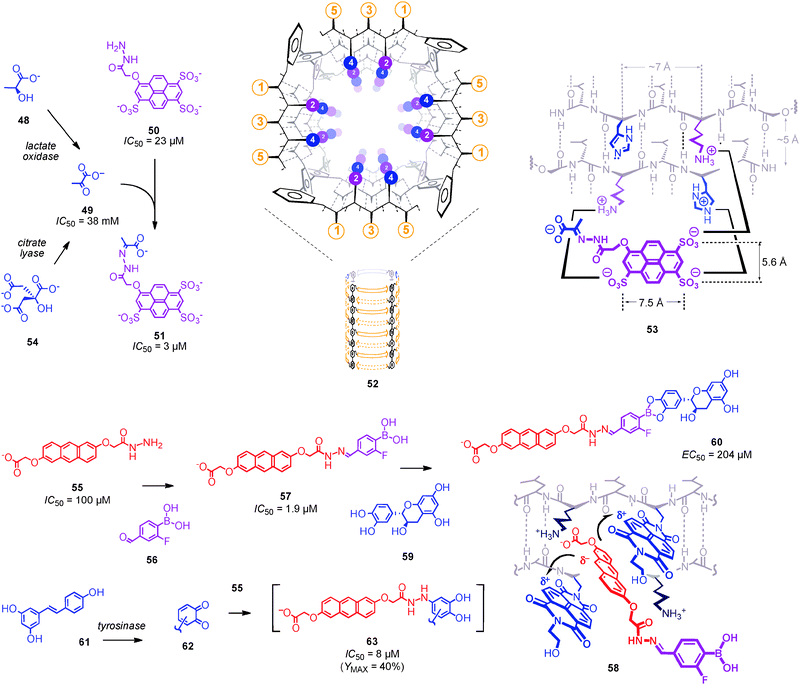 | ||
| Fig. 7 Amplifiers and converters for biosensing with synthetic transport systems (compare Fig. 8). Products of enzymatic signal generation (e.g., pyruvate 49) that fail to, e.g. inactivate pore 52 are captured in situ by an amplifier (e.g. hydrazide 50) to give a conjugate (e.g. hydrazone 51) that is recognized by the synthetic transport system (e.g. inclusion complex 53). Incubation of amplifier 55 and converter 56 gives inactivator 57 (compare inclusion complex 58) for the covalent capture of polyphenols (e.g., catechin 59), which is reported as tyrosinase-dependent activation because product 60 is too large to inactivate the transporter (e.g. pore 52). To sense catechol-free polyphenols (e.g., resveratrol 61), incubation with tyrosinase is followed by covalent capture with hydrazide amplifier 55 to give inactivators of the general structure 63. | ||
To detect otherwise elusive products of enzymatic signal generation, signal amplifiers have been introduced (Fig. 8).10 Signal amplifiers are bifunctional molecules that, firstly, modulate the activity of the synthetic transport system and, secondly, react in situ with the product of enzymatic signal generation. For lactate sensing, the hydrazide 50 was introduced as a signal amplifier (Fig. 7). Covalent capture of pyruvate 49 affords hydrazone 51, which inactivates artificial β-barrel pores 52 with internal lysine-histidine (KH) dyads with an IC50 = 3 μM (Table 1, entry 16). Signal amplification thus improved the sensitivity of the lactate sensors by more than four orders of magnitude! Inclusion complex 53 with a preorganized three-point ion pairing to K2H2 quartets on one side of a β-sheet at the inner surface of the pore was proposed to account for this outstanding molecular recognition (Fig. 7). The additional charge in conjugate 51 was likely to account for the discrimination against unreacted amplifier 50 at IC50 = 23 μM. The same recognition motif has been used to create so far unique catalytic pores.45
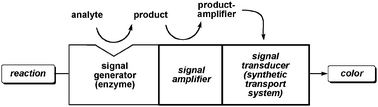 | ||
| Fig. 8 General scheme for biosensing with synthetic transport systems: the latter serve as signal transducers, enzymes serve as signal generators, signal amplifiers are bifunctional molecules that usually catch otherwise elusive analytes after enzymatic signal generation and make them recognizable for the signal transducer. | ||
Lactate sensing was realized with lactate oxidase as a signal generator, catalase for peroxide removal, hydrazide 50 as a signal amplifier and β-barrel pore 52 (2,4 = K,H) or polyguanidino-oxanorbornene transporters as signal transducers (Table 1, entry 16).10,15,16 In excellent agreement with the literature, concentrations from 54 to 72 mM were determined in sour milk.10,15 The stereoselectivity of the enzymatic signal generator provided access to chirality sensing with synthetic transport system 52 (2,4 = K,H) and amplifier 50. Studies with mixtures of L- and D-lactate demonstrated detectability of enantiopurities up to at least 99.6% ee.16 This high sensitivity was in agreement with earlier results concerning the detection of extreme ee's up to 99.9975% with synthetic transport systems.11 These results were obtained for protein biosensing with the protease subtilisin as an enantiospecific signal generator (Table 1, entry 8).
The same signal amplifier 50 was all that was needed to build a biosensor for citrate 54, the sour component in orange juice (Table 1, entry 17, Fig. 7).10 Signal generation with citrate lyase gave oxaloacetate, which may or may not decarboxylate spontaneously to pyruvate 49, a question that is irrelevant to biosensing applications. Covalent capture of pyruvate (or oxaloacetate) by amplifier 50 gave the inactivator needed for signal transduction with pore 52. A citrate concentration of 46 mM in orange juice was in perfect agreement with specifications of the vendors.
Compatibility of the same amplifier strategy with other analytes was confirmed. This includes glutamate, the main origin of the umami flavour, with either glutamate oxidase or transaminase for signal generation.
Polyphenol sensing was chosen to expand the amplifier methodology beyond analytes that contain aldehydes and ketones (Fig. 7, Table 1, entry 18).13 Polyphenols are “super-antioxidants” of medicinal relevance that occur in red wine, fruits and even chocolate. To covalently capture polyphenols, signal amplifier 55 was introduced. Amplifier 55 emerged as best from an extensive screening of π-basic inactivators of pore 52 (2,4 = K,πA) with internal π-acidic clamps or tweezers.48 Incubation of amplifier 55 with converter 56 gave hydrazone 57. This “converted” amplifier 57 inactivated pore 52 (2,4 = K,πA) with an IC50 = 1.9 μM (Fig. 7, Table 1, entry 18).13 Formal double mutant cycles demonstrated that aromatic electron donor–acceptor complexes 58 account for this excellent sensitivity.
In amplifier 57, the hydrazide in amplifier 55, ideal for the capture of aldehydes and ketones, is converted into an unreactive hydrazone. However, amplifier 57 now contains a boronic acid. Boronic acids are ideal for the covalent capture of vicinal diols as well as catechols.46,47 Covalent capture of catechol 59 as representative polyphenol inactivated inactivator 57 with an EC50 = 204 μM (Fig. 7, Table 1, entry 18). This suggested that product 60 is probably too large to interact strongly with pore 52 (2,4 = K,πA).
The presence of polyphenols should thus be detectable as formal activation of pores 52 (2,4 = K,πA) that are blocked by inactivator 57. Pore activation with polyphenon, a green tea extract with known polyphenol content, turned out to be much more effective than expected from the EC50 = 204 μM obtained for catechin 59. Structural studies by CD spectroscopy revealed that this unexpectedly powerful sensitivity of our polyphenol sensors originates from the binding of multivalent catechols such as epigallocatechin gallate (EGCG) to amplifier 57 with an EC50 = 5 μM (Table 1, entry 18).49
For biosensing, tyrosinase was introduced as a signal generator. Incubation of catechol-containing analytes with tyrosinase gave the reactive ortho-quinones, which were removed by covalent capture with a thiol-containing resin. The difference in pore activation before and after treatment with tyrosinase revealed the content of polyphenols that, firstly, contain catechols and, secondly, are tyrosinase substrates. This biosensing strategy was sufficient to detect the polyphenol content in green tea with synthetic transport systems. High quality Japanese shincha leaves turned out to contain more than twice as much polyphenols as green tea bags purchased in Geneva supermarkets (Table 1, entry 18).
However, the biosensing strategy with boronic acid 57 as a signal amplifier and tyrosinase as a signal generator captures only catechol-containing polyphenols. This excludes important members of this family such as resveratrol 61, the polyphenol in red wine that possibly accounts for the much discussed “French paradox” (Fig. 7).14 For the biosensing of catechol-free polyphenols with synthetic transport systems, oxidation with tyrosinase was followed by covalent capture of the produced ortho-quinones 62 with hydrazide 55. The obtained products with the tentative structure 63 efficiently inactivated pore 52 with an IC50 = 8 μM (2,4 = K,πA; Fig. 7, Table 1, entry 19). However, resveratrol sensing in red wine was not meaningful because only partial pore inactivation was achieved in this proof-of-principle study (YMAX = 40%).
The development of biosensors for inositol phosphates was of practical interest for several reasons.12 For instance, phytate 46, the compound that captures most organic phosphate in a non-recyclable form, is at the heart of the current phosphate crisis (Fig. 6). Phytate sensing and screening of bioengineered phytases is thus of high scientific interest. Moreover, phytate 46 and IP745 have been implied as equivalents of ADP and ATP with regard to many aspects including bioenergetics and protein phosphorylation, but the couple remains poorly explored because direct detection is difficult (Fig. 6).50
Synthetic pores 52 (2,4 = K,H) turned out to be inactivated by phytate with an excellent efficiency (IC50 = 45 nM, Table 1, entry 20).12 Phosphate removal catalyzed by phytase gradually reduced pore inactivation over the intermediate IP347 (IC50 = 910 nM) to the fully inactive inositol. Phytate sensing with phytase as a signal generator and pore 52 (2,4 = K,H) as a signal transducer was thus possible with exquisite sensitivity. The phytate content determined in almond, lentil and soybean extracts was in good agreement with expectations based on reports in the literature.
The discrimination between phytate 46 and IP745 was not possible because stoichiometric binding obscured all eventual differences. To discriminate between the two and detect the activity of IP6 kinase, binding to pore 52 (2,4 = K,H) had to be weakened. This was achieved with a zinc filter. In the presence of increasing concentrations of zinc cations, affinities for both partners decreased, leading to differences up to IC50 = 700 nM for IP745 and IC50 = 3.1 μM for IP646 that were ideal for the development of IP7 biosensors.
Hydrophobic analytes remained a big challenge for biosensing with synthetic pores of the general structure 52 because they prefer to disappear within the hydrophobic membrane rather than interacting with the signal transducer. This problem was solved with hydrazide 50 for signal amplification and polyion–counterion transporters for signal transduction.17 The construction of a cholesterol sensor was selected as an example. Signal generation with cholesterol oxidase gave cholestenone, a ketone that could be captured in situ by hydrazide 50. The obtained conjugate is an anionic amphiphile, known to activate cell-penetrating peptides as anion transporters in fluorogenic vesicles (Fig. 2).51 When carefully delivered with submicellar triton X-100, the amphiphile obtained from cholestenone and 50 activated polyarginine with an outstanding EC50 = 600 nM. Biosensing of cholesterol in egg yolk, caviar or blood serum with this sensing system gave excellent results. Cholesterol biosensors operating with cholesterol oxidase for signal generation, hydrazide 50 for signal amplification and polyarginine for signal transduction are mechanistically related to the strategy conceived for differential sensing, although the two approaches could not be more different from a conceptual point of view.1,17
It was amusing to learn that covalent capture strategies operate in gustatory sensing systems as well. Discovered around the time covalent capture has been introduced for signal amplification in biosensors that operate with synthetic transport systems,10 the “spicy” compounds 64 and 65 in mustard and cinnamon oil have been found to react with accessible thiols of the transmembrane channels TRPA1 to make the hot sensation really last (Fig. 9).33 This mechanistic insight allowed for the rational design of a “superspicy” cinnamaldehyde. All that was needed was an activated Michael acceptor with an otherwise similar structure, i.e., enone 66. Receptor activation increased from EC50 = 19 μM for 65 to EC50 = 800 nM for 66.
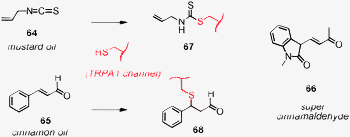 | ||
| Fig. 9 Covalent capture in biology to make hot experiences last longer, and application of the lessons learned to the design of a super cinnamaldehyde. | ||
Conclusions
The feature article summarizes sensing applications of synthetic transport systems comprehensively. The development of strategies to adapt the three fundamental sensing principles to synthetic transport systems is described.Differential sensing was accomplished with reactive cations and anions that capture hydrophobic analytes to give amphiphiles that activate DNA or cell-penetrating peptides as ion transporters in fluorogenic vesicles.1 Patterns are then generated from the different activities obtained with different reactive ions. Pattern recognition by principal component analysis is routine. All perfumes tested with this “molecular nose” were cleanly recognized in the virtual PC space.
The aptamer version of immunosensing was realized with sticky-end polymers of DNA duplexes composed of aptamer and anti-aptamer.4 The high activity of these supramolecular polymers as counterion-activated cation transporters disappeared upon disassembly in response to analyte binding. This approach has so far not been applied to sensing in complex matrices because aptamers with higher sensitivity will be needed first. However, from the point of view of sensing in bilayer membranes, aptamerosensing deserves further attention because it is universal and signal generation and transduction are unified in the same molecule.
The essence of biosensing, that is the coupling of enzymatic activity with activation or inactivation of synthetic transport systems, was illustrated initially with a “molecular tongue,” covering analytes that account for sweet, sour and umami sensations.10 The concept of signal amplification with bifunctional molecules interacting with both the product of enzymatic signal generation and the synthetic transport system was of highest importance to also create biosensors for cholesterol, polyphenols or inositol phosphates, including the otherwise elusive IP7.
Compared to other sensing techniques, it cannot be said that synthetic transport systems sense better or worse. All that can be said is that they are different. They operate with different chemistry that works very well, is easy to use and highly adaptable to tackle different tasks (biosensing, immuno-/aptamerosensing, differential sensing; colorimetric, fluorometric, electric detection, etc.). And they are obviously as biomimetic as it gets.
The main purpose to write this account was to stimulate further developments and invite for practical applications in the broadest sense. Significant opportunities to expand the introduced concepts promise inspiring and fruitful adventures. This can include innovation with regard to signal generation, signal amplification, signal transduction or signal detection, not to speak of more analytes. Promising perspectives beyond sensing naturally exist as well. For instance, the studies on biosensing demonstrate that synthetic transport systems provide ample opportunity to assemble cheap, mix-and-measure assays for the fluorometric or colorimetric detection of the activity of almost any desired enzyme: a gold mine that calls for commercialization. The dynamic amphiphiles introduced to create a molecular nose are currently used to build large libraries and screen for cellular uptake of siRNA and genes. Preliminary results for “fragrant delivery” contain hits that are as good as the best commercial agents and reveal structures that would have been predictable neither from the literature nor from activities in vesicles, i.e., confirm the importance of libraries for screening.
Acknowledgements
We thank all past and present co-workers and collaborators for their contributions, particularly the groups of Jiri Mareda (University of Geneva), Glenn Prestwich (University of Utah), Greg Tew (University of Massachusetts, Amherst), Wolfgang Meier (University of Basel), Francesco Sansone (University of Parma) and Laurent Wunsche (Firmenich), and the University of Geneva, the European Research Council (ERC Advanced Investigator), the National Centre of Competence in Research (NCCR) Chemical Biology and the Swiss NSF for financial support.References
- T. Takeuchi, J. Montenegro, A. Hennig and S. Matile, Chem. Sci., 2011, 2, 303–307 RSC.
- J. Montenegro and S. Matile, Chem.–Asian J., 2011, 2, 281–289 Search PubMed.
- J. Montenegro, J. Braun, O. Fischer-Onaca, W. Meier and S. Matile, Org. Biomol. Chem., 2011, 9, 6623–6628 CAS.
- T. Takeuchi and S. Matile, J. Am. Chem. Soc., 2009, 131, 18048–18049 CrossRef CAS.
- G. Das, P. Talukdar and S. Matile, Science, 2002, 298, 1600–1602 CrossRef CAS.
- N. Sordé, G. Das and S. Matile, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 11964–11969 CrossRef.
- N. Sordé and S. Matile, Biopolymers, 2004, 76, 55–65 CrossRef.
- S. Litvinchuk, N. Sordé and S. Matile, J. Am. Chem. Soc., 2005, 127, 9316–9317 CrossRef CAS.
- T. Miyatake, M. Nishihara and S. Matile, J. Am. Chem. Soc., 2006, 128, 12420–12421 CrossRef CAS.
- S. Litvinchuk, H. Tanaka, T. Miyatake, D. Pasini, T. Tanaka, G. Bollot, J. Mareda and S. Matile, Nat. Mater., 2007, 6, 576–580 CrossRef CAS.
- H. Tanaka and S. Matile, Chirality, 2008, 20, 307–312 CrossRef CAS.
- S. M. Butterfield, D.-H. Tran, H. Zhang, G. D. Prestwich and S. Matile, J. Am. Chem. Soc., 2008, 130, 3270–3271 CrossRef CAS.
- S. Hagihara, H. Tanaka and S. Matile, J. Am. Chem. Soc., 2008, 130, 5656–5657 CrossRef CAS.
- S. Hagihara, H. Tanaka and S. Matile, Org. Biomol. Chem., 2008, 6, 2259–2262 CAS.
- A. Hennig, G. J. Gabriel, G. N. Tew and S. Matile, J. Am. Chem. Soc., 2008, 130, 10338–10344 CrossRef CAS.
- A. Hennig and S. Matile, Chirality, 2009, 21, 145–151 CrossRef.
- S. M. Butterfield, T. Miyatake and S. Matile, Angew. Chem., Int. Ed., 2009, 48, 325–328 CrossRef CAS.
- T. Takeuchi, V. Bagnacani, F. Sansone and S. Matile, ChemBioChem, 2009, 10, 2793–2799 CrossRef CAS.
- S. Matile, A. Vargas Jentzsch, A. Fin and J. Montenegro, Chem. Soc. Rev., 2011, 40, 2453–2474 RSC.
- J. K. Chui and T. M. Fyles, Chem. Soc. Rev., 2012, 41, 148–175 RSC.
- J. T. Davis, O. Okunola and R. Quesada, Chem. Soc. Rev., 2010, 39, 3843–3862 RSC.
- A. P. Davis, D. N. Sheppard and B. D. Smith, Chem. Soc. Rev., 2007, 36, 348–357 RSC.
- P. A. Gale, Acc. Chem. Res., 2011, 44, 216–226 CrossRef CAS.
- G. W. Gokel and N. Barkey, New J. Chem., 2009, 33, 947–963 RSC.
- U. Devi, J. R. Brown, A. Almond and S. J. Webb, Langmuir, 2011, 15, 1448–1456 CrossRef.
- N. Sakai, J. Mareda and S. Matile, Acc. Chem. Res., 2005, 38, 79–87 CrossRef CAS.
- N. Sakai, J. Mareda and S. Matile, Acc. Chem. Res., 2008, 41, 1354–1365 CrossRef CAS.
- R. E. Dawson, A. Hennig, D. P. Weimann, D. Emery, V. Ravikumar, J. Montenegro, T. Takeuchi, S. Gabutti, M. Mayor, J. Mareda, C. A. Schalley and S. Matile, Nat. Chem., 2010, 2, 533–538 CrossRef CAS.
- A. Hennig, L. Fischer, G. Guichard and S. Matile, J. Am. Chem. Soc., 2009, 131, 16889–16895 CrossRef CAS.
- A. Vargas Jentzsch, D. Emery, J. Mareda, S. K. Nayak, P. Metrangolo, G. Resnati, N. Sakai and S. Matile, Nat. Commun., 2012, 3, 905 CrossRef.
- L. B. Buck, Angew. Chem., Int. Ed., 2005, 44, 6128–6140 CrossRef CAS.
- J. Chandrashekar, M. A. Hoon, N. J. Ryba and C. S. Zuker, Nature, 2006, 444, 288–294 CrossRef CAS.
- L. J. Macpherson, A. E. Dubin, M. J. Evans, F. Marr, P. G. Schultz, B. F. Cravatt and A. Patapoutian, Nature, 2007, 445, 541–545 CrossRef CAS.
- S. Matile, H. Tanaka and S. Litvinchuk, Top. Curr. Chem., 2007, 277, 219–250 CrossRef CAS.
- J. Clarke, H.-C. Wu, L. Jayasinghe, A. Patel, S. Reid and H. Bayley, Nat. Nanotechnol., 2009, 4, 265–270 CrossRef CAS.
- J. J. Lavigne and E. V. Anslyn, Angew. Chem., Int. Ed., 2001, 40, 3118–3130 CrossRef CAS.
- N. A. Rakow and K. S. Suslick, Nature, 2000, 406, 710–703 CrossRef CAS.
- J. Montenegro, E.-K. Bang, N. Sakai and S. Matile, Chem.–Eur. J., 2012, 18, 10436–10443 CrossRef CAS.
- S. M. Butterfield, A. Hennig and S. Matile, Org. Biomol. Chem., 2009, 7, 1784–1792 CAS.
- A. Hennig and S. Matile, Chirality, 2008, 20, 932–937 CrossRef CAS.
- D. E. Huizenga and J. W. Szostak, Biochemistry, 1995, 34, 656–665 CrossRef CAS.
- J. Liu and Y. Lu, Angew. Chem., Int. Ed., 2006, 45, 90–94 CrossRef CAS.
- L. C. Clark Jr and C. Lyons, Ann. N. Y. Acad. Sci., 1962, 102, 29 CrossRef CAS.
- N. Sakai, B. Baumeister and S. Matile, ChemBioChem, 2000, 1, 123–125 CrossRef CAS.
- N. Sakai, N. Sordé and S. Matile, J. Am. Chem. Soc., 2003, 125, 7776–7777 CrossRef CAS.
- T. D. James, K. R. A. S. Sandanayake and S. Shinkai, Nature, 1995, 374, 345–347 CrossRef CAS.
- G. Springsteen and B. Wang, Tetrahedron, 2002, 58, 5291–5300 CrossRef CAS.
- S. Hagihara, L. Gremaud, G. Bollot, J. Mareda and S. Matile, J. Am. Chem. Soc., 2008, 130, 4347–4351 CrossRef CAS.
- A. Hennig, S. Hagihara and S. Matile, Chirality, 2009, 21, 826–835 CrossRef CAS.
- A. Saiardi, R. Bhandari, A. C. Resnick, A. M. Snowman and S. H. Snyder, Science, 2004, 306, 2101–2105 CrossRef CAS.
- N. Sakai and S. Matile, J. Am. Chem. Soc., 2003, 125, 14348–14356 CrossRef CAS.
Footnote |
| † Current address: National Institute of Neuroscience, National Center of Neurology and Psychiatry, Tokyo, Japan. |
| This journal is © The Royal Society of Chemistry 2013 |
