Self-assembled oligomeric procyanidin–insulin hybrid nanoparticles: a novel strategy for controllable insulin delivery†
Rui
Liu‡
a,
Li-Bing
Wang‡
b,
Ren-Liang
Huang
a,
Rong-Xin
Su
*a,
Wei
Qi
a,
Yan-Jun
Yu
a and
Zhi-Min
He
a
aState Key Laboratory of Chemical Engineering, Tianjin Key Laboratory of Membrane Science and Desalination Technology, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, P. R. China. E-mail: surx@tju.edu.cn; Fax: +(86)022-27407599; Tel: +(86)022-27407599
bHunan Entry–Exit Inspection and Quarantine Bureau, Changsha 410001, China
First published on 3rd May 2013
Abstract
Natural oligomeric procyanidin (OPC) with high biological and pharmacological activities was successfully used to synthesize OPC–insulin (OPC–INS) nanoparticles with different aggregation sizes for sustained and controlled delivery of hydrophilic insulin. The aggregation size of OPC–INS nanoparticles was regulated by OPC concentration, pH value, and incubation time. The fabrication mechanism would be that OPC and insulin self-assembled into a mixture of insulin aggregates via intermolecular interactions. In the self-assembly of insulin, OPC could serve both in the encompassing of insulin aggregates as a stabilizer and cross-linking different amounts of insulin aggregates into OPC–INS nanoparticles as interphase. OPC–INS nanoparticles not only demonstrated effective insulin drug loading but also exhibited aggregation-size-dependent and controlled insulin release performance in vitro. In the best case for OPC–INS nanoparticles, only ∼21% of insulin was released in 37 days. This study showed that the OPC–INS nanosystem is promising to serve as a long-acting insulin release formulation, and OPC has great potential as a drug carrier for nanomedicine.
Introduction
The development of long-acting insulin drugs is one of the main obstacles to effective treatment of diabetes mellitus. Currently, multiple daily injections of insulin are necessary for maintaining acceptably low glucose levels. Taking such uncomfortable demanding regimens into account, it is very attractive to develop a means to reduce the administration frequency of insulin drugs via delayed release or diffusion.1 In this regard, previous studies have focused on the insulin co-crystallization, soluble insulin assemblies induced by covalently attached polymer chains, and controlled self-assembly of re-generation of insulin through metal–ligand coordination.2 However, only limited success was achieved in clinical use. For example, even Lantus, a long-acting insulin through the clinical trials, should be taken at least once daily.3 Unfortunately, it is still a big challenge to elaborately design a prolonged and controlled insulin release system as an alternative to multiple daily injections.The nanoparticle system has shown promise in addressing the challenge by first providing a way to encapsulate insulin and release it over extended periods of time, thereby potentially reducing the dosage frequency. In particular, it provides numerous advantages as a drug delivery vehicle such as more favorable pharmacokinetics, well-established indications, and extra-enhanced bioactivities compared with the free drug.4,5 Attempts to encapsulate insulin in nanoparticles are currently mainly limited to self-assembled insulin aggregates evaluated in a rat model, biopolymer nanoparticles as colloidal systems for encapsulation of insulin, and polysaccharide nanoparticles, such as alginate and chitosan, for pharmaceutical formulations.6–9 The practical use of these nanoparticle systems also faces a number of difficulties such as biocompatibility, drug instability, or denaturation and increase in drug volumes.1 Therefore, it is highly desirable to develop new strategies for long-lasting and controlled insulin release and to explore new biocompatible carrier materials for insulin delivery.
The oligomeric procyanidins (OPCs), such as epicatechin/epicatechin dimers, are naturally occurring plant metabolites widely available in fruits, vegetables, seed, and barks.10,11 They have been demonstrated to have a high-level spectrum of biological and pharmacological activities, such as antioxidant, antiallergic, and anticancer.12–15 Furthermore, they do not have acute toxicity, and their metabolism ways have been well known.16,17 Due to their good biocompatibility and biodegradability, OPCs have attracted growing interest in biomedicine and nanomaterials since Bate-Smith and Swain showed that water-soluble phenolic compounds can precipitate alkaloids, gelatin, and other proteins.18–22 Recently, Zhai et al. have reported that OPC could be used as a novel crosslinking reagent for preparation of heart valve xenografts efficiently without toxicity, and other procyanidin-crosslinked gelatin conduits have been proposed to promote peripheral nerve regeneration.23,24 Yet until now, the use of OPC is limited to crosslinking agents interacting with proline-rich proteins. It is necessary to explore other applications of OPC in biomedical materials, such as drug carriers or additives for proteins.
Herein we employed OPC as a new building block for pharmaceutical engineering to fabricate OPC–INS nanoparticles for a long-acting insulin release formulation as shown in Fig. 1. OPC has been proven to possess bioavailability levels as high as 95% and can be rapidly absorbed and transported throughout the body.25 Therefore, this simple method allowed for achieving the benefits of relative safety of natural occurring materials and high hydrophilicity of OPC compared to biopolymer carriers.26 Regulation of the aggregation size of OPC–INS nanoparticles allows for modulating the insulin drug release rate. The nanostructures were characterized in terms of size, polydispersity, morphology, and drug release rate, and the interactions between OPC and the protein drug were extensively evaluated to clarify the formation mechanism and the structural information of OPC–INS nanoparticles.
 | ||
| Fig. 1 Schematic illustration of controllable fabrication of OPC–INS nanoparticles and chemical structure of OPC. | ||
Materials and methods
Materials
Unless otherwise noted, all reagents and materials were purchased from commercial sources and used as received. Bovine insulin (∼5800 g mol−1, 98%) was purchased from Sigma-Aldrich. Grape seed-derived OPCs (95%) were generously provided by Tianjin Jianfeng Natural Product R&D Co., Ltd, China and further purified before use.27 An epicatechin/epicatechin dimer (m/z 578.38, Fig. S1 in ESI†), the most abundant form of oligomer in grape seed-derived polyphenols, was used to calculate the molarity of OPC. All other reagents and chemicals were of analytical or HPLC grade. Ultrapure deionized water was obtained from a Millipore purification system (18.2 MΩ cm resistivity).Preparation of OPC–insulin nanoparticles
The OPC used in this study was mainly (4→8) or (4→6)-linked procyanidin dimer based on matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF) estimation (ESI, Fig. S1†). A previously published procedure was modified to prepare the insulin nanoparticles.6 In a typical experiment, 2 mg of insulin was dissolved in 1 mL of PBS buffer (20 mM sodium phosphate, 100 mM NaCl, pH 7). Then, under constant stirring at 37 °C, 20 μL of the OPC was rapidly added to the solution, and pH was adjusted using 0.1 M HCl and 0.1 M NaOH. The solution was continuously stirred for 1 min, after which, the solution was immediately placed in a shaking water bath and incubated at 37 °C and 150 rpm. OPC–insulin samples were obtained at designated time intervals and deposited into a −50 °C deep freeze refrigerator to quench the reaction until further study.Characterization of OPC–insulin nanoparticles
AFM images were obtained using an NTEGRA platform (NT-MDT, Moscow, Russia) in semi-contact mode. The samples were diluted 20-fold with ultrapure deionized water, and immobilized on freshly cleaved mica for 5 min. Then, the samples were rinsed with water to remove any non-adsorbed material, dried under nitrogen, and subjected to AFM analysis.DLS measurements were taken on a Zetasizer Nano ZS (Malvern Instruments, Worcestershire, UK) system with a 4 mV He–Ne laser (λ = 633 nm) using a non-invasive backscatter method (detection at 173° scattering angle). The temperature of the sample holder was controlled at 25 °C. Correlation data were fitted, using the cumulant method, to the logarithm of the correlation function, yielding the diffusion coefficient, D. The hydrodynamic diameters dH of the insulin nanoparticles were calculated using D and Stokes–Einstein equation. The polydispersity index (PDI) of insulin nanoparticles was calculated using PDI = 2c/b2, where b and c are the first- and second-order coefficients, respectively. Unless otherwise noted, all samples were dissolved in 20 mM PBS buffer (pH 7, 100 mM NaCl), and all DLS measurements were completed within 5 min.
TEM was performed as previously described.26 Briefly, a 5 μL portion of OPC–INS sample solution was placed on a 300 mesh carbon-coated copper grid (Beijing, China), and the excess sample was blotted with a filter paper. The samples were then negatively stained with 5% freshly prepared phosphotungstic acid solution for 2 min and allowed to dry. The stained grids were analyzed using a JEOL JEM100CXII transmission electron microscope (JEOL, Tokyo, Japan) at an accelerating voltage of 100 kV. TEM images were captured using an AMT Advantage digital CCD camera system.
SEC was performed using an Agilent 1200 high-performance liquid chromatography system (Agilent Technologies, Santa Clara, USA) equipped with a UV absorbance detector, a differential refractometer (Agilent Technologies), and a Wyatt Mini-Dawn MALLS detector. The columns employed were Shodex Protein KW-G (60 mm × 6.0 mm, 7 μm) and KW-803 (300 mm × 8 mm, 5 μm). Column temperature was maintained at 25 °C ± 0.1 °C using an Autoscience AT-132 temperature control module. The mobile phase was 20 mM PBS buffer and 0.02 wt% sodium azide, pH 7, at a flow rate of 1 mL min−1. The insulin sample concentration was 2 mg mL−1 with an injection volume of 100 μL. Molecular weights and molecular weight distributions were calculated using Astra 5.3.14 software (Wyatt Technology Corporation) based on Zimm's equation with a refractive index increment (dn/dc) of 0.185 mL g−1.
Interactions between OPC and insulin
CD spectra were recorded on a JASCO-810 spectropolarimeter (JASCO, Tokyo, Japan) operating at room temperature under a constant flow of N2. A 0.1 mL of OPC–insulin sample solution was directly added to a 0.2 mm quartz cuvette. A background CD spectrum of 20 mM PBS buffer in the absence or the presence of OPCs was subtracted from the sample spectra for baseline correction. The spectra were recorded between 180 nm and 250 nm with 0.2 nm resolution with 50 nm min−1 scanning speed, 1 s response time, and 2 nm bandwidth. Each result is given as the average of three measurements. CDPro was used to analyze spectra to determine the secondary structure content using CONTINLL, SELCON3, and CDSSTR methods.FTIR measurements were performed using a MAGNA-560 spectrometer (Nicolet, Madison, USA). The samples were gently mixed with 300 mg of KBr powder after freeze-drying and compressed into discs at a force of 10 kN for 2 min. For each spectrum, a 256-scan, double-sided interferogram was collected with a 4 cm−1 resolution in the mid-IR region at room temperature. Insulin-free systems and water vapor spectra were collected under identical conditions for blank subtraction.
DSC thermograms were obtained using a TGA/DSC1 system (Mettler Toledo, Greifensee, Switzerland). The samples were lyophilized, and 1.0 mg of lyophilized powder crimped in a standard aluminum pan and heated from 30 °C to 400 °C at a heating constant rate of 10 °C min−1 under constant purging of nitrogen at 20 mL min−1. All samples were run in duplicate.
Insulin release from nanoparticles
The in vitro release test of insulin from the hybrid nanoparticles was evaluated using the dialysis method. A dialysis bag filled with 0.2 mL of insulin-loaded nanoparticle dispersion was immersed in 8 mL of 20 mM PBS buffer solutions at pH 7. The released OPCs/insulin outside the dialysis bag was sampled at defined time intervals and assayed using a UV–vis absorption spectrometer at 280 nm. Cumulative release is expressed as the total percentage of drug released through the dialysis membrane over time. Release experiments were also performed to show the evolution of the release kinetics in response to OPC concentration.Results and discussion
Synthesis and characterization of OPC–INS nanoparticles
OPC–INS nanoparticles with different aggregation were successfully synthesized by a simple incubation method (Fig. 1). By the addition of dissolved OPC to the insulin solution and subsequent adjustment of the pH value using HCl and NaOH, PBS buffer dispersions of OPC–INS nanoparticles were formed. The effect of OPC concentration on the size of OPC–INS nanoparticles was studied. The AFM results show that the aggregation size increased along with the amount of OPC (Fig. 2). As shown in Fig. 2A and B, OPC–INS nanoparticles exhibited a highly crosslinking morphology with increased aggregation in the presence of 1 mM OPC, whereas the addition of 100 μM OPC to insulin solution formed dispersive spherical nanoparticles. At a lower OPC concentration of 10 μM, the particle size was deformed and not sufficiently large for a long-acting protein drug. The OPC–INS nanoparticles synthesized with 100 μM OPC showed a relatively uniform size distribution, with heights ranging from 12 nm to 25 nm and an average diameter of 80 nm to 130 nm (Fig. 2D–F). Assuming that the actual size of the nanoparticle is equal to its hydrodynamic diameter, the OPC–INS nanoparticles will collapse and further aggregate while they are deposited from an aqueous suspension and N2 dried on a mica support. The AFM image clearly demonstrated that several or dozens of associated OPC–INS small-sized nanoballs (<30 nm) formed a large cluster of nanoparticles (>100 nm) on the mica surface (ESI, Fig. S2†). Then we employed DLS and SEC-MALLS to investigate the particle size and molecular weight of the OPC–INS nanoparticle in solution.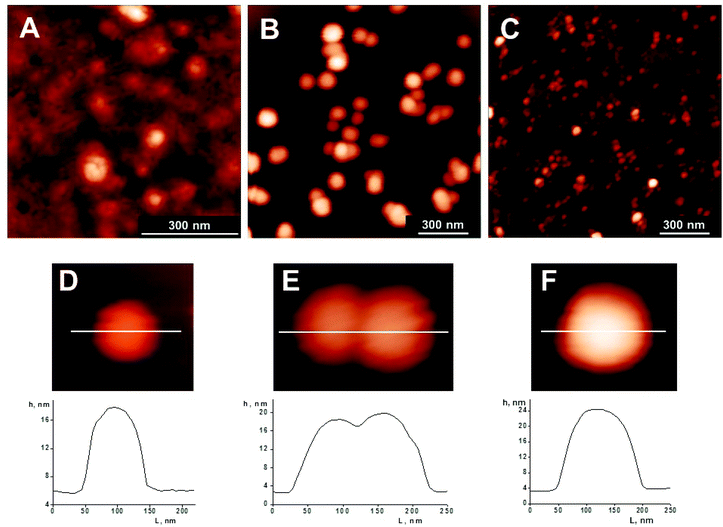 | ||
| Fig. 2 AFM images of OPC–INS nanoparticles prepared from insulin (2 mg mL−1) in PBS buffer (20 mM) with different concentrations of OPC solutions: (A) 1 mM, (B, D–F) 100 μM, and (C) 10 μM. Samples were incubated for 6 h at pH 7. The corresponding profiles show the sample elevation across the indicated lines. | ||
The DLS results showed that the size increased as the OPC concentration increased from 10 μM to 1 mM, which agreed with the AFM data. The OPC–INS nanoparticles synthesized with 10 μM OPC showed a larger particle size (5.6 nm) compared with the size of insulin nanostructures (2.8 nm) (Fig. 3). Contrary to the 10 μM OPC, the OPC–INS nanoparticles displayed relatively narrow size distributions with average hydrodynamic diameters of 11.4 nm and 33.4 nm for the OPC concentrations studied (100 μM and 1 mM), respectively. Particles with diameters below 1000 nm are desirable because they are better absorbed in the intestinal tract.28
 | ||
| Fig. 3 Size distributions of insulin aggregates (INSAs) and OPC–INS nanoparticles synthesized with different concentrations of OPC solutions. Samples were incubated for 6 h at pH 7. | ||
Further insight into the structure of these hybrid nanoparticles is obtained by size exclusion chromatography coupled with multi-angle laser light scattering (SEC-MALLS) (Fig. 4, Fig. S3 and S4† and Table 1). The weight-average molecular weight (Mw) of the OPC–INS nanoparticle increased with increasing pH. The value of Mw/Mn varied between 1.49 and 1.72, indicating that the OPC–INS nanoparticles formed at pH 7 had a relatively uniform size distribution. The number of OPC–INS per aggregate (NINS) was calculated from the ratio of the apparent molecular weight of OPC–INS to INS, ranging from 4.1 to 13.0. The highest Mw/Mn value, but the lowest NINS, occurred under weak acidic conditions (pH 6.5). Higher pH values (7 and 8) were used to prepare the OPC–INS nanoparticles to increase the aggregation level. However, polyphenols become less stable and easily oxidized at pH values higher than 8.22 After 70 h incubation, the OPC–INS nanoparticle suspension at pH 7 became transparent (ESI, Fig. S5†), whereas both OPC–INS solutions at pH 6.5 and pH 8, as well as the solution of insulin aggregates at pH 7, presented a turbid suspension because of the formation of numerous large aggregates (ESI, Fig. S6†). This finding implied that pH was important to the state of the particle suspension. The neutral PBS buffer tested was used for all further studies. For pH 7, one OPC–INS self-aggregate consisted of 2.2 ± 0.1, 6.2 ± 0.2, or 13.6 ± 0.5 insulin molecules dispersed in PBS buffer (Fig. 4), which showed that the sample was a mixture of insulin dimer, hexamer, and polymer. On the other hand, the Mw of OPC–INS nanoparticles increased while increasing the OPC concentration. Although addition of 10 μM OPC had a relatively uniform size distribution, insufficient OPC resulted in a low aggregation level, predominantly in the trimeric state. While the addition of 1 mM OPC caused a precipitation of large OPC–INS aggregates, which were filtered out prior to the sample injection, leading to a low SEC-MALLS intensity (Fig. S4C†). We considered that the appropriate OPC concentration of 100 μM (OPC–INS molar ratio of ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.4) should be selected for all further studies to obtain the long-acting insulin nanoparticles because relatively insufficient OPC resulted in a mixture of OPC–INS aggregates without precipitation, as well as increased the mean particle size and particle aggregation compared with 10 μM OPC.
3.4) should be selected for all further studies to obtain the long-acting insulin nanoparticles because relatively insufficient OPC resulted in a mixture of OPC–INS aggregates without precipitation, as well as increased the mean particle size and particle aggregation compared with 10 μM OPC.
 | ||
| Fig. 4 Elution profiles of OPC–INS nanoparticles prepared from insulin (2 mg mL−1) in PBS buffer (20 mM, pH 7) and the molecular weight distribution calculated using ASTRA software. | ||
| pH | OPC conc. | M w | M w/Mn | N INS |
|---|---|---|---|---|
| 6.5 | 100 μM | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.72 | 4.1 |
| 10 μM | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 210 210 |
1.03 | 3.3 | |
| 7 | 100 μM | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.49 | 7 |
| 1 mM | 530![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.17 | 91.4 | |
| 8 | 100 μM | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.69 | 13.0 |
To characterize the growth of OPC–INC nanoparticles, TEM was used to observe the change in size and morphology as the incubation proceeded. Consistent with the DLS and SEC–MALLS results, TEM images of OPC–INS nanoparticles incubated for 6 h exhibited a limited aggregation (Fig. 5A). Several large-sized species (>100 nm) were evident in the TEM images (ESI, Fig. S7A†). With increasing incubation time, the size of the OPC–INS nanoparticles increased, but less uniform nanoclusters were formed (Fig. 5B–D). Insulin self-assembles into insoluble amyloid fibrils after incubation in solutions at low pH and temperatures above 30 °C.29–31 For the 20 mM PBS buffer at pH 7, the insulin aggregates exhibited a mixture of different structures, including nanoballs and nanorods (ESI, Fig. S7B†). The OPC most likely self-positioned at the interphase, promoting the formation of OPC–INS nanoclusters rather than amyloid fibril or rod-like structures32 and allowing the particle suspension in water. These nanoparticles easily dissolved in aqueous solution because of the high hydrophilicity of OPC used in this study.
![TEM images of OPC–INS nanoparticles incubated for (A) 6, (B) 15, (C) 30, and (D) 70 h at pH 7. [OPC] = 100 μM. TEM images were acquired with a scale bar of 100 nm.](/image/article/2013/BM/c3bm60066a/c3bm60066a-f5.gif) | ||
| Fig. 5 TEM images of OPC–INS nanoparticles incubated for (A) 6, (B) 15, (C) 30, and (D) 70 h at pH 7. [OPC] = 100 μM. TEM images were acquired with a scale bar of 100 nm. | ||
The OPC–INS nanoparticle suspension was transparent and stable when stored at 4 °C without light exposure even after 30 days. Chalasani et al. recently outlined a novel synthetic route for generating a water-soluble vitamin B12-coated dextran nanosphere (150 nm to 300 nm).33 Cui et al. prepared a biodegradable nanocapsule system using a novel reverse micelle-solvent evaporation method (200 nm).34 Both systems were focused on loading insulin into nanoparticles such as alginate/chitosan nanogel and poly(lactic-co-glycolic acid) nanocomplex.35–39 All the above insulin nanoparticles exhibited a relatively narrow size distribution, whereas our OPC–INS nanoparticles exhibited a mixture of insulin dimer, hexamer, and polymer (dH = 11.4 nm). Recently, a rapid- and long-acting insulin was formulated by preparing insulin–phospholipid complex-loaded poly(hydroxybutyrate-co-hydroxyhexanoate) nanoparticles (INS-PLC NPs, 186.2 nm). Although both INS–PLC and OPC–INS nanoparticles showed a polydispersive particle size distribution, their fabrication mechanisms were different.7 The INS–PLC–NPs were produced using a solvent evaporation method, whereas OPC and insulin self-assembled into a structure containing dimers, hexamers, and polymers through intermolecular interactions. OPC–INS nanoparticles prepared under the optimum conditions were used for further interaction analysis.
Interactions between OPC and insulin
The protein structure may have a role in forming different species through various molecular interactions. CD, Fourier transform infrared spectroscopy (FTIR), and DSC were used to characterize the structural information and molecular interaction of OPC–INS nanoparticles. The FTIR spectrum of insulin had absorption bands at wavenumbers 3303 and 2962 cm−1, corresponding to the stretching vibrations of the N–H and C–H groups, respectively (Fig. 6A). The amide I band at 1658 cm−1 is dominantly attributed to the stretching vibrations of peptide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, and the amide II band at 1542 cm−1 arises from the N–H bending vibrations coupled to C–N stretching vibrations. The amide III centered at 1243 cm−1 is assigned to the C–N stretching and N–H bending vibrations from amide linkages. OPC had absorption bands at 3396 and 1608 cm−1 corresponding to the association of O–H and C
O groups, and the amide II band at 1542 cm−1 arises from the N–H bending vibrations coupled to C–N stretching vibrations. The amide III centered at 1243 cm−1 is assigned to the C–N stretching and N–H bending vibrations from amide linkages. OPC had absorption bands at 3396 and 1608 cm−1 corresponding to the association of O–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations, respectively, indicative of the polyhydroxy aromatic properties of OPC. The FTIR spectrum of OPC–INS nanoparticles had a relatively sharp absorption band at 3463 cm−1 corresponding to the association of O–H and N–H stretching vibrations. The positions of the major amide bands changed slightly from that of insulin indicating the hydrogen bonding interactions between OPC and insulin. The side chain hydroxyl, carboxyl, and amide groups of insulin may provide the interacting sites for the formation of new hydrogen bonds with the phenolic hydroxyl groups of procyanidin. In these cases, the insulin domains are likely to be more readily dispersed in the continuous OPC phase as a result of the protein–polyphenol interaction. In other words, the OPC molecules surround the insulin species and act as cross-linkers, which would be further confirmed by DSC.
C stretching vibrations, respectively, indicative of the polyhydroxy aromatic properties of OPC. The FTIR spectrum of OPC–INS nanoparticles had a relatively sharp absorption band at 3463 cm−1 corresponding to the association of O–H and N–H stretching vibrations. The positions of the major amide bands changed slightly from that of insulin indicating the hydrogen bonding interactions between OPC and insulin. The side chain hydroxyl, carboxyl, and amide groups of insulin may provide the interacting sites for the formation of new hydrogen bonds with the phenolic hydroxyl groups of procyanidin. In these cases, the insulin domains are likely to be more readily dispersed in the continuous OPC phase as a result of the protein–polyphenol interaction. In other words, the OPC molecules surround the insulin species and act as cross-linkers, which would be further confirmed by DSC.
![(A) FTIR spectra and (B) DSC thermograms of INS, OPC, and OPC–INS nanoparticles. [OPC] = 100 μM, incubation time = 6 h, pH = 7.](/image/article/2013/BM/c3bm60066a/c3bm60066a-f6.gif) | ||
| Fig. 6 (A) FTIR spectra and (B) DSC thermograms of INS, OPC, and OPC–INS nanoparticles. [OPC] = 100 μM, incubation time = 6 h, pH = 7. | ||
DSC was performed to investigate further the OPC interaction with insulin over the temperature range of 30 °C to 350 °C (Fig. 6B). Consistent with the results from previous studies, insulin and OPC exhibited endothermic transitions approximately at 65 and 91 °C, corresponding to their glass transition temperatures (Tg), respectively. However, the endothermic peaks of OPC and insulin could not be observed from the DSC thermogram of insulin-loaded nanoparticles, suggesting that OPC strongly interacts with insulin. Meanwhile, the OPC–INS composites exothermically cross-linked between 50 °C and 200 °C. The thermal performance of OPC–INS nanoparticles implied that insulin was successfully incorporated into the OPC surroundings, consistent with the FTIR results.
The secondary structure of insulin complexed with OPC was investigated by CD measurement (Fig. 7). The CD spectrum of native insulin has a large negative n–π* transition at 222 nm and a π–π* transition splitting into a positive peak at 199 nm and a negative peak at 208 nm, which are characteristics of typical α-helical structure.40 The CD spectrum of the OPC–INS complex varied significantly from that of insulin. The CD spectrum of OPC–INS nanoparticles showed a positive n–π* transition at 200 nm and a negative π–π* transition at 218 nm. The analysis results indicated an approximately 47% decrease in the helix content as well as 50%, 5%, and 15% increase in β-sheet, turn, and random coil contents, respectively (Table S1†). Although the transformation was basically a transition of helix-sheet type, OPC–INS nanoparticles displayed a predominantly random coil conformation. As a result, the presence of OPC converted the helical insulin into an unordered protein because insulin was incorporated into the OPC surroundings and strongly interacted with the hydrophobic domain.
![CD spectra of INS and OPC–INS nanoparticles. [INS] = 2 mg mL−1, [OPC] = 100 μM, incubation time = 6 h, pH = 7.](/image/article/2013/BM/c3bm60066a/c3bm60066a-f7.gif) | ||
| Fig. 7 CD spectra of INS and OPC–INS nanoparticles. [INS] = 2 mg mL−1, [OPC] = 100 μM, incubation time = 6 h, pH = 7. | ||
Procyanidins can interact with proteins, in particular with digestive enzymes.41 Previous studies on protein–polyphenol interactions indicated that proteins complexed with polyphenols can be stabilized by hydrophobic association and hydrogen bonding.42,43 The results from the current study indicate the following two main modes of interactions between insulin and OPC: (i) OPC molecules surround the insulin species, displace the water, and create new hydrogen bond interactions with insulin, which help stabilize the self-assembled OPC–INS nanoparticles; and (ii) the OPC molecules interact with the insulin hydrophobic nucleation domain, resulting in an α-to-β structural transition and aggregation of insulin.44 By contrast, Simon et al. observed that the secondary structure of procyanidin–saliva protein complexes did not change from the native saliva structure,45 which disagrees with the results reported in the present study. This difference may have been caused by the conformation dependency, rather than sequence dependency, of the OPC binding. In the self-assembly of insulin, OPC could serve both in the encompassing of insulin aggregates as a stabilizer and cross-linking different amounts of insulin aggregates into OPC–INS nanoparticles as interphase.
Insulin release from OPC–INS nanoparticles
To prove our hypothesis that OPC–INS nanoparticles can act as a reservoir for sustained release of the hormone for a longer period, the release of insulin monomers from the OPC complexes through an 8 kDa cutoff membrane was examined (Fig. 8). In the absence of OPC, 71.1% of the insulin monomer was rapidly released from native insulin solution during the first 8 h (Fig. S8†), whereas for insulin aggregates incubated for 6 h, the insulin monomer was gradually released up to 93% for 37 d. In contrast, insulin released from the OPC–INS nanoparticles was less than 30% for 37 days and inversely proportional to the OPC concentration. The release profile can be divided into two phases, i.e., the fast-release phase, which lasts for 24 h, and the slow-release phase, which starts after the first 24 h. During the 24 h fast-release, the insulin aggregates released 16.1% of insulin. By contrast, the 10 μM, 100 μM and 1 mM OPC-coupled insulin released 10.2%, 11% and 12.3% of insulin, respectively. The initial fast release of insulin may be a result of some free insulin not incorporated into the OPC–INS nanoparticles, contributing to the rapid efficacy of insulin formulation. During the second release phase, a linear increase in the release of insulin over a period of 2 days to 30 days was observed in the absence of OPC, whereas only 18.8%, 9.7%, and 7.2% of insulin were released at a very low rate with an OPC concentration ranging from 10 μM to 1 mM, suggesting the tight association of OPC and insulin aggregates. The slow release rate of insulin and the relatively high insulin load would be the main reasons for the long-term efficacy of the OPC–INS nanoparticles. Furthermore, the controllable release of insulin would be achieved by tuning the OPC concentration, thereby regulating the aggregation size of OPC–INS nanoparticles.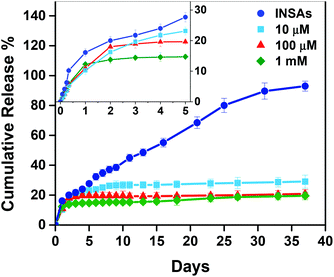 | ||
| Fig. 8 In vitro release profiles of insulin from insulin aggregates (INSAs) and OPC–INS nanoparticles in PBS buffer (pH 7) at 37 °C under the stirring rate of 60 rpm. The inset graph was the detailed release profiles of insulin in the first five days. Each data presented as mean ± S.D. (n = 3). | ||
Conclusions
Natural and biodegradable polyphenol OPC was attached to insulin species, such as dimers, hexamers, and polymers, forming aggregation-size-controllable OPC–INS nanoparticles via hydrogen bonding and hydrophobic interaction. In addition, OPC–INS nanoparticles not only demonstrated effective insulin drug loading but also exhibited controlled insulin release performance in vitro with a very slow rate and a low burst release. In the best case for OPC–INS nanoparticles, only ∼21% of insulin was released in 37 days. In summary, OPC can cross-link insulin aggregates into a novel long-acting insulin release formulation and serve as a potential vehicle for sustained and controlled delivery of insulin. OPCs should offer more opportunities as building blocks for medical and pharmaceutical applications because patient compliance could be significantly improved using OPC-based nanoparticles.Acknowledgements
This work was supported by the Natural Science Foundation of China (Nos. 20806057, 21276192 and 31071509), the Ministry of Science and Technology of China (Nos. 2012YQ090194, 2012BAD29B05 and 2012AA06A303), and the Ministry of Education (Nos. B06006 and NCET-11-0372).Notes and references
- T. Hoeg-Jensen, S. Havelund, P. K. Nielsen and J. Markussen, J. Am. Chem. Soc., 2005, 127, 6158–6159 CrossRef CAS.
- H. K. Munch, S. T. Heide, N. J. Christensen, T. Hoeg-Jensen, P. W. Thulstrup and K. J. Jensen, Chem.–Eur. J., 2011, 17, 7198–7204 CrossRef CAS.
- G. B. Bolli and D. R. Owens, Lancet, 2000, 356, 443–445 CrossRef CAS.
- T. M. Allen and P. R. Cullis, Science, 2004, 303, 1818–1822 CrossRef CAS.
- J. Panyam and V. Labhasetwar, Adv. Drug Delivery Rev., 2003, 55, 329–347 CrossRef CAS.
- S. Gupta, T. Chattopadhyay, M. Pal Singh and A. Surolia, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 13246–13251 CrossRef CAS.
- Q. Peng, Z. R. Zhang, T. Gong, G. Q. Chen and X. Sun, Biomaterials, 2011, 33, 1583–1588 CrossRef.
- F. A. Dorkoosh, J. Coos Verhoef, M. H. C. Ambagts, M. Rafiee-Tehrani, G. Borchard and H. E. Junginger, Eur. J. Pharm. Sci., 2002, 15, 433–439 CrossRef CAS.
- Y. Pan, Y. J. Li, H. Y. Zhao, J. M. Zheng, H. Xu, G. Wei, J. S. Hao and F. D. Cui, Int. J. Pharm., 2002, 249, 139–147 CrossRef CAS.
- W. Tückmantel, A. P. Kozikowski and L. J. Romanczyk, J. Am. Chem. Soc., 1999, 121, 12073–12081 CrossRef.
- M. Karonen, J. Loponen, V. Ossipov and K. Pihlaja, Anal. Chim. Acta, 2004, 522, 105–112 CrossRef CAS.
- E. Q. Xia, G. F. Deng, Y. J. Guo and H. B. Li, Int. J. Mol. Sci., 2010, 11, 622–646 CrossRef CAS.
- H. Schroeter, C. Heiss, J. Balzer, P. Kleinbongard, C. L. Keen, N. K. Hollenberg, H. Sies, C. Kwik-Uribe, H. H. Schmitz and M. Kelm, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 1024–1029 CrossRef CAS.
- S. K. Mantena, M. S. Baliga and S. K. Katiyar, Carcinogenesis, 2006, 27, 1682–1691 CrossRef CAS.
- H. Fujii, T. Yokozawa, Y. A. Kim, C. Tohda and G. Nonaka, Biosci., Biotechnol., Biochem., 2006, 70, 2104–2111 CrossRef CAS.
- C. Santos-Buelga and A. Scalbert, J. Sci. Food Agric., 2000, 80, 1094–1117 CrossRef CAS.
- M.-P. Gonthier, J. L. Donovan, O. Texier, C. Felgines, C. Remesy and A. Scalbert, Free Radical Biol. Med., 2003, 35, 837–844 CrossRef CAS.
- A. E. Hagerman, M. E. Rice and N. T. Ritchard, J. Agric. Food Chem., 1998, 46, 2590–2595 CrossRef CAS.
- A. Edelmann and B. Lendl, J. Am. Chem. Soc., 2002, 124, 14741–14747 CrossRef CAS.
- E. C. Bate-Smith and T. Swain, in Comparative Biochemistry, ed. H. S. Mason and A. M. Florkin, Academic Press, New York, 1962, vol. 3, ch. 14, pp. 755–809 Search PubMed.
- T. Zou, Z. Li, S. S. Percival, S. Bonard and L. Gu, Food Hydrocolloids, 2012, 27, 293–300 CrossRef CAS.
- T. G. Shutava, S. S. Balkundi, P. Vangala, J. J. Steffan, R. L. Bigelow, J. A. Cardelli, D. P. O'Neal and Y. M. Lvov, ACS Nano, 2009, 3, 1877–1885 CrossRef CAS.
- W. Zhai, J. Chang, K. Lin, J. Wang, Q. Zhao and X. Sun, Biomaterials, 2006, 27, 3684–3690 CAS.
- B. S. Liu, J. Biomed. Mater. Res., Part A, 2008, 87A, 1092–1102 CrossRef CAS.
- S. Deprez, I. Mila, J. F. Huneau, D. Tome and A. Scalbert, Antioxid. Redox Signaling, 2001, 3, 957–967 CrossRef CAS.
- S. Sundar, J. Kundu and S. C. Kundu, Sci. Technol. Adv. Mater., 2010, 11, 014104–014115 CrossRef.
- J. Yamakoshi, M. Saito, S. Kataoka and M. Kikuchi, Food Chem. Toxicol., 2002, 40, 599–607 CrossRef CAS.
- N. Hussain, V. Jaitley and A. T. Florence, Adv. Drug Delivery Rev., 2001, 50, 107–142 CrossRef CAS.
- D. F. Waugh, J. Am. Chem. Soc., 1946, 68, 247–250 CrossRef CAS.
- L. Nielsen, R. Khurana, A. Coats, S. Frokjaer, J. Brange, S. Vyas, V. N. Uversky and A. L. Fink, Biochemistry, 2001, 40, 6036–6046 CrossRef CAS.
- M. Bouchard, J. Zurdo, E. J. Nettleton, C. M. Dobson and C. V. Robinson, Protein Sci., 2000, 9, 1960–1967 CrossRef CAS.
- K. Ono, M. M. Condron, L. Ho, J. Wang, W. Zhao, G. M. Pasinetti and D. B. Teplow, J. Biol. Chem., 2008, 283, 32176–32187 CrossRef CAS.
- K. B. Chalasani, G. J. Russell-Jones, A. K. Jain, P. V. Diwan and S. K. Jain, J. Controlled Release, 2007, 122, 141–150 CrossRef CAS.
- F. Cui, K. Shi, L. Zhang, A. Tao and Y. Kawashima, J. Controlled Release, 2006, 114, 242–250 CrossRef CAS.
- B. Sarmento, A. Ribeiro, F. Veiga, P. Sampaio, R. Neufeld and D. Ferreira, Pharm. Res., 2007, 24, 2198–2206 CrossRef CAS.
- D. R. Bhumkar, H. M. Joshi, M. Sastry and V. B. Pokharkar, Pharm. Res., 2007, 24, 1415–1426 CrossRef CAS.
- Y. Han, H. Tian, P. He, X. Chen and X. Jing, Int. J. Pharm., 2009, 378, 159–166 CrossRef CAS.
- Z. M. Wu, L. Zhou, X. D. Guo, W. Jiang, L. Ling, Y. Qian, K. Q. Luo and L. J. Zhang, Int. J. Pharm., 2012, 425, 1–8 CrossRef CAS.
- X. Y. Xiong, Y. P. Li, Z. L. Li, C. L. Zhou, K. C. Tam, Z. Y. Liu and G. X. Xie, J. Controlled Release, 2007, 120, 11–17 CrossRef CAS.
- Y. Pocker and S. B. Biswas, Biochemistry, 1980, 19, 5043–5049 CrossRef CAS.
- R. M. Gonçalves, N. Mateus and V. De Freitas, J. Agric. Food Chem., 2010, 58, 11924–11931 CrossRef.
- K. J. Siebert, N. V. Troukhanova and P. Y. Lynn, J. Agric. Food Chem., 1996, 44, 80–85 CrossRef CAS.
- N. J. Baxter, T. H. Lilley, E. Haslam and M. P. Williamson, Biochemistry, 1997, 36, 5566–5577 CrossRef CAS.
- Y. Takahashi, A. Ueno and H. Mihara, Bioorg. Med. Chem., 1999, 7, 177–185 CrossRef CAS.
- C. Simon, K. Barathieu, M. Laguerre, J. M. Schmitter, E. Fouquet, I. Pianet and E. J. Dufourc, Biochemistry, 2003, 42, 10385–10395 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: MALDI-TOF MS spectrum of OPC, AFM images of OPC-INS nanoclusters formed on the mica surface, Elution profiles of insulin aggregates and OPC-INS nanoparticles, photographs of OPC-INS nanoparticles suspension, TEM images of OPC-Ins nanoparticles, and secondary structure of INS and OPC-INS nanoparticles. See DOI: 10.1039/c3bm60066a |
| ‡ These authors contribute equally to the work of the manuscript. |
| This journal is © The Royal Society of Chemistry 2013 |
