DOI:
10.1039/C3BM60128E
(Paper)
Biomater. Sci., 2013,
1, 918-923
Traceless protein delivery with an efficient recyclable nanocarrier†
Received
14th May 2013
, Accepted 6th June 2013
First published on 3rd July 2013
Abstract
Intracellular delivery is a prerequisite for the efficacy of many pharmaceutical proteins. Herein, vitamin B6 (pyridoxal-5′-phosphate, PLP) functionalized calcium phosphate (CP) is used as the bio-recyclable nanocarrier for delivery of proteins into cells. Proteins could be loaded on/released from PLP-CP via formation/hydrolysis of pH sensitive aldimine bridging lysine and surface-displayed PLP. The loaded proteins could be delivered into the cytosol of HeLa, HepG2 and L929 cells where the carrier could be metabolized into endogenous metabolites of Ca2+, HPO42−, and vitamin B6. PLP-CP mediated cell transduction is 10–40 folds more efficient than TAT which is a widely used cell penetrating peptide, demonstrating the utility of PLP-CP as the traceless platform for high-efficiency delivery of proteins into mammalian cells.
Introduction
Introduction of bioactive proteins into cells is fundamental for a number of biomedical applications such as enzyme replacement therapy and immunotherapy targeting subcellular antigens.1 Given the impermeability of cell membranes to macromolecular proteins, tools enabling efficient cellular delivery would greatly advance the utility of many proteins.2 Accordingly, distinct strategies relying on synthetic carriers or cell penetrating peptides,3e.g. TAT derived from the transduction domain of HIV, have been developed to facilitate intracellular protein delivery.
Nanomaterials are promising drug carriers and yet there is increasing concern regarding their biosafety.4 For instance, mesoporous silica nanoparticles (MSNs), widely considered to be biocompatible,5 were shown to be resistant to biodegradation within cells and were accumulated in various organs.6 Recently, chemically modified MSNs were employed for delivery of proteins into cells via endocytosis, an intrinsic cellular pathway that can actively transport nanoparticles into the cells.7 We reported the cytosolic delivery of proteins into mammalian cells with aldehyde-displaying MSNs via lysosomal pH triggered protein release from the silica carrier.7e Herein, a bio-recyclable nanocarrier, assembled from calcium phosphate (CP) and pyridoxal-5′-phosphate (PLP), is used for highly efficient and traceless delivery of proteins into living cells where the carrier could be degraded into Ca2+, HPO42−, and PLP which is the biologically active form of vitamin B6 (Scheme 1).
 |
| | Scheme 1 Intracellular protein delivery mediated by PLP-CP. Proteins are loaded on PLP-CP via formation of multivalent aldimines featuring an intra-molecular H-bond (shown in red). The protein/PLP-CP composite is subjected to lysosomal pH promoted protein release and dissociation of PLP-CP into endogenous metabolites. | |
Results and discussion
Synthesis and characterization of PLP-CP
Calcium phosphate (CP), a constituent of bones, is an ideal biocompatible material. Apart from being widely used for DNA transfections in cell biology studies,8 nanosized CP was recently employed as the carriers of dyes or chemotherapeutics for imaging or treatment of cancers.9 As the cofactor of aminotransferases, PLP interacts with the ε-amino group of the lysine residue located in the catalytic sites to give aldimine. The aldimino nitrogen forms an internal hydrogen bond with the neighbouring phenolic moiety of PLP, which stabilizes the PLP–lysine complex.10 Inspired by the unique aldimine chemistry of PLP and in order to avoid the use of inert MSNs, we sought to construct PLP functionalized CP (PLP-CP) which could be used as a biocompatible nanocarrier for efficient protein loading driven by formation of aldimines and release of the payload inside cells via lysosomal acidity mediated dissociation of the protein–carrier composites (Scheme 1).
PLP-CP was easily prepared via a one-step reaction by neutralization of an aqueous solution of CaCl2–Na2HPO4–PLP (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, molar ratio) (pH 1) with addition of triethylamine under vigorous stirring. The nanocarrier was collected by centrifugation, washed with water and then lyophilized. The scanning electron microscopy (SEM) image showed that PLP-CP was rod-like particles with a typical length of 150 nm (Fig. 1A). PLP-CP displayed the yellow color of PLP whereas CP free of PLP was colorless, suggesting the incorporation of PLP into the as-prepared carrier. To quantify the levels of doped PLP, PLP-CP was treated with an EDTA solution to liberate the embedded PLP by competitive chelation of Ca2+ (ESI†). UV-vis absorption of the resulting solution showed that 4% PLP (w/w) was present in the nanocarrier (Fig. S2, ESI†). Additionally, it was shown that the zeta potential of PLP-CP in distilled water is −9.0 mV whereas that of CP is 3.5 mV (Fig. S3, ESI†), suggesting that anionic PLP was displayed on the surface of PLP-CP. These data demonstrated that PLP was successfully incorporated into PLP-CP. It is noteworthy that the contents of PLP in the nanocarrier can be easily modulated by altering the ratio of PLP to Na2HPO4 in the aforementioned reaction medium (Fig. S1, ESI†).
1, molar ratio) (pH 1) with addition of triethylamine under vigorous stirring. The nanocarrier was collected by centrifugation, washed with water and then lyophilized. The scanning electron microscopy (SEM) image showed that PLP-CP was rod-like particles with a typical length of 150 nm (Fig. 1A). PLP-CP displayed the yellow color of PLP whereas CP free of PLP was colorless, suggesting the incorporation of PLP into the as-prepared carrier. To quantify the levels of doped PLP, PLP-CP was treated with an EDTA solution to liberate the embedded PLP by competitive chelation of Ca2+ (ESI†). UV-vis absorption of the resulting solution showed that 4% PLP (w/w) was present in the nanocarrier (Fig. S2, ESI†). Additionally, it was shown that the zeta potential of PLP-CP in distilled water is −9.0 mV whereas that of CP is 3.5 mV (Fig. S3, ESI†), suggesting that anionic PLP was displayed on the surface of PLP-CP. These data demonstrated that PLP was successfully incorporated into PLP-CP. It is noteworthy that the contents of PLP in the nanocarrier can be easily modulated by altering the ratio of PLP to Na2HPO4 in the aforementioned reaction medium (Fig. S1, ESI†).
 |
| | Fig. 1 Characterization of PLP-CP. (A) SEM images of PLP-CP and CP (scale bar: 100 nm); (B) SEM images of PLP before and after storage in PBS at rt for one month (bar: 100 nm). | |
Colloidal stability is a key factor for the applications of nanoscaled materials. The morphology of PLP-CP was monitored using SEM over the storage time. No agglomeration was observed on PLP-CP before and after storage at rt in PBS for one month (Fig. 1B), revealing the beneficial role of PLP in maintaining the high colloidal stability of PLP-CP.
Interaction of proteins with PLP-CP
Traditional imines are labile to hydrolysis in aqueous media. In contrast, PLP readily complexes with lysine in the enzyme active sites under physiological conditions. In addition, the pyridoxal–polysaccharide conjugate has been employed for protein cross-linking.11 As such, we anticipated that loading of proteins on PLP-CP could be facilitated owing to the formation of multivalent PLP–lysine complexes at the interface of protein–nanocarrier. To verify the role of PLP in protein capturing, PLP-CP and CP were respectively incubated with bovine serum albumin (BSA), fluorescein isocyanate-labelled BSA (BSA-FITC), bovine pancreatic ribonuclease (RNase), and FITC-labelled RNase (RNase-FITC) in PBS for 12 h. The resulting protein/carrier composite was harvested by centrifugation, rinsed in PBS, and then analyzed by the Bradford assay to quantitate the levels of proteins absorbed on the carriers. As shown in Fig. 2, the amounts of proteins on each mg of PLP-CP were significantly higher (40–80 μg mg−1) than that on CP (0–10 μg mg−1). Additionally, the levels of loaded proteins closely correlated with the contents of PLP doped in the nanocarrier (Fig. S5, ESI†). In a separate experiment, PLP-CP/eGFP was incubated in fetal bovine serum (FBS). The time course study showed that the amounts of eGFP released into FBS were small post 6 h incubation (Fig. S6, ESI†), suggesting the stability of the protein–carrier composites in serum. Collectively, the results confirmed the critical role of PLP in efficient protein loading on the nanocarrier.
 |
| | Fig. 2 Efficiency of protein loading on PLP-CP vs. CP. PLP-CP and CP were respectively added to PBS containing BSA, BSA-FITC, RNase, or RNase-FITC (10 mg ml−1) to a final concentration of 1 mg ml−1. The solutions were incubated at 4 °C for 12 h, and then centrifuged. The pellets were re-dispersed in water and then analyzed by the Bradford assay to determine the levels of proteins loaded on carriers. The experiments were repeated three times and the error bars show the standard deviations. | |
Lysosomes are characterised by acidic luminal pH (6.0–4.0). Linkers labile to acidic pH, e.g. hydrazones and orthoesters, are often integrated with various pH-responsive drug delivery systems.12 Albeit poised to hydrolysis in acidic media, imines have been largely unexplored in delivery of therapeutic entities.13 To probe pH mediated protein release, PLP-CP/RNase-FITC was incubated in Na2HPO4–H3PO4 buffer of various pHs where FITC served as the fluorescence tag to allow tracking of proteins. A portion of the mixture was centrifuged, and the levels of protein that remained on the pellet were determined by fluorometry as a function of incubation time. Fig. 3 revealed the accelerated release of RNase-FITC from PLP-CP under acidic conditions, which is consistent with the proposed lysosomal acidity mediated protein release (Scheme 1).
 |
| | Fig. 3 pH dependent protein release from PLP-CP. PLP-CP/RNase-FITC was spiked into Na2HPO4–H3PO4 (100 mM) of indicated pHs to a final concentration of 2.5 mg ml−1 and then incubated for 0–30 min. The fluorescence emission of protein that remained on the carrier was acquired at the indicated time points of incubation (λex at 488 nm). | |
PLP-CP mediated delivery of protein into mammalian cells
PLP-CP was assessed for its capability to deliver proteins into living cells. Human hepatoma cells (HepG2) were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with RNase-FITC or PLP-CP/RNase-FITC for 4 h. Fluorescence microscopic images showed that FITC signal was present in cells treated with PLP-CP/RNase-FITC whereas no fluorescence was observed in cells cultured with RNase-FITC (Fig. S7–S8, ESI†), demonstrating the effectiveness of PLP-CP in the delivery of hydrophilic protein into live cells.
We further explored the impact of cell lines on PLP-CP mediated protein transduction. L-Asparaginase (ASP) fused with N-terminal eGFP (ASP-eGFP) and RNase-FITC were used as the model proteins. RNase is a small protein (13.7 kDa) whereas ASP is a large protein composed of four identical subunits (160 kDa) and has been marketed as an anticancer drug for the treatment of acute lymphoblastic leukemia. HeLa, HepG2 and L929 cells pre-loaded with DAPI were respectively cultured with PLP-CP/RNase-FITC or PLP-CP/ASP-eGFP for 2–10 h and then stained with LysoTracker Red for 15 min. The signals of RNase-FITC and ASP-eGFP were both clearly and universally present in the cell populations after 2 h of incubation (Fig. S9, ESI†), revealing the capability of PLP-CP to ferry different sized proteins into a variety of mammalian cells. LysoTracker Red is a lysosome-specific dye and DAPI is a nucleus-staining dye. Confocal microscopy images of individual cells of HeLa, HepG2 and L929 at different stages of incubation showed that the colocalization of RNase-FITC or ASP-eGFP signals with LysoTracker Red was negligible post 2 h incubation. The colocalization increased substantially after 4 h culturing and was then attenuated upon prolonged 10 h incubation (Fig. 4). The time dependent colocalization patterns suggested that the payloads were initially internalized into lysosomes and then translocated into the cytosol. Taken together, these data indicated that PLP-CP could be used for the delivery of proteins into the cytosol of mammalian cells.
 |
| | Fig. 4 Time dependent intracellular distribution of PLP-CP/RNase-FITC and PLP-CP/ASP-eGFP. HeLa, HepG2 and L929 cells pre-stained with DAPI (1 μM) were respectively incubated in DMEM containing PLP-CP/RNase-FITC (25 μg ml−1) or PLP-CP/ASP-eGFP (25 μg ml−1) for 2–10 h, and then stained with LysoTracker Red (1 μM) for 15 min. The cells were analyzed by fluorescence confocal microscopy to pinpoint the subcellular locations of internalized proteins. A merge of Lysotracker Red (in red) and ASP-eGFP/RNase-FITC (in green) is shown in yellow. | |
Reactivity of proteins delivered into cells by PLP-CP
Proteases are abundantly located in lysosomes. It is critical that the internalized PLP-CP/protein nanocomposites could relocate from lysosomes into the cytosol to avoid significant lysosomal proteolysis. As eGFP fluorescence is dependent on the structural integrity,14 the intense eGFP fluorescence within cells transduced with PLP-CP/eGFP or PLP-CP/ASP-eGFP suggested that the internalized eGFP remained structurally intact (Fig. 4). To further determine whether a protein delivered by PLP-CP remained bioactive inside cells, HeLa, L929 and HepG2 cells were respectively cultured with PLP-CP/β-galactosidase and then stained with X-Gal which is a chromogenic substrate of β-galactosidase. Deep-blue staining was observed in all the cell lines that have been treated with PLP-CP/β-galactosidase while no color formation could be detected in the control cells (Fig. 5). Compared to L929 cells, HepG2 is a cell line derived from macrophages which efficiently internalize various vesicles such as pathogens and viruses. The intense color within HepG2 relative to that of L929 shown in Fig. 5 implies that cellular uptake of protein-loaded PLP-CP may differ among cells lines. The differential staining patterns of the cells treated with or without β-galactosidase/PLP-CP showed that β-galactosidase delivered into the mammalian cells retained its catalytic activity.
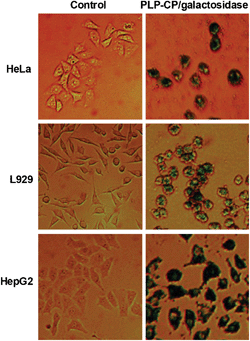 |
| | Fig. 5 Activity of β-galactosidase delivered into cells by PLP-CP. HeLa, HepG2 and L929 cells were respectively cultured in DMEM spiked with free β-galactosidase (25 μg ml−1) or β-galactosidase loaded on PLP-CP (25 μg ml−1) for 4 h. The cells were washed with PBS, incubated in fresh DMEM containing X-gal (100 μM) for 12 h and then photographed. | |
Efficiency of protein delivery mediated by PLP-CP vs. TAT
TAT peptide has been widely used to facilitate cellular delivery of various cargos.3g To discern protein transduction mediated by PLP-CP vs. TAT, HepG2 cells were respectively transduced with eGFP, PLP-CP/eGFP or TAT-eGFP which is a fusion protein with NH2-terminal TAT peptide. As expected, cells treated with TAT-eGFP exhibited considerable fluorescence emission intensity whereas no fluorescence was observed in cells treated with eGFP (Fig. 6). Fig. 6 illustrates the unbiased subcellular distribution of TAT-eGFP, e.g. in the cytosol and the nucleus, which is consistent with previous observations.3i In contrast, cells cultured with PLP-CP/eGFP displayed intense punctate fluorescence largely located in the cytosol (Fig. 4 and 6), suggesting that PLP-CP preferentially ferries proteins into the cytosol, which is desirable for the efficacy of many therapeutic proteins.
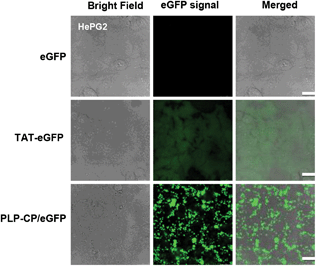 |
| | Fig. 6 Intracellular delivery of eGFP mediated PLP-CP vs. TAT. L929 cells were respectively cultured for 4 h in DMEM (1 ml) supplemented with eGFP (5 μg), TAT-eGFP (5 μg) or eGFP (5 μg) loaded on PLP-CP. The cells were washed with PBS, and examined by confocal fluorescence microscopy. | |
We then quantify protein internalization by flow cytometry. HeLa, L929 and HepG2 cells were independently cultured with equal amounts of TAT-eGFP, free eGFP, or eGFP pre-loaded on PLP-CP. As anticipated, the overall fluorescence intensity within cells treated with TAT-eGFP is moderately higher than control cells treated with or without eGFP (Fig. 7). Surprisingly, the uptake of PLP-CP/eGFP into the three cell lines was dramatically greater than TAT-eGFP under the assay conditions. The mean intracellular fluorescence of HeLa and HepG2 cells treated with PLP-CP/eGFP was roughly 40 folds higher than that of the corresponding cells treated with TAT-eGFP while L929 cells transduced with PLP-CP/eGFP displayed 10-fold enhancement in the intracellular fluorescence as compared to L929 cells cultured with TAT-eGFP (Fig. 7). The superior efficacy of PLP-CP over TAT proves that PLP-CP is a highly efficient carrier for the delivery of proteins into mammalian cells.
 |
| | Fig. 7 Flow cytometric quantitation of protein transduction mediated by PLP-CP vs. TAT. HeLa (A), HepG2 (B) and L929 cells (C) were respectively incubated for 4 h in DMEM (1 ml) containing eGFP (5 μg), TAT-eGFP (5 μg) or eGFP (5 μg) loaded on PLP-CP. The cells were washed with fresh DMEM, and then analyzed by flow cytometry. The viable cells were gated under identical conditions and the fluorescence emission was recorded using λex at 488 nm. | |
Cytotoxicity of PLP-CP
Lastly, PLP-CP was evaluated for its potential cytotoxicity by a trypan blue exclusion test. No toxic effects of PLP-CP were identified on the viability of HepG2 cells at doses up to 100 μg ml−1 after incubation for 0–24 h (Fig. 8), suggesting that PLP-CP is of low cytotoxicity.
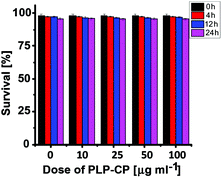 |
| | Fig. 8 Cytotoxicity of PLP-CP. HepG2 cells were incubated in DMEM containing various amounts of PLP-CP (0–100 μg ml−1) for 0–24 h. Cell viability was determined by a trypan blue exclusion test. | |
Conclusions
PLP-CP, vitamin B6 functionalized calcium phosphate, effectively delivers proteins into the cytosol of mammalian cells with an efficiency 10–40 folds higher than that of TAT, a cell penetrating peptide widely used for cellular delivery. Compared with previous efforts utilizing biologically/chemically modified proteins (e.g. TAT fused protein) or synthetic vectors comprised of abiotic components,3 our system uses intact proteins, which could be released from PLP-CP in a pH dependent manner, and a “green” carrier that could be metabolized into safe and endogenous species of living systems. With the ease of carrier synthesis and the ubiquitous presence of lysine in proteins, PLP-CP would be of practical utility as the traceless nanocarrier for high-efficiency delivery of various proteins into mammalian cells.
Acknowledgements
We thank Professor Xiaomei Yan of Xiamen University for providing β-galactosidase. Dr S. Han was supported by grants from NSF China (21272196, 21072162) and the Fundamental Research Funds for the Central Universities (2011121020); Dr J. Han was supported by grants from NSF China (31221065, 91029304, 81061160512) and the 973 program (2009CB522200).
Notes and references
-
(a) Y. Lee, T. Ishii, H. J. Kim, N. Nishiyama, Y. Hayakawa, K. Itaka and K. Kataoka, Angew. Chem., Int. Ed., 2010, 49, 2552 CrossRef CAS;
(b) K. G. Ford, B. E. Souberbielle, D. Darling and F. Farzaneh, Gene Ther., 2001, 8, 1 CrossRef CAS;
(c) S. R. Schwarze and S. F. Dowdy, Trends Pharmacol. Sci., 2000, 21, 45 CrossRef CAS.
- B. Leader, Q. J. Baca and D. E. Golan, Nat. Rev. Drug Discovery, 2008, 7, 21 CrossRef CAS.
-
(a) Y. Lee, T. Ishii, H. Cabral, H. J. Kim, J. H. Seo, N. Nishiyama, H. Oshima, K. Osada and K. Kataoka, Angew. Chem., Int. Ed., 2009, 48, 5309 CrossRef CAS;
(b) M. Yan, J. Du, Z. Gu, M. Liang, Y. Hu, W. Zhang, S. Priceman, L. Wu, Z. H. Zhou, Z. Liu, T. Segura, Y. Tang and Y. Lu, Nat. Nanotechnol., 2010, 5, 48 CrossRef CAS;
(c) S. K. Kim, M. B. Foote and L. Huang, Biomaterials, 2012, 33, 3959 CrossRef CAS;
(d) G. A. Ellis, M. J. Palte and R. T. Raines, J. Am. Chem. Soc., 2012, 134, 3631 CrossRef CAS;
(e) L. N. Patel, J. L. Zaro and W. C. Shen, Pharm. Res., 2007, 24, 1977 CrossRef CAS;
(f) M. Mae and U. Langel, Curr. Opin. Pharmacol., 2006, 6, 509 CrossRef;
(g) V. P. Torchilin, Adv. Drug Delivery Rev., 2008, 60, 548 CrossRef CAS;
(h) A. Joliot and A. Prochiantz, Nat. Cell Biol., 2004, 6, 189 CrossRef CAS;
(i) S. R. Schwarze, A. Ho, A. Vocero-Akbani and S. F. Dowdy, Science, 1999, 285, 1569 CrossRef CAS.
- D. Peer, J. M. Karp, S. Hong, O. C. Farokhzad, R. Margalit and R. Langer, Nat. Nanotechnol., 2007, 2, 751 CrossRef CAS.
- J. L. Vivero-Escoto, I. I. Slowing, B. G. Trewyn and V. S. Lin, Small, 2010, 6, 1952 CrossRef CAS.
-
(a) X. Huang, L. Li, T. Liu, N. Hao, H. Liu, D. Chen and F. Tang, ACS Nano, 2011, 5, 5390 CrossRef CAS;
(b) J. Lu, M. Liong, Z. Li, J. I. Zink and F. Tamanoi, Small, 2010, 6, 1794 CrossRef CAS;
(c) K. K. Pohaku Mitchell, A. Liberman, A. C. Kummel and W. C. Trogler, J. Am. Chem. Soc., 2012, 134, 13997 CrossRef CAS.
-
(a) J. S. Lim, K. Lee, J. N. Choi, Y. K. Hwang, M. Y. Yun, H. J. Kim, Y. S. Won, S. J. Kim, H. Kwon and S. Huh, Nanotechnology, 2012, 23, 085101 CrossRef;
(b) I. I. Slowing, B. G. Trewyn and V. S. Lin, J. Am. Chem. Soc., 2007, 129, 8845 CrossRef CAS;
(c) S. S. Bale, S. J. Kwon, D. A. Shah, A. Banerjee, J. S. Dordick and R. S. Kane, ACS Nano, 2010, 4, 1493 CrossRef CAS;
(d) J. Mendez, A. Monteagudo and K. Griebenow, Bioconjugate Chem., 2012, 23, 698 CrossRef CAS;
(e) X. Wu, S. Wu, L. Yang, J. Han and S. Han, J. Mater. Chem., 2012, 22, 17121 RSC.
-
(a) C. A. Chen and H. Okayama, BioTechniques, 1988, 6, 632 CAS;
(b) W. Song and D. K. Lahiri, Nucleic Acids Res., 1995, 23, 3609 CrossRef CAS.
-
(a) E. I. Altinoglu, T. J. Russin, J. M. Kaiser, B. M. Barth, P. C. Eklund, M. Kester and J. H. Adair, ACS Nano, 2008, 2, 2075 CrossRef CAS;
(b) B. M. Barth, E. I. Altinoglu, S. S. Shanmugavelandy, J. M. Kaiser, D. Crespo-Gonzalez, N. A. DiVittore, C. McGovern, T. M. Goff, N. R. Keasey, J. H. Adair, T. P. Loughran Jr., D. F. Claxton and M. Kester, ACS Nano, 2011, 5, 5325 CrossRef CAS;
(c) M. Kester, Y. Heakal, T. Fox, A. Sharma, G. P. Robertson, T. T. Morgan, E. I. Altinoglu, A. Tabakovic, M. R. Parette, S. M. Rouse, V. Ruiz-Velasco and J. H. Adair, Nano Lett., 2008, 8, 4116 CrossRef CAS;
(d) T. T. Morgan, H. S. Muddana, E. I. Altinoglu, S. M. Rouse, A. Tabakovic, T. Tabouillot, T. J. Russin, S. S. Shanmugavelandy, P. J. Butler, P. C. Eklund, J. K. Yun, M. Kester and J. H. Adair, Nano Lett., 2008, 8, 4108 CrossRef CAS.
-
(a) H. H. Limbach, M. Chan-Huot, S. Sharif, P. M. Tolstoy, I. G. Shenderovich, G. S. Denisov and M. D. Toney, Biochim. Biophys. Acta, 2011, 1814 Search PubMed , 1426; ;
(b) L. Liu and R. Breslow, J. Am. Chem. Soc., 2002, 124, 4978 CrossRef CAS;
(c) L. Liu, W. Zhou, J. Chruma and R. Breslow, J. Am. Chem. Soc., 2004, 126, 8136 CrossRef CAS.
- F. Sasaki, Y. Tsuchido, S. Sawadaa and K. Akiyoshi, Polym. Chem., 2011, 2, 1267 RSC.
-
(a) R. V. Chari, Acc. Chem. Res., 2008, 41, 98 CrossRef CAS;
(b) M. C. Garnett, Adv. Drug Delivery Rev., 2001, 53, 171 CrossRef CAS;
(c) A. Kakinoki, Y. Kaneo, Y. Ikeda, T. Tanaka and K. Fujita, Biol. Pharm. Bull., 2008, 31, 103 CrossRef CAS;
(d) T. Etrych, M. Jelinkova, B. Rihova and K. Ulbrich, J. Controlled Release, 2001, 73, 89 CrossRef CAS;
(e) Z. Huang, X. Guo, W. Li, J. A. MacKay and F. C. Szoka Jr., J. Am. Chem. Soc., 2006, 128, 60 CrossRef CAS;
(f) E. R. Gillies, A. P. Goodwin and J. M. Frechet, Bioconjugate Chem., 2004, 15, 1254 CrossRef CAS;
(g) W. Gao, J. M. Chan and O. C. Farokhzad, Mol. Pharm., 2010, 7, 1913 CrossRef CAS.
-
(a) L. Matesic, J. M. Locke, K. L. Vine, M. Ranson, J. B. Bremner and D. Skropeta, Bioorg. Med. Chem., 2011, 19, 1771 CrossRef CAS;
(b) Y. H. Kim, J. H. Park, M. Lee, T. G. Park and S. W. Kim, J. Controlled Release, 2005, 103, 209 CrossRef CAS;
(c) S. Duan, W. Yuan, F. Wu and T. Jin, Angew. Chem., Int. Ed., 2012, 51, 7938 CrossRef CAS.
- P. Corish and C. Tyler-Smith, Protein Eng., 1999, 12, 1035 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2013 |
Click here to see how this site uses Cookies. View our privacy policy here. 
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, molar ratio) (pH 1) with addition of triethylamine under vigorous stirring. The nanocarrier was collected by centrifugation, washed with water and then lyophilized. The scanning electron microscopy (SEM) image showed that PLP-CP was rod-like particles with a typical length of 150 nm (Fig. 1A). PLP-CP displayed the yellow color of PLP whereas CP free of PLP was colorless, suggesting the incorporation of PLP into the as-prepared carrier. To quantify the levels of doped PLP, PLP-CP was treated with an EDTA solution to liberate the embedded PLP by competitive chelation of Ca2+ (ESI†). UV-vis absorption of the resulting solution showed that 4% PLP (w/w) was present in the nanocarrier (Fig. S2, ESI†). Additionally, it was shown that the zeta potential of PLP-CP in distilled water is −9.0 mV whereas that of CP is 3.5 mV (Fig. S3, ESI†), suggesting that anionic PLP was displayed on the surface of PLP-CP. These data demonstrated that PLP was successfully incorporated into PLP-CP. It is noteworthy that the contents of PLP in the nanocarrier can be easily modulated by altering the ratio of PLP to Na2HPO4 in the aforementioned reaction medium (Fig. S1, ESI†).
1, molar ratio) (pH 1) with addition of triethylamine under vigorous stirring. The nanocarrier was collected by centrifugation, washed with water and then lyophilized. The scanning electron microscopy (SEM) image showed that PLP-CP was rod-like particles with a typical length of 150 nm (Fig. 1A). PLP-CP displayed the yellow color of PLP whereas CP free of PLP was colorless, suggesting the incorporation of PLP into the as-prepared carrier. To quantify the levels of doped PLP, PLP-CP was treated with an EDTA solution to liberate the embedded PLP by competitive chelation of Ca2+ (ESI†). UV-vis absorption of the resulting solution showed that 4% PLP (w/w) was present in the nanocarrier (Fig. S2, ESI†). Additionally, it was shown that the zeta potential of PLP-CP in distilled water is −9.0 mV whereas that of CP is 3.5 mV (Fig. S3, ESI†), suggesting that anionic PLP was displayed on the surface of PLP-CP. These data demonstrated that PLP was successfully incorporated into PLP-CP. It is noteworthy that the contents of PLP in the nanocarrier can be easily modulated by altering the ratio of PLP to Na2HPO4 in the aforementioned reaction medium (Fig. S1, ESI†).







