High-throughput characterisation of osteogenic differentiation of human mesenchymal stem cells using pore size gradients on porous alumina†
Peng-Yuan
Wang‡§
ab,
Lauren R.
Clements§
b,
Helmut
Thissen
c,
Wei-Bor
Tsai
*a and
Nicolas H.
Voelcker
*d
aDepartment of Chemical Engineering, National Taiwan University, No. 1 Roosevelt Rd. Sec. 4, 106 Taipei, Taiwan. E-mail: weibortsai@ntu.edu.tw
bSchool of Chemical and Physical Sciences, Flinders University, Bedford Park, 5042 SA, Australia
cCSIRO Materials Science and Engineering, Bayview Avenue, Clayton, 3168 VIC, Australia
dMawson Institute, University of South Australia, Mawson Lakes, 5095 SA, Australia. E-mail: nico.voelcker@unisa.edu.au
First published on 18th June 2013
Abstract
The ability to control the cellular response is of critical importance when designing advanced biomaterials for applications in tissue engineering and regenerative medicine. An important aspect of biointerfacial interactions is surface topography at the nanoscale and therefore this needs to be taken into consideration. Here, a pore size gradient in porous alumina (pAl) was fabricated with pore sizes ranging from 50 nm to 3 μm. The attachment behaviour and osteogenesis of human mesenchymal stem cells (hMSCs) was investigated along this topography gradient for up to 2 weeks. Generally, cell attachment density and spreading area decreased with increasing pore size. Pore wall width and solid surface fraction also played a key role in cell adhesion. After 2 weeks, osteogenesis of hMSCs was enhanced by porous topography with a pore size of 120–230 nm in diameter and 10 nm pore wall width, compared with other topographies of the gradient. The results demonstrate that the gradient format allows in-depth high-throughput screening of surface parameters that are important for the control of mammalian cell behaviour, thereby advancing the development of new and improved biomaterials for e.g. orthopaedic and tissue engineering applications.
1. Introduction
The ability to direct the cellular response, and in particular the response of stem cells, by means of biomaterial surface topography is of critical importance for biomedical and tissue engineering applications.1 Substrate surface topography has been shown to be an effective cue for the regulation of cellular responses2 including stem cell fate.3 Cell attachment, migration, proliferation and differentiation can be effectively regulated by various topographic features such as pores4 and grooves5 with dimensions ranging from the nano- to the micro-scale. Since cellular responses to these surface topographies are feature size-dependent, surface topographies with various pore characteristics have been fabricated in order to explore cell–surface topography interactions. However, analysis and screening of discrete, individual samples is a tantalising task due to the large combinatorial space that has to be analysed. Therefore, a high-throughput approach such as the use of surface-bound gradients is desirable, since it significantly reduces the time required for sample preparation and analysis and allows the fine-tuning of surface characteristics in a single experiment.Such gradients have helped define parameters dictating optimal conditions for cell attachment for a variety of cell types. Whilst there have been considerable efforts devoted to the fabrication of chemical gradients,6,7 significantly fewer methods for the generation of physical (topographical and mechanical) gradients have emerged so far.8 One recent example are porous silicon (pSi) gradients,9–11 designed for uses ranging from optical sensors to the modulation of cell behaviour and stem cell differentiation. A decrease in pore size has been shown to increase the attachment of rat mesenchymal stem cells (rMSCs).11 In another study, a pore size of >1 μm and <50 nm in diameter was shown to be optimal for the attachment of neuron-like cells.9 In addition, it has been shown that the pore size not only influences the attachment of cells but also the morphology and differentiation of cells.9,11 Whilst the use of pSi gradients has many advantages in terms of its ease of fabrication and further functionalisation, the stability of the biodegradable substrate is a continuous issue for long term cell culture studies. For this reason, a stable substrate with tuneable porous gradients is highly desirable.
Porous alumina (pAl) is fabricated by means of anodic etching of aluminium foil in aqueous acids. The key advantage of pAl over pSi for long-term cell culture is its high stability in aqueous environments.12 In addition, aluminum based materials have been used as an implant material for a long time.13 For these reasons, the use of pAl has attracted much attention in recent years.12,14,15 In addition, the difficulty to further functionalise the pAl surface has been overcome in recent years, resulting in pAl emerging as an increasingly useful biomaterial.16,17,18 Therefore, the aim of this study was exploring the pore size effect on the osteogenic differentiation of human MSCs using pAl gradients. A novel and facile method was implemented for pAl gradient fabrication. The cellular response in regard to morphology and osteogenesis of human MSCs in response to the various pore diameters and structures were investigated systematically on the pAl gradients over a course of two weeks.
2. Experimental
2.1. Materials
Aluminum (Al) foil (0.1 mm thick, 99.99%) was purchased from Alfa Asear, Ward Hill, MA, USA. Oxalic acid was purchased from Fluka (Milwaukee, WI, USA). Phosphoric acid was provided by Ajax Finechem (Taren Point, Australia). Solutions were prepared in 18.2 MΩ Millipore water (Eschborn, Germany). Modified Eagle's Medium low-glucose (DMEM-LG), MEM non-essential amino acid, L-glutamine, penicillin, streptomycin, and amphotericin were purchased from Invitrogen (Carlsbad, USA). All other reagents for cell culture and characterisation were purchased from Sigma-Aldrich (St. Louis, USA) unless specified otherwise.2.2. Surface preparation
Al foil was sonicated in acetone for 30 min followed by submersion in a 5% sodium hydroxide solution for 10 s and rinsing with copious amounts of Milli-Q water and drying in a nitrogen gas stream. Porous alumina (pAl) with a uniform pore size was generated by anodic etching. The custom made reaction cell was immersed in an oxalic acid solution (0.3 M, 0 ± 0.1 °C) and stirred vigorously while a voltage was applied between the gold back contact and the lead counter electrode (100 V, 2 min). Following etching, the pAl was rinsed with Milli-Q water, then incubated in toluene for 20 min and dried in a nitrogen gas stream. pAl gradients were achieved by means of chemical etching. Fixed to a vertical motorised motion stage, the surfaces were slowly dipped into a solution of 5% phosphoric acid (25 °C, gentle stirring) for a period of 180 min at a constant velocity (0.0012 mm s−1). The dipping velocity was controlled using LabView software. Following complete immersion, the surfaces were immediately washed with copious amounts of Milli-Q water and dried with a gentle nitrogen gas stream.2.3. Human mesenchymal stem cells
Human mesenchymal stem cells (hMSCs) were received from Veteran's General Hospital (Taipei, Taiwan) and maintained according to a previously described protocol.19 hMSCs were cultured and passaged in culture medium (DMEM-LG supplemented with 100 U mL−1 penicillin, 100 μg mL−1 streptomycin, 2 mM L-glutamine and 10% (v/v) fetal bovine serum). Cells were seeded on substrates with a cell density of 2 × 104 cells cm−2. Cell morphology and density were examined after 24 h. After 24 h culture, the medium was changed to osteogenic medium (culture medium supplemented with 50 μg mL−1 ascorbate-2 phosphate, 10 nM dexamethasone and 10 mM β-glycerophosphate) for osteogenic induction. Cell morphology and calcium and phosphorus deposition were examined after a further 7 days and 14 days of induction culture.2.4. Scanning electron microscope and energy dispersive X-ray spectroscopy
pAl gradients were analysed using a Helios NanoLab Dual Beam Focused Ion Beam/Scanning Electron Microscope (FIB/SEM) (FEI, USA). SEM images were collected at 1 mm intervals. Pore size, depth, pore wall width and solid surface fraction (%) were determined from SEM images using NIH ImageJ.After 24 h and 14 day osteogenic differentiation culture, the samples were rinsed with phosphate buffered saline (PBS: 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 1.8 mM KH2PO4 with pH 7.4), and then fixed with 4% (v/v) paraformaldehyde for 10 min. The samples were dehydrated in a graded ethanol solution of 50, 70, 80, 90, 95% ethanol in Milli-Q water and then twice in absolute ethanol. After air-drying, the samples were sputtered with gold and observed using SEM (JSM-5310, JEOL, Japan). Energy dispersive X-ray spectroscopy (EDS, Oxford Instruments) was employed to determine calcium and phosphorus elements on the osteogenic differentiation sample. Three parallel experiments were carried out along the surface and the weight percent of each element was averaged (n = 3).
2.5. Fluorescence staining
For fluorescence staining at 24 h and 7 day cultures, the samples were fixed with 4% (v/v) paraformaldehyde for 10 min, permeated with 0.1% Triton X-100 in PBS for 15 min, followed by incubation with 2% bovine serum albumin for 15 min. F-actin and nuclei were stained with 500 nM Phalloidin-TRITC and 100 nM DAPI, respectively, for 1 h. For calcium staining, Calcein Blue solution (30 μM) was added before fixation as described previously.8 Nuclei and F-actin were counterstained with Pico Green (10 μM) and phalloidin-TRITC (500 nM), respectively. Fluorescence images were captured using a spectral confocal and multiphoton system (TCS SP5, Leica, Germany). Cell density was quantified by counting nuclei along the pAl gradient and flat Al. Three parallel experiments were run and the density was averaged from three images at each location. Cell spreading area was quantified by drawing the cell outline from captured images along the pAl gradient and flat Al using NIH ImageJ software.20 A total of 50–75 cells were analysed at each location.2.6. Statistical analysis
Statistic analysis was performed using GraphPad Instat 3.0 program (GraphPad Software, La Jolla, CA). The statistical analysis between each group was determined with one-way ANOVA and Student–Newman–Keuls multiple comparison tests. P < 0.05 was considered as significant.3. Results
3.1. Analysis of porous alumina gradient surfaces
A uniform pAl layer with a uniform pore size of 41 ± 7 nm was generated in oxalic acid at a current density of 100 V (ESI, Fig. S1A). pAl gradients were subsequently prepared by dipping the uniform pAl layer in 5% aqueous phosphoric acid using a vertical motion stage at a constant velocity. The phosphoric acid acted as a chemical etchant and resulted in pore enlargement that correlated with the immersion time. Since the anodised pAl was quite hydrophilic after the initial anodisation step, dipping the sample into the phosphoric acid solution caused significant wicking, thus making it difficult to generate a gradient of immersion time. However, we observed that by first incubating the as-anodised pAl in toluene for 20 min, followed by drying with nitrogen prior to dip-coating, the wicking was reduced to an acceptable level.A typical pAl gradient shows a continuous surface colour change from silver to dark yellow over a distance of 12 mm (ESI, Fig. S1B). Lateral pAl gradients were visualised via SEM (Fig. 1A). Representative SEM images with 1 mm spacing along the gradient show the change in pore characteristics over the length of the gradient. In the region of the lowest incubation time (Fig. 1A, image i, 0 mm, 0 min) the pore size was found to be 46 ± 8 nm (Fig. 1B). This smallest pore size is slightly larger than the initial uniform pAl surface (that was not exposed to phosphoric acid solution, Fig. S1A) of 41 ± 7 nm, which could be explained by slight wicking of the solution during the dip coating process. Increasing the incubation time to 30 min (image iii, 2 mm, Fig. 1A) resulted in a pore size increase to 61 ± 9 nm (Fig. 1B). Progressing further along the gradient at 4 mm and 6 mm, (image v and vii, Fig. 1A) corresponding to an incubation time of 60 min and 75 min, the pore size was found to be 101 ± 10 nm and 147 ± 14 nm, respectively (Fig. 1B). At a distance of 7 mm (image viii, 105 min, Fig. 1A) the pore opening almost reached critical diameter (229 ± 45 nm) as the pore wall width neared zero (Fig. 1B and 1C). Consequently, the pore walls began to collapse, creating much larger porous structures. At a distance of 10 mm, the pore wall collapse became even more prominent (image xi, Fig. 1A), resulting in pore voids of 1052 ± 939 nm (Fig. 1B). Additionally, brush-like fibres were observed, which may represent the remnants of pore walls that were not strong enough to stand independently, alternatively tilting to form tee pee like structures. In the region of the highest incubation time (image xiii, 12 mm, 180 min, Fig. 1A) the pore walls appeared further corroded with average pore size of 2710 ± 648 nm (Fig. 1B). This phenomenon has been observed by Dronov et. al. during pore opening of uniform pAl surfaces for 90–180 min where the pore size increased with increasing incubation in phosphoric acid.12 A relatively linear increase in pore size was observed for the shorter incubation times from 0 min (0 mm) to 75 min (6 mm), with increasing standard deviations observed for longer incubation times, explained by the collapse of the porous structure and formation of large voids in the film (Fig. 1B). The pore wall width decreased linearly with increasing incubation times for the first 7 mm along the gradient (Fig. 1C). For the shortest incubation time (0 min), the pore wall width was 127 ± 32 nm. Following 60 min incubation (4 mm), the pore wall width decreased to 19 ± 11 nm. Finally at 7 mm, the pore wall width was a mere 4 ± 4 nm. Longer incubation times resulted in pore wall collapse and as a result large voids formed within the porous layer. This resulted in remnants or pore wall structures tipping over to form tee pee like structures, and therefore an increase in the pore wall width to 72 ± 35 nm and 128 ± 75 nm at a distance of 8 mm and 10 mm respectively. The largest pore wall with was observed at the longest incubation time (12 mm, 452 ± 202 nm). Similarly, as expected, the solid surface fraction also decreased with increasing incubation times (Fig. 1D). At the shortest incubation time (0 min), the solid surface fraction was 90 ± 0.6%, and decreased linearly over a distance of 7 mm to 17 ± 2%. The solid surface fraction was then shown to plateau, with a final solid surface fraction of 3.4 ± 0.5% for the longest incubation time (12 mm, 180 min).
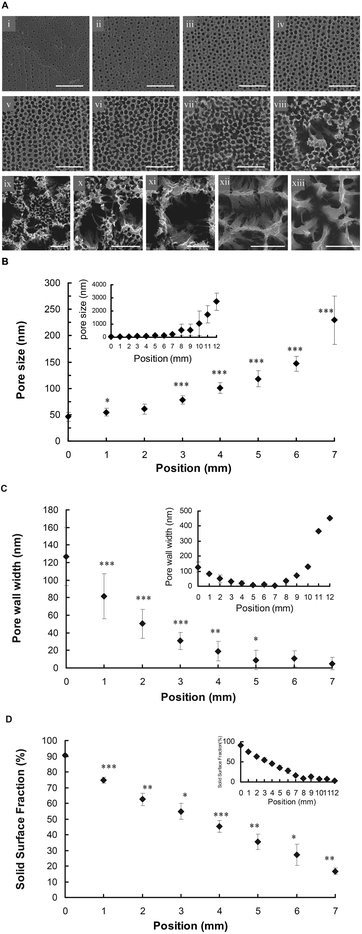 | ||
| Fig. 1 (A) Scanning electron microscopy (SEM) images of pAl gradient at 1 mm intervals along the gradient. Scale bar represents (i–ix) 4 μm, (x–xiii) 1 μm. Images were captured 90° to the substrate surface. (B) Pore size analysis of pAl gradient using captured SEM images from 0 mm (0 min incubation time) to 7 mm (105 min incubation time) along the gradient (number of pores analysed = 50). (C) Pore wall width (n = 3). (D) Solid surface fraction (n = 3). The inserted graphs show the topography change along the entire 12 mm gradient. Error bars = standard deviation. *, **, *** indicate p < 0.05, 0.01, 0.001 compared with the previous position. | ||
The thickness of porous layer was also found to vary across the pAl surface (Fig. 2). The initial layer thickness of the pAl prior to pore enlargement was 4.2 ± 0.1 μm thick (Fig. 2B). Following incubation in phosphoric acid for 30 min (2 mm), the porous layer increased to 4.7 ± 0.04 μm. At an incubation time of 60 min (4 mm), the porous layer was 5.5 ± 0.05 μm thick, whilst after 90 min (6 mm) the porous layer was 5.8 ± 0.03 μm (Fig. 2B). It was unpractical to measure the pore thickness along the pAl gradient at the larger incubation times (8–12 mm) due to the severe pore wall collapse observed, but could assume that the layer thickness may continue increase along the pAl gradient from 8 mm to 12 mm.
 | ||
| Fig. 2 (A) Representative cross-section images for distances of (i) 1 mm, (ii) 4 mm, (iii) 7 mm along the pAl gradient. Scale bar = 4 μm. (B) Porous layer thickness of the pAl gradient analysed using captures SEM images of pAl cross-sections. SEM images were acquired every 1 mm along the gradient from 0 mm (0 min incubation) to 7 mm (105 min incubation). Error bars = standard deviation (n = 5). For larger pore sizes, the pore wall collapse was too severe to determine accurate pAl thicknesses. *, **, *** indicate p < 0.05, 0.01, 0.001 compared with the previous position. | ||
The steepness and pore size range of the pAl gradient could be controlled through adjustment of the dip coating velocity into the phosphoric acid solution, with a velocity of 0.0012 mm s−1 (corresponding to a maximum incubation time of 180 min) proving to be optimal to cover the largest pore range. Longer incubation times (>200 min) led to complete pore wall collapse. Conversely, whilst a shorter maximum incubation time (<150 min) led to the successful preparation of a gradient surface, a smaller pore range was achieved. One of the key advantages of our dipping method is the ability to extend the length of the gradient to any desired length by changing the dipping velocity in accordance with the increased length of the gradient desired. As expected, incubation of pAl gradients in PBS at 37 °C for 21 days showed no visible signs of pore degradation (ESI, Fig. S2), suggesting that pAl is a stable substrate for cell culture.
3.2. Attachment of hMSCs
Attachment of hMSCs to the pAl substrates was dependent on the pore size (Fig. 3). SEM images displayed the cell density across the pAl gradient (Fig. 3A), whilst fluorescence images provided information in regard to cell morphology and cytoskeletal features at higher magnification (Fig. 3B). Cell density at the small pore size end was the highest (0 mm, 11.88 ± 0.88 × 103 cm−2), with the cell density gradually decreasing toward the large pore end (12 mm, 3.60 ± 0.32 × 103 cm−2) (Fig. 3A and 4A). Cell density was significantly higher on the flat Al (17.82 ± 1.23 × 103 cm−2, p < 0.001) compared with cell density on the pAl gradient. Cell density on the small pore end was 34% lower than the flat Al, whilst cell density on the large pore end was 80% lower compared to the flat Al. | ||
| Fig. 3 Cell attachment and morphology of hMSCs along the pAl gradient after 24 h. (A) SEM images and (B) fluorescence images along the gradient from (i) small pore end (0 mm) to (vi) large pore end (12 mm) and (F) flat Al. Images represent 2.4 mm intervals along the gradient. Nucleus (blue) and F-actin (red) was stained with DAPI and Phalloidin-TRITC, respectively. Arrows indicated cell protrusions. Scale bar = 200 μm in SEM images and 100 μm in fluorescence images. | ||
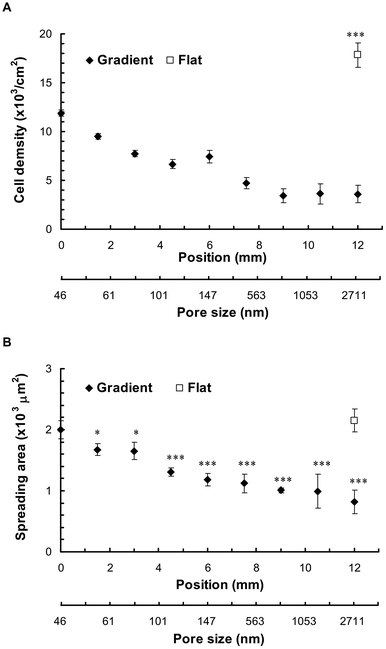 | ||
| Fig. 4 Cell density and spreading area of hMSCs along the pAl gradient after 24 h. (A) Cell density was quantified by counting the nucleus along the gradient and flat Al from three separate samples (n = 3). Error bars = standard deviation. (B) Spreading area was quantified by drawing the cell outline using ImageJ software. Analysed cells were 50–75. Error bars = standard error mean. *, **, *** indicate p < 0.05, 0.01, 0.001 (flat compared with porous regions). | ||
Cytoskeletal staining showed a fully spread-out morphology of attached cells on the flat Al and the small pore end of the pAl gradient (Fig. 3B), whilst cells were small and often had multiple protrusions on the large pore end (12 mm, arrows in Fig. 3B, image vi). The morphology of cells changed gradually along the gradients from the small pore end to the large pore end (Fig. 3B). The averaged cell area on flat Al was 2.15 ± 0.19 × 103 μm2, which was significantly higher than for porous region, except at the small pore size end (0 mm, p < 0.001, Fig. 4B). The degree of cell spreading gradually decreased from 1.99 ± 0.15 × 103 μm2 at the small pore end (0 mm), to 1.60 ± 0.11 × 103 μm2 in the middle region (6 mm), and to 1.32 ± 0.20 × 103 μm2 at the large pore end (12 mm) of the pAl gradient (Fig. 4B).
3.3. Osteogenic differentiation of hMSCs
After 1 day in culture, hMSCs were induced to become osteoblast-like cells using osteogenic medium. After 1 week induction culture, samples were stained with Calcein Blue (for calcium phosphate deposits), PICO Green (nuclei), and Phalloidin-TRITC (F-actin). Calcein Blue staining failed to show any signal along the pAl gradient after 1 week. Fluorescence microscopy images showed that the cell morphology of hMSCs became elongated after induction (Fig. 5A). Quantitative data showed that there still was a cell density gradient along the later part of pAl gradient, i.e. the 7–12 mm region, after 1 week induction (Fig. 5B). Cell density on the flat Al (57.5 ± 5.0 × 103 μm2) was significantly higher than the large pore end (34.3 ± 4.7 × 103 μm2, p < 0.01). Cell density on the small pore region (0–7 mm), was similar to flat Al. | ||
| Fig. 5 Cell morphology and cell density of hMSCs after 1 week induction culture along the pAl gradient. (A) Fluorescence images along the gradient from (i) small pore end to (vi) large pore end and (F) flat Al. Images represent 2.4 mm intervals along the gradient. Nucleus (green) and F-actin (red) was stained with PICO Green and Phalloidin-TRITC, respectively. (B) Cell density was quantified by counting the nucleus along the pAl gradient and flat Al (n = 3). Error bars = standard deviation. *,** indicate p < 0.05, 0.01 (flat compared with porous regions). | ||
After 2 week induction culture, cell morphology and calcium/phosphorous deposition was analysed using SEM and EDS, respectively, along the gradient. In the 0–7 mm region of the gradient, as well as the flat Al surface, cell clusters were observed, whilst there were no cell clusters found in the 7–12 mm region (Fig. 6A). hMSCs were in general well-spread and flat on the surface (appearing as dark patches in the images) except for occasional 3D cell clusters (which appeared brighter than the background). In addition, it was difficult to observe spread cells at the large pore size end (12 mm) after the 2 week induction culture. EDS element analysis showed that calcium (Ca) deposition increased gradually along the gradient from the small pore size end (0 mm, 2.1 ± 0.4 wt% Ca) to the middle region of the gradient (5–7 mm) where the largest intact pore sizes were observed reaching a peak value of 10.7 ± 6.9 wt% Ca (6 mm, Fig. 6B), and decreased again gradually to the region of pore destruction (12 mm, 2.9 ± 2.8 wt% Ca). At region 5–7 mm, Ca was significantly higher than at the small pore size end (1–2 mm, p < 0.05) and the large pore end (11 mm, p < 0.05). However, at this region (5–7 mm), there was no statistical difference in Ca content compared with flat Al (7.9 ± 3.5 wt% Ca). Interestingly, phosphorous (P) was only detected at the central region of the gradient (5–7 mm, Fig. 6B). The amount of phosphorous observed on the surface at a distance of 5 mm, 6 mm and 7 mm along the gradient was 2.4 ± 3.4 wt%, 2.6 ± 4.5 wt%, and 1.5 ± 2.6 wt%, respectively, resulting in the ratio of Ca/P was 2.1, 2.0, and 1.4, respectively.
 | ||
| Fig. 6 Osteogenic differentiation of hMSCs after 2 week induction culture along the pAl gradient. (A) Scanning electron microscopy (SEM) images along the gradient from (i) small pore end to (vi) large pore end and (F) flat Al. Images displayed at 2.4 mm intervals along the gradient. The cells appeared a dark-background and the clusters appeared 3-D bright area due to the charging effect. A typical energy dispersive X-ray spectroscopy (EDS) analysis was used for quantifying the elements Ca and P along the gradient. (B) Element analysis along the pAl gradient. Error bar = standard deviation (n = 3). *,** indicate p < 0.05, 0.01 compared with positions of 1 mm, 2 mm, and 11 mm on the pAl gradient. | ||
4. Discussion
MSCs are a powerful cell source for regenerative medicine and tissue engineering, and also an emerging in vitro study system for drug delivery, immune diseases, and cancer therapy.21–24 It is therefore imperative to gain a better understanding of MSC behaviour, especially human MSCs, on biomaterial surfaces. The choice of the substrate surfaces in this study was motivated by the fact that implants widely used in orthopaedic and dental surgery are made from alumina ceramics (Al2O3).25 Because porous alumina is categorised as “bioinert material”, surface modification of porous alumina is of great interest26,27 for improving the biostability and bioactivity at the cell–material interface due to the excellent biocompatibility and mechanical strength of alumina.13 Another feature of porous alumina is that the porous structure can be directly grown on metal implant surfaces and have a very broad size range (5 nm–10 μm).28,29Porous substrates have demonstrated their intrinsic potential for osteoconductivity in vitro.30,31 The osteogenic differentiation of MSCs on porous surfaces has been reported to respond differently to the feature size of nanopores30 and even the symmetry of nanopores.4 However, the study of cell behaviour on discrete substrates with different properties is not only time-consuming and costly, but also leads to systematic errors between sample sets. Recently, porous silicon (pSi) gradients have been designed and fabricated for studying the MSC–surface topography interaction.10,11,19 However, until the degradation and delamination of the pSi layer can be arrested to a greater extent, longer term cell culture studies are not feasible on this substrate. To this end, we have designed a novel method to fabricate a stable pore size gradient of pAl with pore sizes ranging from 50 nm to 3 μm across a 12 mm distance. Here, the pore size effect of pAl on hMSCs osteogenic differentiation was investigated using a high-throughput screening approach.
Previous research has shown that nanopitted surfaces with 120 nm diameter with 300 nm center–center spacing fabricated by electron beam lithography disrupted cell adhesion, decreased cytoskeleton organisation, and downregulated cell proliferation of osteoblasts.32–34 In those studies, cells formed stellate shaped morphology with long filopodia or spindle shape morphology with the F-actin network orientated in the direction of cell polarity and small spreading area on the porous surfaces. Another study showed that MSC behaviours depended on the feature sizes of nanopitted surface (15–100 nm diameters).35 Nanopits with 15 nm diameter facilitated cell adhesion and osteogenic differentiation, whilst 100 nm diameter pits inhibited both adhesion and differentiation. This is consistent with a previous study suggesting that cell contact guidance on surfaces is sensitive to a critical length scale of around 70 nm.36 The results also suggested that topographic feature size has a tremendous effect on cell behaviour. One advantage of using our gradient format is that cell behaviour can be monitored on various pore sizes at the same time. Our results showed that cell attachment density and spreading area were decreased on nanoscale pores, even the smallest (50 nm) pores, when compared to flat Al. Cell attachment decreased when pore size increased continuously along the gradient, suggesting that there is no “watershed” on cell–surface interaction.
Cell adhesion is not solely dependent on the pore size, but also many other parameters such as the symmetry of the pores,4 edge–edge spacing, solid surface fraction,11 and roughness. Therefore, study of different nanopitted surfaces would help to construct the whole picture of cell–surface interaction on biomaterials. Using a gradient format, this task is readily achievable. For tissue engineering purposes, surface properties should be optimised for enhancing cell adhesion, proliferation, and differentiation. However, the same platform technology could also be applied to other screening purposes such as inhibition of biofilm formation and reduction of immune response.37
The mechanism by which topography affects cell attachment and spreading has been related to the change of focal adhesion formation and adhesive force.33,38 Biggs et al. summarised that focal adhesion formation changed when the filopodia are in contact with nanoscale pores.38 To facilitate integrin clustering in cells adhering to an array of nanoprotrusions, the feature diameter must exceed 70 nm. In another word, focal adhesion will be perturbed on nanoprotrusion with size smaller than 70 nm. Therefore, nanoscale pits >70 nm in diameter perturb integrin clustering, forcing focal adhesion formation to occur at the interpit regions. In our study, we found that 50 nm nanopits with 120 nm edge-to-edge spacing (solid surface fraction = 90.6%) did not disturb cell adhesion significantly, suggesting that focal adhesions were forced forming at the interpit regions. When the pore size increased to 150 nm accompanying by the pore wall width decreasing to 10 nm (solid surface fraction = 27.3%), both cell attachment density and spreading area decreased. When the pore wall collapse occurs along the latter half (7–12 mm) of the gradients, average pore size increased dramatically accompanying by solid surface fraction decreasing. Focal adhesion was thus disturbed by the surface topography changing. Using single cell force spectroscopy, it was found that the adhesive force of hMSCs on a porous TiO2 surface with a pore size of 45 nm diameter and pore wall width of 19 nm (detachment force of 268 ± 5 pN) was lower by approximately 25% compared to the flat surface (detachment force of 360 ± 20 pN) measurements.39 Therefore, focal adhesion formation appears to play a critical role to determine cell adhesion, attachment, and spreading on the porous surfaces.
A porous surface with optimal geometry could stimulate osteogenic differentiation of hMSCs. Indeed, Dalby et al. showed that nanopits with the same pore size (120 nm diameter) but various symmetries (square with 300 nm centre–centre spacing, displaced square 20 nm, displaced square 50 nm, and random placements) influence osteogenic differentiation of hMSCs distinctly. Displaced square 50 nm surfaces stimulated osteogenic differentiation without osteogenic supplements compared with the other geometries.4 Another study showed that porous surfaces 80–100 nm diameter, with a 20–50 nm pore wall width, promoted osteogenic differentiation of rat MSCs.40 The present study showed the highest Ca and P deposition at the central part of the gradient (5–7 mm) with Ca/P ratio of about 2.1. The inorganic part of bone matrix is hydroxyapatite, Ca10(PO4)6(OH)2, and the Ca/P ratio in human bone is 2.5. The ratio balance between Ca and P at the central part of the gradient (5–7 mm) is close to this ratio. The amount of Ca and P and the ratio of Ca/P are usually represented as the indicators of osteogenesis in vitro,41 and the ratio is essential for the development and maintenance of a strong and healthy skeletal structure.42
Not only does the cell–substrate interaction play an important role in mechanotransduction for osteogenesis, but cell–cell interactions are also crucial for regulating the autocrine signalling.43 The formation of bone nodule-like morphology is a common index for judging osteogenesis in vitro. Our result shows that cell clusters were only found at 0–7 mm regions and flat Al. However, cell density on the 0–4 mm regions was higher than on 5–7 mm region. The formation of cell clusters may be due to higher cell density, but not osteogenesis, resulting in lower Ca deposition in the regions of 0–4 mm. Furthermore, it is noteworthy that cells were confluent on the flat Al after 1 day culture (Fig. 3), whilst cells density on the small pore end (the highest area on pAl gradient) were 66% of the flat Al. After 1 week induction culture, the cell density on flat Al and the small pore end was similar. This result suggested that cells grew faster on the flat Al and entered the differentiation stage earlier than those on the small pore end, resulting in higher Ca deposition on flat Al than the small pore end. Therefore, our results indicated that the pore size ranging 120–230 nm with about 10 nm pore wall width may stimulate osteogenic hMSCs to deposit the bone matrix apatite compared with other topographical feature sizes across the pAl gradient and the flat control. Using the pAl gradient, we were not only able to explore cell–surface behaviour on different feature sizes, but also a series of successive behaviours of hMSCs on a single sample, which is difficult to achieve using discrete substrates.
5. Conclusion
This study revealed the importance of surface topography for the control of hMSC behaviour using a pAl pore size gradient. The pAl gradient allowed us to characterise cell–surface interactions on biomaterial surfaces in a more systematic and reliable manner compared with using discrete samples. Pore size-dependent attachment behaviour of hMSCs was observed along the pAl gradient. Large pores and low solid surface fraction resulted in a low cell attachment density and spreading area of hMSCs. Osteogenic differentiation of hMSCs was optimal in the middle part of the gradient where the pore sizes was 120–230 nm in diameter, pore wall width was around 10 nm, and solid surface fraction was around 27.3%. This study provides a novel high-throughput format for long-term screening and studying of topography effects on mammalian cell behaviour. The results presented in this study are expected to facilitate the development of advanced biomaterials for orthopaedic, tissue engineering and stem cell biology applications.Acknowledgements
The authors gratefully acknowledge funding from the Australian Research Council and the CSIRO Food Futures Flagship. P.Y.W. acknowledges support from the National Science Council (Taiwan) via the award of a travel scholarship under the Graduate Program for Studying (GPS) in Australia/New Zealand scheme (98-2911-I-002-056).References
- C. J. Bettinger, R. Langer and J. T. Borenstein, Angew. Chem., Int. Ed., 2009, 48, 5406 CrossRef CAS.
- P. Y. Wang, T. H. Wu, P. H. Chao, W. H. Kuo, M. J. Wang, C. C. Hsu and W. B. Tsai, Biotechnol. Bioeng., 2013, 110, 327 CrossRef CAS.
- P. Y. Wang, W. T. Li, J. Yu and W. B. Tsai, J. Mater. Sci.: Mater. Med., 2012, 23, 3015 CrossRef CAS.
- M. J. Dalby, N. Gadegaard, R. Tare, A. Andar, M. O. Riehle, P. Herzyk, C. D. W. Wilkinson and R. O. C. Oreffo, Nat. Mater., 2007, 6, 997 CrossRef CAS.
- P.-Y. Wang, J. Yu, J.-H. Lin and W.-B. Tsai, Acta Biomater., 2011, 7, 3285 CrossRef CAS.
- S. Morgenthaler, C. Zink and N. D. Spencer, Soft Matter, 2008, 4, 419 RSC.
- J. Genzer and R. R. Bhat, Langmuir, 2008, 24, 2294 CrossRef CAS.
- P.-Y. Wang, W.-B. Tsai and N. H. Voelcker, Acta Biomater., 2012, 8, 519 CrossRef CAS.
- Y. L. Khung, G. Barritt and N. H. Voelcker, Exp. Cell Res., 2008, 314, 789 CrossRef CAS.
- L. R. Clements, P. Y. Wang, W. B. Tsai, H. Thissen and N. H. Voelcker, Lab Chip, 2012, 12, 1480 RSC.
- P.-Y. Wang, L. R. Clements, H. Thissen, A. Jane, W.-B. Tsai and N. H. Voelcker, Adv. Funct. Mater., 2012, 22, 3414 CrossRef CAS.
- R. Dronov, A. Jane, J. G. Shapter, A. Hodges and N. H. Voelcker, Nanoscale, 2011, 3, 3109 RSC.
- E. Gultepe, D. Nagesha, S. Sridhar and M. Amiji, Adv. Drug Delivery Rev., 2010, 62, 305 CrossRef CAS.
- S. H. Chung, S. J. Son and J. Min, Nanotechnology, 2010, 21, 125104 CrossRef CAS.
- C. J. Ingham, J. Maat and W. M. de Vos, Biotechnol. Adv., 2012, 30, 1089 CrossRef CAS.
- A. M. M. Jani, D. Losic and N. H. Voelcker, Prog. Mater. Sci., 2013, 58, 636 CrossRef.
- A. Mutalib Md Jani, I. M. Kempson, D. Losic and N. H. Voelcker, Angew. Chem., Int. Ed., 2010, 49, 7933 CrossRef.
- A. Mutalib Md Jani, E. J. Anglin, S. J. McInnes, D. Losic, J. G. Shapter and N. H. Voelcker, Chem. Commun., 2009, 3062 RSC.
- P.-Y. Wang, L. R. Clements, H. Thissen, S.-C. Hung, N.-C. Cheng, W.-B. Tsai and N. H. Voelcker, RSC Adv., 2012, 2, 12857 RSC.
- P.-Y. Wang, H.-T. Yu and W.-B. Tsai, Biotechnol. Bioeng., 2010, 106, 285 CrossRef CAS.
- A. Torsvik and R. Bjerkvig, Cancer Treat. Rev., 2013, 39, 180 CrossRef CAS.
- Q. Bao, Y. Zhao, H. Niess, C. Conrad, B. Schwarz, K. W. Jauch, R. Huss, P. J. Nelson and C. J. Bruns, Stem Cells Dev., 2012, 21, 2355 CrossRef CAS.
- M. Maumus, D. Guerit, K. Toupet, C. Jorgensen and D. Noel, Stem Cell Res. Ther., 2011, 2, 14 CrossRef.
- R. M. Dwyer, S. Khan, F. P. Barry, T. O'Brien and M. J. Kerin, Stem Cell Res. Ther., 2010, 1, 25 CrossRef.
- T. I. Berge and A. G. Gronningsaeter, Clin. Oral Implants Res., 2000, 11, 154 CAS.
- J. ter Maat, R. Regeling, C. J. Ingham, C. A. Weijers, M. Giesbers, W. M. de Vos and H. Zuilhof, Langmuir, 2011, 27, 13606 CrossRef CAS.
- E. E. Swan, K. C. Popat and T. A. Desai, Biomaterials, 2005, 26, 1969 CrossRef.
- H. Wieneke, O. Dirsch, T. Sawitowski, Y. L. Gu, H. Brauer, U. Dahmen, A. Fischer, S. Wnendt and R. Erbel, Catheter. Cardiovasc. Interventions, 2003, 60, 399 CrossRef.
- K. C. Popat, G. Mor, C. A. Grimes and T. A. Desai, Langmuir, 2004, 20, 8035 CrossRef CAS.
- M. J. Dalby, N. Gadegaard, A. S. Curtis and R. O. Oreffo, Curr. Stem Cell Res. Ther., 2007, 2, 129 CrossRef CAS.
- K. C. Popat, K. I. Chatvanichkul, G. L. Barnes, T. J. Latempa Jr., C. A. Grimes and T. A. Desai, J. Biomed. Mater. Res., Part A, 2007, 80, 955 CrossRef.
- M. J. Biggs, R. G. Richards, N. Gadegaard, R. J. McMurray, S. Affrossman, C. D. Wilkinson, R. O. Oreffo and M. J. Dalby, J. Biomed. Mater. Res., Part A, 2009, 91, 195 CrossRef.
- A. Hart, N. Gadegaard, C. D. Wilkinson, R. O. Oreffo and M. J. Dalby, J. Mater. Sci.: Mater. Med., 2007, 18, 1211 CrossRef CAS.
- F. Kantawong, R. Burchmore, N. Gadegaard, R. O. C. Oreffo and M. J. Dalby, J. R. Soc. Interface, 2009, 6, 1075 CrossRef CAS.
- J. Park, S. Bauer, K. A. Schlegel, F. W. Neukam, K. von der Mark and P. Schmuki, Small, 2009, 5, 666 CrossRef CAS.
- E. Lamers, R. van Horssen, J. te Riet, F. C. van Delft, R. Luttge, X. F. Walboomers and J. A. Jansen, Eur. Cell Mater., 2010, 20, 329 CAS.
- K. Anselme, P. Davidson, A. M. Popa, M. Giazzon, M. Liley and L. Ploux, Acta Biomater., 2010, 6, 3824 CrossRef CAS.
- M. J. P. Biggs, R. G. Richards and M. J. Dalby, Nanomedicine, 2010, 6, 619 CrossRef CAS.
- P. Bertoncini, S. Le Chevalier, S. Lavenus, P. Layrolle and G. Louarn, J. Mol. Recognit., 2012, 25, 262 CrossRef CAS.
- L. Zhao, L. Liu, Z. Wu, Y. Zhang and P. K. Chu, Biomaterials, 2012, 33, 2629 CrossRef CAS.
- L. E. Rodriguez-Vilchis, R. Contreras-Bulnes, O. F. Olea-Mejia, I. Sanchez-Flores and C. Centeno-Pedraza, Photomed. Laser Surg., 2011, 29, 493 CrossRef CAS.
- R. Wojnar, Bone and Cartilage – its Structure and Physical Properties, in Biomechanics of Hard Tissues. Wiley-VCH Verlag GmbH & Co. KGaA., 2010, p. 1 Search PubMed.
- M. Kabiri, B. Kul, W. B. Lott, K. Futrega, P. Ghanavi, Z. Upton and M. R. Doran, Biochem. Biophys. Res. Commun., 2012, 419, 142 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3bm60026b |
| ‡ Current address: Industrial Research Institute Swinburne (IRIS), Faculty of Engineering and Industrial Sciences, Swinburne University of Technology, Hawthorn, 3122 VIC, Australia. |
| § These authors have contributed equally. |
| This journal is © The Royal Society of Chemistry 2013 |
