In vitro growth and differentiation of primary myoblasts on thiophene based conducting polymers†
Anita F.
Quigley
ab,
Klaudia
Wagner
a,
Magdalena
Kita
ab,
Kerry J.
Gilmore
a,
Michael J.
Higgins
a,
Robert D.
Breukers
a,
Simon E.
Moulton
a,
Graeme M.
Clark‡
a,
Anthony J.
Penington
c,
Gordon G.
Wallace
a,
David L.
Officer
a and
Robert M. I.
Kapsa
*abd
aARC Centre of Excellence for Electromaterials Science, Intelligent Polymer Research Institute, University of Wollongong, Northfields Avenue, Wollongong, NSW 2522, Australia. E-mail: rmik@unimelb.edu.au; Fax: +61 3 9288 3350; Tel: +61 3 9288 3344
bCentre for Clinical Neuroscience and Neurology Research, St Vincent's Hospital, 41 Victoria Pde., Melbourne, Victoria 3065, Australia
cDepartment of Surgery, University of Melbourne, St Vincent's Hospital, Melbourne, Victoria 3065, Australia
dDepartment of Medicine, University of Melbourne, St Vincent's Hospital, Melbourne, Victoria 3065, Australia
First published on 10th July 2013
Abstract
Polythiophenes are attractive candidate polymers for use in synthetic cell scaffolds as they are amenable to modification of functional groups as a means by which to increase biocompatibility. In the current study we analysed the physical properties and response of primary myoblasts to three thiophene polymers synthesized from either a basic bithiophene monomer or from one of two different thiophene monomers with alkoxy functional groups. In addition, the effect of the dopants pTS− and ClO4− was investigated. In general, it was found that pTS− doped polymers were significantly smoother and tended to be more hydrophilic than their ClO4− doped counterparts, demonstrating that the choice of dopant significantly affects the polythiophene physical properties. These properties had a significant effect on the response of primary myoblasts to the polymer surfaces; LDH activity measured from cells harvested at 24 and 48 h post-seeding revealed significant differences between numbers of cells attaching to the different thiophene polymers, whilst all of the polymers equally supported cell doubling over the 48 h period. Differences in morphology were also observed, with reduced cell spreading observed on polymers with alkoxy groups. In addition, significant differences were seen in the polymers’ ability to support myoblast fusion. In general pTS− doped polymers were better able to support fusion than their ClO4− doped counterparts. These studies demonstrate that modification of thiophene polymers can be used to promote specific cellular response (e.g. proliferation over differentiation) without the use of biological agents.
Introduction
Effective engineering of muscle tissue facilitates the remodeling of tissues lost due to disease, trauma or surgery and as such, can involve replacement of significant amounts of tissue. To date, muscle remodeling approaches involving cell replacement have shown limited potential due to a variety of issues, including death and/or immune rejection of donor cells, lack of donor cell proliferation and limited migration from the injection site.1,2 Thus many cell transplantation strategies designed to treat severe loss of muscle tissue in conditions such as Duchenne Muscular Dystrophy or trauma, have achieved limited degrees of success.3,4A number of reports have highlighted the diversity of approaches that have been applied to improve skeletal muscle engineering, including the use of micro-patterned polymers,5–7 biological scaffolds8 and synthetic polymers.9–13 Synthetic scaffolds have the advantage over biologic scaffolds in that their characteristics can be defined and manipulated to be inherently pro-myogenic (i.e. “myo-bionic”), thus greatly facilitating their utility in future bionic approaches for skeletal muscle engineering. Such engineered scaffolds may be seeded ex vivo with host or donor cells7 and implanted, or utilized directly in vivo to encourage endogenous muscle regeneration by influencing myoblast division and migration, without the need for cell replacement or cell seeding.
Organic conducting polymers (OCPs) such as polypyrrole, polyaniline and pEDOT have demonstrated reasonable biocompatibility14,15 which has been shown to vary according to the counter-ion used as the dopant, the polymerisation conditions and resulting properties (i.e. surface roughness, surface energy etc.).16 OCPs, such as pEDOT, have been used to control the differentiation and proliferation of epithelial cells.17 In addition, polypyrrole and pEDOT have been used to control protein (fibronectin) conformation and cell adhesion by controlling the potential through the polymer.18–20 In this way, conducting polymers, such as pEDOT, can be used to control the presentation of protein functional groups in order to elicit specific biological responses. These capabilities demonstrate the versatility and potential of these polymers in medical bionics.
OCPs have been shown to support myoblast adhesion and proliferation,21,22 and their facilitation of electrical stimulation23 and controlled release of functional proteins to influence cell growth in vitro and in vivo24,25 offers an exciting new approach for influencing skeletal muscle regeneration.
As an “excitable” tissue, muscle and its resident progenitor cells, myoblasts, have been shown to respond to electrical stimuli both in vitro and in vivo, influencing proliferation26 as well as differentiation.27,28 OCPs thus have potential applications in effecting control of myoblast proliferation and/or differentiation directly as part of implantable scaffold/electrode systems. Alternatively, OCPs may provide a substratum as part of a “myo-reactor” system for ex vivo pre-conditioning of myogenic precursors to optimal regenerative condition prior to implantation.7
Polythiophenes are attractive candidate polymers for use in synthetic cell scaffolds as they are more amenable to modification of functional groups as a means by which to increase biocompatibility than are other OCPs such as polyaniline and polypyrrole. This is highlighted by the synthetic methods that have been described for the introduction of a wide variety of functional groups to polythiophenes.29 Despite this, there are few reports describing the modification and use of polythiophenes for cellular interaction other than for pEDOT.5,22,30–32
Our group has recently investigated the use of an ester-functionalized polythiophene and its hydrolyzed derivative, demonstrating excellent capacity to support myoblast proliferation and differentiation in vitro.12 In addition, this organic solvent-soluble polythiophene was electrospun to provide an aligned nanostructured scaffold that orientated myoblast differentiation, in vitro. We expand on these findings in the current study, where we investigate the capacity of three different polythiophenes to support primary myoblast proliferation and differentiation and the effects of the polymer properties on myoblast behaviour. Poly(bithiophene) (PBTh) was chosen as a control polymer, to examine the effect of the basic polythiophene backbone. Two variants of the basic PBTh chemistry were synthesized by introduction of alkoxy substituents to (i) improve the oxidative stability of the polymers and (ii) provide molecular entities amenable to further functionalization with other molecules. An alkoxy-substituted polythiophene, poly-3-decoxythiophene (P3DTh) was synthesized to explore the effect of the solubilizing functionality that would be required for a processable polymer. The third polymer, poly(decyl 4,4′′-didecoxy-2,2′:5′,2′′-terthiophene-3′-carboxylate, PDTTh3E), incorporates both the solubilizing groups as well as an ester group amenable to subsequent functionalization with peptides. We explored the physical properties of the resulting polythiophene films synthesized with two different dopants, tetrabutylammonium para-toluenesulphonate (pTS−) and tetrabutylammonium perchlorate (ClO4−) to provide surface energy variants in the first instance, and conductivity variants to allow subsequent electrical stimulation studies. In this study, these polymers were evaluated for their influence on the growth and differentiation of primary myoblasts.
Materials and methods
Polythiophene film synthesis, electrochemistry and spectroscopic characterization
The monomers 3-decoxythiophene (3DTh) and decyl 4,4′′-didecoxy-2,2′:5′,2′′-terthiophene-3′-carboxylate (DTTh3E) were synthesized essentially as described by Gambhir and Feldhues.29,33 The polymer films were synthesized galvanostatically at a current density of 1 mA cm−2 on Au-Mylar (CP Films Inc.) and ITO-coated glass (Delta Technologies) in two different electrolytes: 0.1 M tetrabutylammonium perchlorate (ClO4−) or tetrabutylammonium para-toluenesulphonate (pTS−) (Aldrich). The Au-Mylar was cleaned, prior to deposition, by sonication for 2 min in the following solutions: detergent (Triton X-100; 0.05%) water, isopropanol and methanol. Electrochemical polymerization of bithiophene (Aldrich) and 3DTh was carried out from solutions containing 0.1 M of the monomer in acetonitrile for 40 s and 20 s respectively. In the case of DTTh3E, the concentration of the monomer was 20 mM in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of dichloromethane and acetonitrile and polymerization was carried out for 20 s. After synthesis, all films were rinsed in acetonitrile. All electrochemical experiments were performed in a three-electrode cell, with a non-aqueous Ag/Ag+ reference and Pt mesh counter electrode, using an eDAQ system controlled by EChem software. Absorbance spectra were recorded using a Shimadzu UV-1800 spectrophotometer.
1 mixture of dichloromethane and acetonitrile and polymerization was carried out for 20 s. After synthesis, all films were rinsed in acetonitrile. All electrochemical experiments were performed in a three-electrode cell, with a non-aqueous Ag/Ag+ reference and Pt mesh counter electrode, using an eDAQ system controlled by EChem software. Absorbance spectra were recorded using a Shimadzu UV-1800 spectrophotometer.
Water contact angle
The wettability of the films was characterized using a Data Physics OCA20 goniometer. Contact angle was measured using the sessile drop method (2 μL, Milli Q water). Contact angles were determined under a number of conditions including directly after deposition, rinsing and drying; after 24 hours exposure to air or after 19 h incubation in cell culture media at room temperature or 24 h incubation at 37 °C. In each case, an average of 5 measurements ± standard deviation was calculated.Atomic force microscopy (AFM)
Polymer samples were prepared for characterisation by washing with Milli Q water and drying with nitrogen gas. AFM imaging was performed using a JPK Biowizard II AFM (JPK Instruments, Germany). AFM images were obtained in air at 25 °C using a 0.29 N m−1 silicon nitride cantilever in tapping mode with a scan rate of 0.5 Hz. Root Mean Square (R.M.S.) roughness values were calculated from AFM height images using analysis software of the AFM.Myoblast cell culture
Bl10.129S4Gt(ROSA)26 mice (Bioresources Center, St Vincent's Hospital, Melbourne, Australia) were exposed to a 12 hour day/night cycle and fed ad libitum until they were sacrificed for myoblast culture at 5–6 weeks of age. All animal handling was performed according to St Vincent's Hospital Animal Ethics Committee protocol 86/06 in accordance with the Australian Code of Practice for the Care of Animals for Scientific Purposes (NHMRC).After cervical dislocation, skeletal muscle was removed from the hind limbs of mice and primary myoblast cultures prepared essentially as previously described.34 Adherent myoblasts were cultured in proliferation medium (Hams/F10 (Trace Biosciences) supplemented with 20% fetal bovine serum (Invitrogen), 2.5 ng ml−1 bFGF (PeproTech), 2 mM L-glutamine, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin (Invitrogen)). All cell culture was carried out in 5% CO2 at 37 °C.
Quantification of cell adhesion
Circular discs of polythiophene films and Au mylar (6 mm in diameter) were placed into polystyrene 96-well plates (Nunc). Polymer surfaces were sterilized by briefly washing with 70% ethanol and air drying before removal of residual ethanol by soaking in 2 washes of serum-free culture media. Myoblasts were then seeded in proliferation media (100 μl per well) at a density of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per cm2. Cells were allowed to adhere for 2, 24 and 48 h to determine initial attachment (2 h) and rate of proliferation (24 and 48 h). Before quantification of attached cells by lactate dehydrogenase (LDH) assay, the media containing non-adherent cells was removed and the films washed briefly with proliferation media. The remaining attached cells were then assayed for LDH activity using a CytoTox96 Non-Radioactive Cytotoxicity Assay (Promega), according to manufacturer's instructions. Results were analyzed for significance by one-way ANOVA with Bonferroni post-hoc analysis (GraphPad Prism V 5.0.4).
000 cells per cm2. Cells were allowed to adhere for 2, 24 and 48 h to determine initial attachment (2 h) and rate of proliferation (24 and 48 h). Before quantification of attached cells by lactate dehydrogenase (LDH) assay, the media containing non-adherent cells was removed and the films washed briefly with proliferation media. The remaining attached cells were then assayed for LDH activity using a CytoTox96 Non-Radioactive Cytotoxicity Assay (Promega), according to manufacturer's instructions. Results were analyzed for significance by one-way ANOVA with Bonferroni post-hoc analysis (GraphPad Prism V 5.0.4).
Differentiation of myoblasts to myotubes
As described above, circular discs of polythiophene films and Au mylar (5 mm diameter) were placed into sterile polystyrene 96-well plates (Nunc) and sterilized. Myoblasts were seeded in proliferation media at 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per cm2 and allowed to adhere for 24 h. Myotube differentiation was then induced by changing the media to DMEM supplemented with 2% (v/v) horse serum, 4 mM L-glutamine, 100 units ml−1 of penicillin and streptomycin (all reagents from Invitrogen). Myoblasts were allowed to differentiate for 3 days before further analysis was made.
000 cells per cm2 and allowed to adhere for 24 h. Myotube differentiation was then induced by changing the media to DMEM supplemented with 2% (v/v) horse serum, 4 mM L-glutamine, 100 units ml−1 of penicillin and streptomycin (all reagents from Invitrogen). Myoblasts were allowed to differentiate for 3 days before further analysis was made.
Analysis of myoblasts and myotube differentiation
Myoblasts were seeded onto polythiophene disks, as outlined above, and allowed to proliferate under standard tissue culture conditions for 48 h for visualization of actin filaments and Proliferating Cell Nuclear Antigen (PCNA) expression. For phalloidin labelling of actin filaments, the media was removed and cells fixed in 4% paraformaldehyde (Sigma) for 15 minutes before labelling with AlexaFluor 488 Phalloidin (Invitrogen), according to manufacturer's specifications. Cell nuclei were then stained with 1 μg ml−1 DAPI (4,6-diamidino-2-phenylindole dihydrochloride, Sigma) for 5 min and specimens mounted in fluorescent mounting media (Dako) for microscopy.For PCNA immunostaining, the cells were fixed with 100% methanol (BDH) for 15 min, washed in phosphate-buffered saline (PBS, pH 7.4) and permeabilized with 0.01% Triton-x 100 (Sigma). Non-specific sites were blocked in 10% donkey serum (Millipore) in PBS (Sigma) for 60 min, then incubated with mouse anti-PCNA (Sigma) at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 dilution in blocking solution for 60 min at 37 °C, followed by two washes with PBS. Cells were then incubated with donkey anti-mouse Alexa Fluor 594 (Invitrogen) diluted 1
1000 dilution in blocking solution for 60 min at 37 °C, followed by two washes with PBS. Cells were then incubated with donkey anti-mouse Alexa Fluor 594 (Invitrogen) diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 in blocking solution for 60 minutes at 37 °C. Cell nuclei were stained with DAPI and mounted for microscopy as described above.
2000 in blocking solution for 60 minutes at 37 °C. Cell nuclei were stained with DAPI and mounted for microscopy as described above.
Differentiating myoblasts were stained for desmin expression as follows. After fixation with 100% ice cold methanol (BDH) for 15 min and washing in PBS, cells were immunolabelled as described above using a mouse anti-desmin monoclonal antibody (NovoCastra) at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 dilution followed by donkey anti-mouse Alexa Fluor 594 (Invitrogen). Nuclei were labelled with DAPI and processed for microscopy as described above. All specimens were visualized using an Olympus IX-70 fluorescent microscope and images obtained using Spot Advanced 4.0.9 software (Diagnostic Instruments). Random non-overlapping fields of fluorescently labelled myotubes were taken at 100× magnification. Myotube differentiation was assessed by calculating the percentage of nuclei within myotubes (fusion of two or more myoblasts) as a percentage of total nuclei (from both myoblasts and myotubes) per field. Cell numbers were quantified using the cell count function in ImageJ (NIH).35
100 dilution followed by donkey anti-mouse Alexa Fluor 594 (Invitrogen). Nuclei were labelled with DAPI and processed for microscopy as described above. All specimens were visualized using an Olympus IX-70 fluorescent microscope and images obtained using Spot Advanced 4.0.9 software (Diagnostic Instruments). Random non-overlapping fields of fluorescently labelled myotubes were taken at 100× magnification. Myotube differentiation was assessed by calculating the percentage of nuclei within myotubes (fusion of two or more myoblasts) as a percentage of total nuclei (from both myoblasts and myotubes) per field. Cell numbers were quantified using the cell count function in ImageJ (NIH).35
Statistical analyses
Analysis of LDH activity and cell differentiation were carried out using one way ANOVA with Bonferroni post-hoc analysis. Linear regression analysis was used to determine the relationship between nuclei per cm2 and degree of multinucleation. Non-linear relationships (i.e. correlations with RMS and contact angle) were analyzed using parametric two-tailed Pearsons Product-Moment Correlation analysis for contact angle and Spearman's non-parametric analysis for correlation with roughness. All analyses were carried out using GraphPad Prism (Version 6.0.1).Results
Polythiophene film synthesis
Bithiophene (BTh), alkoxy-derived thiophene (3DTh) and an ester functionalized terthiophene (DTTh3E) were polymerised by galvanostatic deposition with either ClO4− or pTS− as dopants. In all cases, coherent uniform films were deposited at constant current density. Deposition times were short to limit the polymer film thickness to give sufficient transparency for optical microscopy.Surface hydrophobicity
Water contact angle measurements of all polythiophene films deposited on Au-Mylar and ITO were measured under the following conditions: just after electrodeposition, after 24 h in air (for films on ITO), after 19 h soaking in DMEM (CM1) and after 24 h soaked at 37 °C in HAMS F10 media (CM2). The results are summarized in Table 1. The hydrophobicity of the films electrodeposited on Au-Mylar was measured using goniometry, with supplementary results from films deposited on ITO providing further insights into the films’ de-doping dynamics by UV-visible spectroscopy.| Monomer |
|
|
|
|||
|---|---|---|---|---|---|---|
| Dopant | ClO4− | pTS− | ClO4− | pTS− | ClO4− | pTS− |
| Contact angle (°) | ||||||
| Films on Au-Mylar after deposition | 13.7 ± 4.2 | 37.9 ± 1.2 | 110.7 ± 7.8 | 96.7 ± 8.7 | 141.4 ± 4.7 | 98.6 ± 2.3 |
| 19 h soaking in culture media | 22.8 ± 8.3 | 55.7 ± 0.3 | 116.6 ± 1.1 | 120.7 ± 7.0 | 130.1 ± 2.3 | 103.1 ± 2.8 |
| Films on ITO after deposition | 11.1 ± 1.4 | 56.4 ± 2.7 | 123.7 ± 1.8 | 93.2 ± 3.3 | 144.6 ± 1.8 | 99.8 ± 7.9 |
| 24 h in air | 25.3 ± 2.0 | 62.5 ± 3.5 | 119.0 ± 4.3 | 92.9 ± 3.4 | 143.5 ± 3.0 | 99.2 ± 4.9 |
| 19 h soaking in culture media 1 | 46.1 ± 6.6 | 83.6 ± 1.9 | 92.0 ± 6.5 | 98.9 ± 1.1 | 130.6 ± 2.5 | 106.0 ± 3.4 |
| 24 h, 37 °C in culture media 2 | 81.3 ± 5.3 | 77.1 ± 3.5 | 103.0 ± 0.8 | 97.7 ± 0.9 | 120.4 ± 5.8 | 100.7 ± 2.3 |
| Roughness (nm) | ||||||
| (RMS at 10 μm) | 347.2 | 32.3 | 114.9 | 59.4 | 383.0 | 82.0 |
| (RMS at 100 μm) | 574.2 | 60.4 | 217.0 | 74.5 | 585.9 | 94.23 |
| LDH activity (48 h) | ||||||
| Absorbance (490 nm) | 0.629 | 0.771 | 0.887 | 0.971 | 0.899 | 0.859 |
| Myotube formation post differentiation (% of nuclei within myotubes >2 nuclei) | 50.14 | 59.55 | 42.95 | 51.55 | 18.69 | 44.21 |
| Total nuclei post differentiation (nuclei per cm2 % PS control) | 4.46 × 104 (83.31) | 6.80 × 104 (127.04) | 3.62 × 104 (67.72) | 6.38 × 104 (119.31) | 2.55 × 104 (47.70) | 5.29 × 104 (98.81) |
The use of two different dopants for each polymer resulted in significantly different contact angles. ClO4− polymers initially tended to be more hydrophobic than pTS− polymers, when the polymer has hydrophobic substituents. For unsubstituted PBTh, this trend was reversed. The variation in contact angle of both P3DTh and PDTTh3E was much smaller between dopants than that seen for PBTh, for which both ClO4− and pTS− variants remained hydrophilic. It must be noted however, that the films were fully oxidized in solution before the first measurement. As discussed in the next section, spontaneous reduction (de-doping) of the films over the course of the contact angle measurements reduced the influence of the dopant.
Polymer film oxidative stability
To probe the oxidative stability of the polymer films in the dry state and after exposure to culture media, the PBTh, P3DTh and PDTTh3E polymer films were examined by UV-vis spectroscopy. Fig. 1 shows representative UV-vis spectra, since all the polymer films showed similar characteristics. This series of spectra from PBTh-ClO4, recorded just after electrodeposition and then after exposure of the film to air and culture media, are generally representative of the other thiophene/dopant species evaluated in this study. Very broad bands between 600 to 1500 nm, characteristic of polaron (or bipolaron) absorption, can be seen for the freshly prepared film (Fig. 1, black line). After exposure to air for 24 h, these bands reduced in intensity, while the peak at 490 nm corresponding to the neutral polythiophene increased. Interactions of the films with culture media were shown to accelerate these changes, indicative of polymer reduction. The corresponding increase in PBTh film hydrophobicity with dopant loss is evident in the contact angle measurements (Table 1).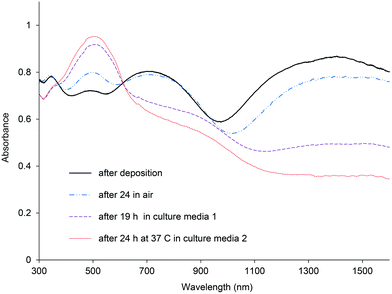 | ||
| Fig. 1 UV-vis spectra of PBTh doped with ClO4 after electrodeposition, after 24 h in air, after 19 h soaking in culture media 1 and after 24 h soaked in 37 °C in culture media 2. | ||
Atomic force microscopy (AFM): surface topography
Initial attempts were made to obtain AFM images of the polymer surfaces in solution so as to more accurately reflect the actual surfaces exposed to cells within a cell culture environment. However, the nodular and friable nature of the surfaces, particularly the PBTh, prevented wet environmental surface measurement and consequently, only dry surface measurements were made. The surface roughness of the films, measured within 100 and 10 μm squares on each of the respective films is shown in Table 1. Images made within 10 μm squares are presented in Fig. 2A–F. Polymers synthesized with ClO4− as the counter ion had a general morphology that consisted of a continuous underlying film (i.e. primary surface features) with particulate structures (secondary features) on the surface. The roughness of each film was primarily determined by the number and size of these particulate surface structures. The particulate structures of the PBTh were not well adhered to the smooth underlying film and were easily swept away by the AFM tip during imaging (results not shown). At 100 μm resolution, PBTh-ClO4 and PDTTh3E-ClO4 were both found to be relatively rough with RMS of 574.2 and 585.9 nm respectively. P3DTh-ClO4 was relatively smoother with a RMS of 217.0 nm at 100 μm resolution (Fig. 2C).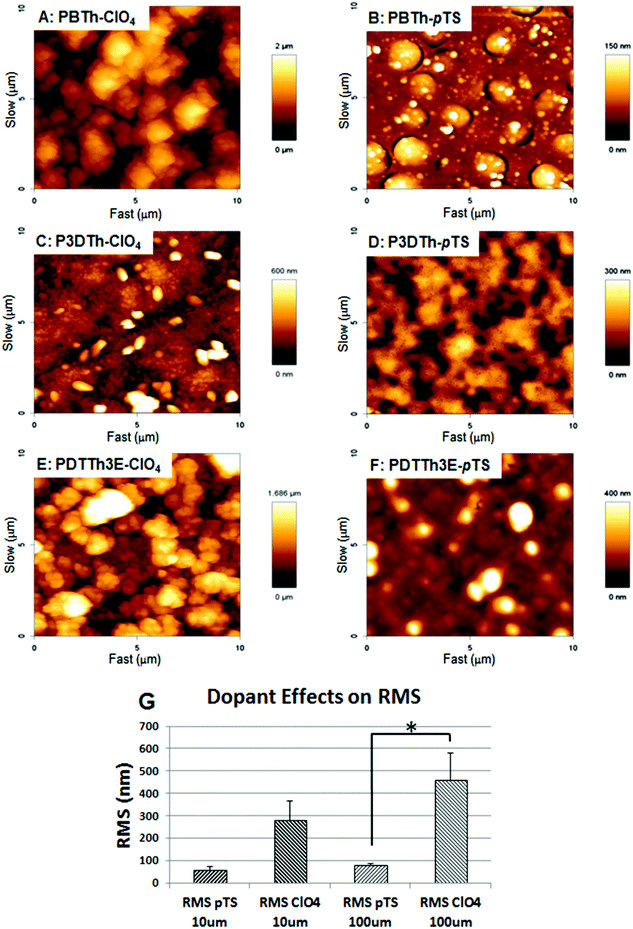 | ||
| Fig. 2 AFM imaging of polymer surface topography. PBTh-ClO4 (A), PBTh-pTS (B), P3DTh-ClO4 (C), P3DTh-pTS, PDTTh3E-ClO4 (E) and PDTTh3E-pTS (F). ClO4− doped polymers were generally rougher than pTS− doped polymers reaching statistical significance at 100 μm2 resolution (G). | ||
The use of pTS− as a dopant resulted in the production of smoother film surfaces (Fig. 2B, D and F; Table 1) when compared to ClO4− doped polymers, which could also influence their wettability. This effect reached statistical significance (p < 0.05) over 100 μm areas (Fig. 2G). A similar trend (not statistically significant) was evident at 10 μm resolution. In the pTS− doped group, the smoothest polymer was PBTh-pTS, followed by P3DTh-pTS and then PDTTh3E-pTS at both 10 and 100 μm (see Table 1).
Adhesion and proliferative metabolism of myoblasts
The affinity of myoblasts for the polythiophene films was assessed by quantification of lactate dehydrogenase (LDH) activity measured in attached cells at 2, 24 and 48 h. LDH was used as an indicator of relative cell numbers on the basis of its constitutive expression as a metabolic enzyme component of the glycolytic cycle. The results for initial cell adhesion, analyzed at 2 h post cell seeding, show that myoblasts adhere to the range of polymer films included in this study to a similar extent, with no significant difference between cellular LDH activities detected on the respective substrates (including tissue culture polystyrene (PS) or Au-mylar control surfaces, data not shown). At 24 h post seeding, lower LDH activities were evident in cell populations grown on PBTh-ClO4 and PDTTh3E-pTS (p < 0.05) compared to the other polymer formulations (Fig. 3A), indicative of reduced cell attachment or otherwise reduced cellular metabolic activity.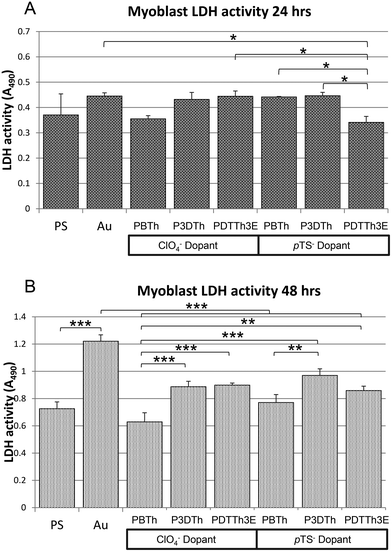 | ||
| Fig. 3 Myoblast LDH activity at 24 (A) and 48 h (B). At 24 h (A), LDH activity was significantly lower in myoblasts grown on PDTTh3E compared to the remaining two polymers of the pTS− doped group (p < 0.05). Myoblasts grown on ClO4− doped polymers did not show any significant difference in LDH activity. By 48 hours (B), differences between polymers were more apparent, indicating variation of effect on cell metabolism according to differences between the pTh formulations. All polymers supported lower LDH activity than gold mylar (Au) surfaces, with significant differences evident between the Au and PBTh-ClO4− and PDTTh3E-pTS surfaces (p < 0.001). In the ClO4− doped group, both P3DTh and PDTTh3E had significantly higher activity than PBTh (p < 0.001). In the pTS− doped group, P3DTh supported the highest activity, significantly higher than PBTh doped with pTS− (p < 0.01). No differences in support of myoblast LDH activity occur between any of the polymer substrata and standard tissue culture polystyrene. In general, the PBTh surfaces (both dopant groups) facilitated less metabolic activity than their similarly doped P3DTh and PDTTh3E counterparts. | ||
By 48 h, differences between the respective affinities of the cells for the polymers became more apparent. P3DTh-pTS yielded cells with the highest mean LDH activity overall (indicating the largest numbers of cells attached), significantly higher than on PBTh-pTS (p < 0.01), or PBTh-ClO4 (p < 0.001) (Fig. 3B). Cells grown on PBTh-ClO4 yielded significantly lower LDH activity than on all other polymers tested with the exception of PBTh-pTS. In the ClO4− doped group, P3DTh-ClO4 and PDTTh3E-ClO4 demonstrated better cell attachment performing equally well at 48 h, both with significantly higher LDH activity than PBTh-ClO4 (p < 0.001). The LDH activity recovered from myoblasts on all of the respective polymer surfaces was doubled at 48 h from that at 24 h, indicating that all of the polymers equally facilitated metabolic expansion of attached myoblasts. In agreement with this, PCNA staining at 48 h revealed that at least a sub-population of myoblasts on each surface were actively proliferating (ESI Fig. 1†).
Actin labeling with phalloidin-Alexafluor 488 demonstrated some variation in cell morphology. The majority of cells on PBTh showed a stellate morphology (Fig. 4A and B) similar to that observed on the control Au film, while more rounded cells were apparent on P3DTh and PDTTh3E (Fig. 4C–F). However, whilst this may be an indicator of generally more favorable proliferative status on the P3DTh and PDTTh3E species, specific detailed quantitative confirmation of this aspect of myogenic cell growth behavior fell beyond the scope of this study and will be reported elsewhere.
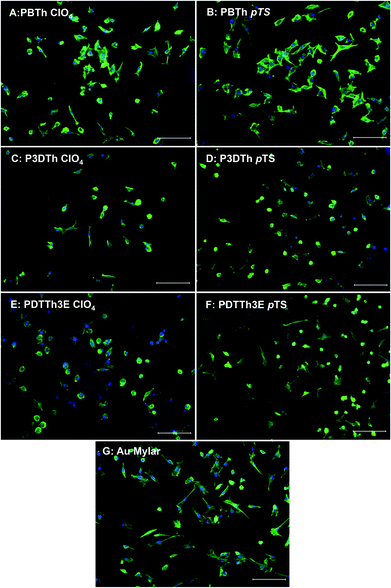 | ||
| Fig. 4 Phalloidin labelling of actin filaments demonstrates differences in cell morphology after 24 h growth on the different surfaces. On PBTh polymers (A and B), the majority of cells appear spread out with well-defined actin filaments indicative of pre-fusional myogenic behavior and show similar morphology to cells grown on gold coated mylar (G), while on P3DTh and PDTTh3E cells appeared more rounded with contracted cytoplasm and condensed cytoskeletal arrangement (C–F). Scale bars represent 100 μm. | ||
Differentiation of myoblasts to myotubes
Myoblasts were cultured in growth medium on polythiophene films and control (PS and Au) substrata for 24 h and then induced to differentiate into myotubes for 72 h in low serum medium. The cells were fixed and immunostained and myoblast differentiation quantified by scoring the proportion of cell nuclei within multinucleate myotubes as a percentage of total nuclei per field.Desmin immunolabelling and DAPI nuclear staining demonstrated that myotubes and undifferentiated myoblasts were present on all the polymer films (Fig. 5A–G), however there was significant variation in myotube formation between polymer types. Tissue culture polystyrene (PS) and Au-Mylar were used as reference substrata for myoblast differentiation, having shown satisfactory facilitation of myoblasts in previous experiments. No significant difference in myoblast differentiation was observed on these two substrata, with both supporting multinucleation (myotube formation) at approximately 64% (Fig. 5H). Only two of the tested polymers, PTTh3E-ClO4 (p < 0.001) and P3DTh-ClO4 (p < 0.05) showed significantly lower percentage myoblast differentiation than these controls. Further to this, myoblasts differentiated on ClO4− doped polymers generally showed lower levels of multinucleation compared to myoblasts differentiated on their pTS− doped counterparts (Fig. 5H), however this trend only reached statistical significance on PPTTh3E films (p < 0.01) films. In the ClO4− group of polymers, PBTh-ClO4 showed the highest level of myotube formation, reaching significance when compared to PTTh3E-ClO4 (p < 0.001), which showed significantly lower capacity to support myotube formation compared to all the other substrata included in this study. The same trend was observed for pTS− doped polymers, however the reduced myotube formation on PPTTh3E-pTS films was not statistically different to that on the remaining pTS− polymers.
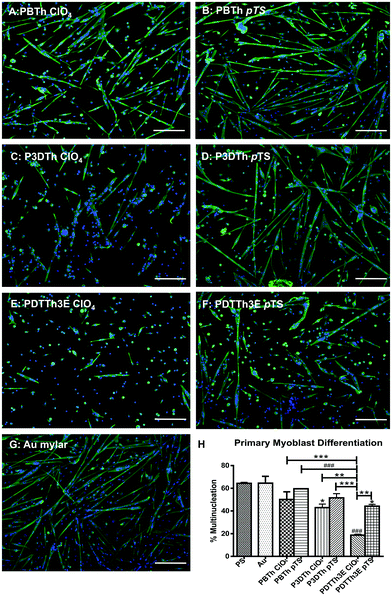 | ||
| Fig. 5 Myoblast differentiation was expressed as the percentage of total nuclei included in multinucleate myofibres containing more than 2 nuclei. Gold coated mylar supported the same level of myoblast differentiation as tissue culture plastic (PS). P3DTh doped with ClO4− and PDTTh3E doped with pTS− and ClO4− showed significantly lower levels of myofibre differentiation than gold mylar and PS (p ≤ 0.05, and p ≤ 0.0001 respectively). Scale bars represent 100 μm. * = p ≤ 0.05; ** = p ≤ 0.01; *** = p ≤ 0.001 and ### = p ≤ 0.0001. | ||
The order of progression from highest to lowest degree of myotube formation on pTh doped with either of the two counter-ions was PBTh > P3DTh > PDTTh3E (Fig. 5H). Myoblasts grown on PBTh underwent the highest levels of myoblast differentiation overall. However, this polymer chemistry supported the lowest numbers of myoblasts by 48 h (Fig. 3), further supporting a biomimetic role for the PBTh polymer chemistry towards induction of myogenic differentiation compared to the other thiophene formulations used here.
Nuclear density was analysed by counting the total nuclei present in myoblasts that were subjected to 24 h of attachment in growth media, followed by 3 days of differentiation on the polymers in low serum media. The densities of DAPI-stained nuclei (nuclei per cm2) were calculated for each polymer (Fig. 6A). Not unexpectedly, this revealed a similar profile to that observed for myoblast differentiation (Fig. 5H), yielding a significant correlation (R = 0.87, p < 0.03; P) between cell density (nuclei per cm2) and myotube formation (Fig. 6B). In all cases, pTS− doped polymers facilitated significantly higher cell densities compared to their ClO4− doped counterparts, under differentiation conditions. In both dopant series, the PBTh substrata yielded higher nuclear densities under differentiation conditions (low serum) than did the other two thiophene species used. This suggests that at least in part, the higher myo-differentiation propensity of myoblasts grown on PBTh species suggested by other data presented in this study (Fig. 4 and 5H) may nevertheless involve some cell proliferation under differentiation conditions to critical contact densities that enhance differentiation. P3DTh-ClO4 and PDTTh3E-ClO4 supported significantly lower nuclei per cm2 under differentiation conditions than control tissue culture PS (Fig. 6), used as a control substrate (p < 0.05 and p < 0.001 respectively) whilst PBTh-pTS supported the highest nuclear density of all of the formulations, with the exception of the P3DTh-pTS substratum.
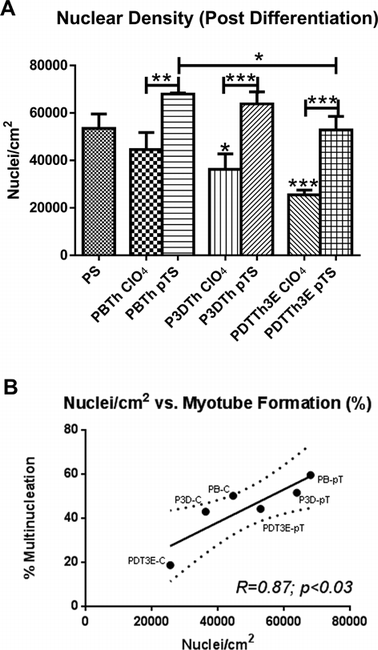 | ||
| Fig. 6 Nuclear density (nuclei per cm2) after differentiation followed a similar profile to myoblast differentiation on the various substrata (A). All pTS− doped polymers showed significantly higher nuclear density than their ClO4− doped counterparts (p < 0.01 for PBTh and p < 0.001 for the others). P3DTh doped with ClO4− and PDTTh3E ClO4− showed significantly lower nuclear density than tissue culture plastic control surfaces (PS) (p < 0.05 and p < 0.001 respectively). A significant correlation (R = 0.87) was observed between nuclear density and myotube formation (B) (p < 0.03). Polymer species were denoted as: PBTH (PB), P3DTh (P3D) and PDTTh3E (PDT3E), with suffixes of C and pT denoting ClO4− and pTS− dopants respectively. | ||
Effect of polymer properties on myoblast and myotube behavior
In order to gain some understanding of the effects of polymer properties on cell behavior, contact angle and roughness (RMS) were correlated with myoblast LDH activity, multinucleation and nuclear density after differentiation. Contact angle did not show a significant correlation with roughness although a positive trend was evident at both 10 μm and 100 μm resolution (data not shown).Likewise, myoblast LDH activity did not show a significant correlation with roughness (data not shown), however significant correlations were observed between LDH activity after 48 h and both contact angle measured on freshly prepared polymers (t = 0; R2 = 0.73, p < 0.03) and polymers exposed to DMEM for 19 h (t = 19 h; R2 = 0.73, p < 0.03, Fig. 7A). These data both yielded peak myoblast adhesion on surfaces with contact angles greater than ∼90°. Furthermore, the general similarity of the relationship displayed on the thiophene surfaces immediately after deposition and after 19 h in DMEM indicates that evaluation of this relationship is equally valid on dry and biological media-wetted surfaces respectively.
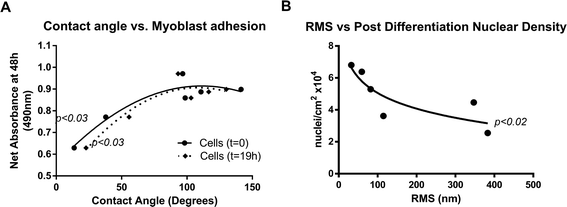 | ||
| Fig. 7 The effects of surface roughness and hydrophobicity on myoblast behavior. Significant trends were observed between contact angle and myoblast adhesion (p < 0.03) (A) and post-differentiation nuclear density (B, p < 0.02). | ||
Myoblast differentiation (represented by % multinucleation) was not found to correlate significantly with surface contact angle, although a trend approaching significance (p < 0.059, data not shown) was evident. Again, similarity in relational trends between cell differentiation and contact angles that were measured as dry films and after soaking in culture media for 19h respectively, means that cell differentiation behaviour on the polymers can be predicted using contact angles measured in the dry state.
A semi-logarithmic trend approaching statistical significance (p < 0.059, data not shown) was observed between surface roughness (RMS) and percentage of cells that differentiated on the thiophene surfaces. This (inverse) trend coincided with a statistically significant correlation (p < 0.02) between RMS and post-differentiation nuclear density (Fig. 7B). Collectively, these differentiation data suggest that (i) the polythiophene surfaces may support a weak myonuclear differentiation effect over a range of surface roughnesses up to around 400 nanometres where this effect appears to plateau/alter, and that (ii) this effect correlates with an increased nuclear density during differentiation (Fig. 7B). However, these interesting effects require more detailed investigation to fully develop the specific nature of surface topographical effects on myoblast attachment, proliferation and differentiation to myotubular form. In particular, the likelihood that known molecular (polymer and dopant) chemistries underlying surface property variations of the polymers studied here will have direct effects on cell behaviour makes definitive identification of the individual effects of these factors challenging.
Discussion
Polymer properties
While a wide variety of synthetic polymers have been investigated for the engineering of muscle tissue, conducting polymers have distinct advantages in that they offer a platform for the simultaneous electrochemical, biological and mechanical stimulation of muscle cells. Polythiophenes, in particular, can be readily synthetically modified to tailor polymer properties such as morphology, surface energy and tensile strength.36 Variation of substituents and dopants also enhances the versatility of these materials as tissue platforms.In our previous investigations into the use of polythiophene substrates for the in vitro growth of skeletal muscle cells, we utilized two chemically prepared substituted polythiophenes of distinctly different surface characteristics.12 It was demonstrated that, despite the diverse hydrophilic character of the films, both films supported the proliferation and differentiation of primary and transformed skeletal muscle myoblasts. In addition, we manipulated these polymers into aligned electrospun fibers for the orientation of differentiated primary myotubes, an important requirement for skeletal muscle engineering. However, this work raised a number of questions regarding the effect of the dopant on cell growth and differentiation, the stability of the conducting form of the polymer and the influence of the different substituents on cell performance.
In this investigation, we have attempted to probe these issues, using electrochemically-prepared polymer films in which the dopant was varied, and alkoxy groups incorporated into the polymer backbone to enhance the oxidative stability of the polymer. The current study compares the properties of polymers synthesized from the electrochemical oxidation of three thiophene monomers, using both ClO4− and pTS− as counter anions and their effect(s) on the growth and differentiation of primary myoblasts in vitro. The different thiophene chemistries yielded a variety of surface energy (represented as surface contact angle of a water droplet) and roughness (surface RMS by AFM) effects between the different substituent groups. Likewise, variations in surface energy and roughness were also evident between the ClO4− and pTS− doped polymers (Table 1).
Contact angle measurements were made on films exposed to air as well as cell culture media in order to investigate how the effects of media exposure might affect the surface available for cell growth (Table 1). With regard to the dopant, pTS− doped thiophenes tended to be more hydrophilic than the analogous ClO4− doped polymers with the exception of PBTh, whose surfaces yielded the lowest contact angle measurements for both dopants. However, for both PBTh polymers, a reduction in hydrophilicity was observed over time either in air or culture media presumably due to de-doping.
Spontaneous reduction with concomitant de-doping of the thiophene polymers under ambient conditions and in culture media has been reported previously.12,37 In an attempt to improve the oxidative stability of the polymers, we introduced alkoxy substituents.38 The UV-vis spectroscopic analysis of the films (Fig. 1) exposed to both air and culture media show, however, that the polythiophene films are still unstable in the oxidized form. This indicates partial de-doping, which can alter the surface wettability. Only in the case of the PBTh polymers (PBTh-ClO4 and PBTh-pTS) was the increase in hydrophobicity clearly influenced by de-doping of the polymers. This has been observed previously by Kossmehl et al.39 The variation in contact angles for the other two polymers, P3DTh and PDTTh3E, for both dopants, is much smaller and does not follow any overt trend. The presence of the alkoxy groups thus appears to dominate the surface hydrophobicity, negating the effect of the dopant. Interactions of the films with culture media accelerated these changes, indicating progressive polymer reduction. Against expectations, the polymers with alkoxy substituents (P3DTh and PDTTh3E) did not show improvement in oxidative stability.
The morphology of the polymers was also clearly affected by the dopants with significant variations in surface roughness occurring between polymers (Fig. 2 and Table 1). All of the pTS− doped polymers were much smoother than their ClO4− doped counterparts. In comparison with the chemically-prepared polythiophenes that we used in our previous study of muscle cell growth,12 these electrochemically grown films are up to five times rougher. Therefore, it was of interest to ascertain whether this increased roughness would have an impact on cell growth and differentiation as it is well documented that roughness, as well as wettability, can effect cell adhesion to and migration on substrata.40,41
Primary myoblast adhesion, proliferation and differentiation on thiophene polymers
Primary myoblasts were seeded on the polymer surfaces for analysis of myoblast attachment, proliferation and differentiation. A general bio-affinity of the polymer surfaces for primary myoblasts was evident, with proliferative cellular metabolism occurring on all polymers for at least 48 hours. However, differences in cell number evident on the polymers by 48 h are nevertheless suggestive of some variation in proliferative metabolic rate effect(s) imparted by polymer chemistry (Fig. 3). Immunofluorescent staining of PCNA in the cells confirmed that proliferating cells were present on all polymers by 48 h, indicating that progression though the cell cycle and effective cell division was taking place and in general all of the thiophene polymers evaluated in this study facilitated effective cell growth.Despite this, extensive morphological differences were observed between cells proliferating on the different polymer types. On PBTh polymers, myoblasts generally demonstrated a stellate morphology, with well-defined actin filaments by phalloidin staining and showed similar morphology to cells grown on gold-coated mylar (Fig. 4A, B and G). This morphology is characteristic of membrane lacunae formation and activation of stochastic radial filopodial extensions, suggesting myoblast progression towards fusion and exit from proliferative cell cycling.42 In contrast, significant numbers of the myoblasts grown on P3DTh and PDTTh3E polymers compared to the PBTh species appeared more rounded with contracted cytoplasm and generally condensed actin distribution, suggesting lower surface compatibility, reduced attachment and a more proliferative metabolic status. These observations are significant as actin is known to play an important role in myoblast adhesion, motility and fusion.43 Extensive actin remodeling is required to promote effective myoblast alignment and fusion,44,45 and disruption of actin polymerization could potentially cause perturbation of the differentiation process.
This interpretation of morphological differences was indeed supported by higher myo-differentiation on PBTh surfaces (along with control Au-mylar and PS), compared to their likewise-doped polythiophene counterparts (Fig. 5H), which demonstrated a lower capacity to support myoblast fusion and differentiation. However, the greater post-differentiation nuclear densities observed on the two PBTh dopant species (Fig. 6A) suggest that the greater myo-differentiation on PBTh substrata compared to similarly doped P3DTh and PDTTh3E may involve a proliferative component (Fig. 6B) that selectively promotes differentiation-resistant cells from the myoblast population. Whilst the specific mechanisms behind this are unclear, the results yielded in this study suggest that in addition to potential perturbation of actin polymerization, the greater pro-differentiation propensity of myoblasts grown on PBTh may be mediated by this polymer’s biomimetic support for attainment of critical cell densities and its surface properties that collectively enhance myo-differentiation response. In addition, obvious differences were evident between myogenic cell behaviors promoted by the polythiophene species doped with ClO4− and pTS− respectively. In particular, the polythiophene species doped with pTS− supported greater differentiation (Fig. 5H) and nuclear densities (Fig. 6), than did the ClO4−-doped polymers. This confirms polythiophene chemistry as amenable to being tailored both by monomer derivatisation and by dopant chemistry to affect myoblast proliferation or differentiation, possibly even prospectively once the mechanisms are better understood.
Effects of polymer properties on myoblast behavior
Surface energy and roughness are important considerations in polymer chemistry, particularly for the interaction of biological agents and entities with polymer materials developed for bio-medical applications. The chemical and physical properties of surfaces are known to play a critical role in regulating cellular behavior. In particular, surface energy and micro/nanoscale roughness have been shown to exert effects on cellular adhesion and proliferation, motility and differentiation.40,46,47 These two properties are not mutually exclusive as the topographical features of a surface can have a large influence on the surface energy. A well-known example of this is the superhydrophobic lotus leaf effect which has been “mimicked” for synthesis of materials.48 A general increase in contact angle with increasing roughness was observed in this study (with the exception of PBTh-ClO4 which exhibited high roughness but low contact angle) and concurs with other reports (with some exceptions) of correlation of modulated roughness with hydrophobicity.49Both wettability and roughness have been shown to play a direct role in protein adsorption onto substrate surfaces. In cell culture, high concentrations of fetal bovine serum, which contains numerous proteins, lipids and growth factors, are used. The influence of roughness on albumin adsorption has been widely studied, with a number of reports demonstrating an increase in adsorption with increasing roughness.50,51 In addition, we have observed that adsorption of proteins such as fibronectin and albumin, can be affected differently by surface roughness51 whereby albumin adsorption increases with polypyrrole roughness, while fibronectin adsorption is independent of polypyrrole surface roughness. This yields a possibility that surface roughness and wettability may at least in part, exert their effect on cell attachment and differentiation through differential binding of proteins present in serum. In the current study, there was no correlation seen between surface roughness and cell attachment/proliferation (represented by LDH activity), despite reports to the contrary.52–54 It is noteworthy that the specific direct effects of roughness on in vitro cell adhesion may be masked due to adsorption of proteins within the media or alternatively the effects of substrate roughness may be cell specific.55 A detailed study on myoblast adhesion, proliferation and the effects of protein adsorption on thiophene polymers would be of interest but is beyond the scope of the current study.
A significant correlation occurred between substratum contact angle and LDH activity recovered from cells on the surface suggesting an influence of polymer surface energy on myoblast adhesion (Fig. 7A). The PBTh polymers presented a soft, powdery top layer that could be easily dislodged by AFM stylus, a characteristic previously reported by our group and others.56,57 Whilst this did not appear to have any effect on the general affinity of the cells for the PBTh surfaces (Fig. 3), this may have a bearing on some of the subsequent cell differentiation behaviors on these compared to the other thiophene surfaces.
Myoblast adhesion and to a lesser extent differentiation (trend only, p < 0.059; data not shown) were observed to be influenced by contact angle (CA), with maximal adhesion occurring at CA of around 90° (Fig. 7A). Surface roughness appeared to show a weak inverse relationship trend with differentiation (trend only, p < 0.059), along with an inverse effect on numbers of nuclei attached to the substratum (Fig. 7B).
The nature of any conducting polymer surface is determined by interplay between multiple factors mediated by the base monomer and dopant chemistries and modulated by the polymerisation process. With regard to the salient features of a polymer surface that determine the biomimetic cellular response to contact with the polymer, surface energy, modulus and surface topography interplay to yield an overall environment that affects cell/surface interaction.41 These (amongst other) highly variable influencing factors can make it difficult to identify the precise mechanisms affecting cellular behavior on these polymers. It is noteworthy from this context that the results presented here showed that these relationships can be equally established using substrata analysed as dry surfaces immediately post-synthesis as when using wetted surfaces representative of the growth environment (Fig. 7A). This provides some indication that these complex effects persist reliably throughout the exposure of the material substrate to an aqueous environment and confirm (at least for the materials studied here) the validity of material characterization immediately post-synthesis in non-biological conditions.
It is thus evident from this study that polythiophene chemistry provides a potential mechanism by which the biomimetic properties of biosynthetic scaffolds can be tailored to suit the biological needs of specific cell types (such as the myoblasts used here) if these needs are known. In this study myogenic precursors were observed to react differentially to growth and differentiation on various polythiophene surfaces according to monomer and dopant chemistries. With further work aimed towards understanding the precise mechanisms by which polymer and dopant chemistries promote biomimetic effects in specific cell types, this study provides a basis by which myogenic behavior may be controlled by polymer chemistries to attain desired outcomes for skeletal muscle engineering.
Conclusions
This study has highlighted that polythiophene polymers provide a versatile platform for the directed growth and differentiation of muscle precursors. Primary myoblasts were observed to proliferate and differentiate on the polymers as effectively as on control (polystyrene and gold-mylar) substrata with the added advantage of being able to modulate myogenic behavior by variation of dopant and incorporating alkoxy modifications to the polymer backbone. The specific mechanism(s) by which these changes in polythiophene chemistry mediate biomimetic responses in myogenic precursors are largely unclear. It is however evident from this study that these changes in myogenic behavior are mediated at least in part, via effects on growth and replication responses of cells growing on the polythiophene surfaces. In turn, these effects appear to be mediated through changes in the physical properties of the polymer, such as the wettability (contact angle), which has more pronounced effect on cell adhesion than on differentiation. In this study, decreased surface roughness supported higher numbers of cells, which facilitated cell fusion into myotubes.Further understanding of the potential of polymer chemistry to effect changes in the molecular biology of the cell should reveal the precise mechanisms by which cell growth and development affected by the chemical structure and physico-chemical properties of biosynthetic scaffolds. These phenomena can potentially be exploited by chemical variation to tailor polymer properties to achieve a given desired cellular response. In turn, this will facilitate the development of more effective scaffolds by which to replace and/or restore damaged or diseased tissues.
Acknowledgements
This research was supported by the Australia Research Council and the National Health and Medical Research Council, Australia. ARC fellowships to Simon E. Moulton (ARC QEII Fellow) and Gordon G. Wallace (Australia Laureate Fellow) are also gratefully acknowledged.Notes and references
- A. D. Bach, J. P. Beier, J. Stern-Staeter and R. E. Horch, J. Cell. Mol. Med., 2004, 8(4), 413–422 CrossRef CAS.
- J. Huard, R. Roy, B. Guerette, S. Verreault, G. Tremblay and J. P. Tremblay, Muscle Nerve, 1994, 17(2), 224–234 Search PubMed.
- E. Gussoni, H. M. Blau and L. M. Kunkel, Nat. Med., 1997, 3(9), 970–977 Search PubMed.
- L. Morandi, P. Bernasconi, M. Gebbia, M. Mora, F. Crosti, R. Mantegazza and F. Cornelio, Neuromuscular Disord., 1995, 5(4), 291–295 Search PubMed.
- N. F. Huang, R. G. Thakar, M. Wong, D. Kim, R. J. Lee and S. Li, Conf. Proc. IEEE Eng. Med. Biol. Soc., 2004, 7, 4966–4969 Search PubMed.
- N. F. Huang, S. Patel, R. G. Thakar, J. Wu, B. S. Hsiao, B. Chu, R. J. Lee and S. Li, Nano Lett., 2006, 6(3), 537–542 CrossRef CAS.
- J. M. Razal, M. Kita, A. F. Quigley, E. Kennedy, S. E. Moulton, R. M. I. Kapsa, G. M. Clark and G. G. Wallace, Adv. Funct. Mater., 2009,(19), 3381–3388 Search PubMed.
- J. P. Beier, D. Klumpp, M. Rudisile, R. Dersch, J. H. Wendorff, O. Bleiziffer, A. Arkudas, E. Polykandriotis, R. E. Horch and U. Kneser, BMC Biotechnol., 2009, 9, 34 CrossRef.
- L. Boldrin, N. Elvassore, A. Malerba, M. Flaibani, E. Cimetta, M. Piccoli, M. D. Baroni, M. V. Gazzola, C. Messina, P. Gamba, L. Vitiello and C. P. De, Tissue Eng., 2007, 13(2), 253–262 Search PubMed.
- L. Boldrin, A. Malerba, L. Vitiello, E. Cimetta, M. Piccoli, C. Messina, P. G. Gamba, N. Elvassore and C. P. De, Cell Transplant, 2008, 17(5), 577–584 Search PubMed.
- L. Thorrez, J. Shansky, L. Wang, L. Fast, T. VandenDriessche, M. Chuah, D. Mooney and H. Vandenburgh, Biomaterials, 2008, 29(1), 75–84 Search PubMed.
- R. D. Breukers, K. J. Gilmore, M. Kita, K. K. Wagner, M. J. Higgins, S. E. Moulton, G. M. Clark, D. L. Officer, R. M. Kapsa and G. G. Wallace, J. Biomed. Mater. Res., Part A, 2010, 95(1), 256–268 CrossRef CAS.
- A. K. Saxena, J. Marler, M. Benvenuto, G. H. Willital and J. P. Vacanti, Tissue Eng., 1999, 5(6), 525–532 CAS.
- X. Wang, X. Gu, C. Yuan, S. Chen, P. Zhang, T. Zhang, J. Yao, F. Chen and G. Chen, J. Biomed. Mater. Res., Part A, 2004, 68(3), 411–422 Search PubMed.
- Z. Wang, C. Roberge, L. H. Dao, Y. Wan, G. Shi, M. Rouabhia, R. Guidoin and Z. Zhang, J. Biomed. Mater. Res., Part A, 2004, 70(1), 28–38.
- K. J. Gilmore, M. Kita, Y. Han, A. Gelmi, M. J. Higgins, S. E. Moulton, G. M. Clark, R. Kapsa and G. G. Wallace, Biomaterials, 2009, 30(29), 5292–5304 CrossRef CAS.
- K. Svennersten, M. H. Bolin, E. W. Jager, M. Berggren and A. Richter-Dahlfors, Biomaterials, 2009, 30(31), 6257–6264 CrossRef CAS.
- A. M. Wan, R. M. Schur, C. K. Ober, C. Fischbach, D. Gourdon and G. G. Malliaras, Adv. Mater., 2012, 24(18), 2501–2505 Search PubMed.
- A. M. Wan, D. J. Brooks, A. Gumus, C. Fischbach and G. G. Malliaras, Chem. Commun., 2009,(35), 5278–5280 RSC.
- A. Gelmi, M. J. Higgins and G. G. Wallace, Small, 2013, 9(3), 393–401 Search PubMed.
- P. R. Bidez III, S. Li, A. G. MacDiarmid, E. C. Venancio, Y. Wei and P. I. Lelkes, J. Biomater. Sci., Polym. Ed., 2006, 17(1–2), 199–212 CrossRef.
- R. M. Miriani, M. R. Abidian and D. R. Kipke, Conf. Proc. IEEE Eng. Med. Biol. Soc., 2008, 2008, 1841–1844 Search PubMed.
- A. F. Quigley, J. M. Razal, B. C. Thompson, S. E. Moulton, M. Kita, E. L. Kennedy, G. M. Clark, G. G. Wallace and R. M. I. Kapsa, Adv. Mater., 2009, 21(43), 4393–4397 CrossRef CAS.
- R. T. Richardson, A. K. Wise, B. C. Thompson, B. O. Flynn, P. J. Atkinson, N. J. Fretwell, J. B. Fallon, G. G. Wallace, R. K. Shepherd, G. M. Clark and S. J. O'Leary, Biomaterials, 2009, 30(13), 2614–2624 CrossRef CAS.
- R. T. Richardson, B. Thompson, S. Moulton, C. Newbold, M. G. Lum, A. Cameron, G. Wallace, R. Kapsa, G. Clark and S. O'leary, Biomaterials, 2007, 28(3), 513–523 CrossRef CAS.
- D. M. Pedrotty, J. Koh, B. H. Davis, D. A. Taylor, P. Wolf and L. E. Niklason, Am. J. Physiol.: Heart Circ. Physiol., 2005, 288(4), H1620–H1626 Search PubMed.
- Y. Kawahara, K. Yamaoka, M. Iwata, M. Fujimura, T. Kajiume, T. Magaki, M. Takeda, T. Ide, K. Kataoka, M. Asashima and L. Yuge, Pathobiology, 2006, 73(6), 288–294 Search PubMed.
- M. L. Langelaan, K. J. Boonen, K. Y. Rosaria-Chak, D. W. van der Schaft, M. J. Post and F. P. Baaijens, J. Tissue Eng. Regener. Med., 2011, 5(7), 529–539 Search PubMed.
- S. Gambhir, K. Wagner and D. L. Officer, Synth. Met., 2005, 154, 117–120 CrossRef.
- T. Neumann, S. D. Hauschka and J. E. Sanders, Tissue Eng., 2003, 9(5), 995–1003 Search PubMed.
- I. Jun, S. Jeong and H. Shin, Biomaterials, 2009, 30(11), 2038–2047 Search PubMed.
- P. Y. Lee, E. Cobain, J. Huard and L. Huang, Mol. Ther., 2007, 15(6), 1189–1194 CAS.
- M. Feldhues, G. Kampf, H. Litterer, T. Mecklenburg and P. Wegener, Synth. Met., 1989, 28(1–2), 487–493 CrossRef.
- R. M. Kapsa, A. F. Quigley, J. Vadolas, K. Steeper, P. A. Ioannou, E. Byrne and A. J. Kornberg, Gene Ther., 2002, 9(11), 695–699 Search PubMed.
- M. D. Abramoff, P. J. Magalhaes and S. J. Ram, Biophotonics Int., 2004, 11(1), 36–42 Search PubMed.
- G. Barbarella, M. Melucci and G. Sotgiu, Adv. Mater., 2005, 17, 1581–1593 CrossRef CAS.
- L. Zhou, X. Liu and G. Xue, Spectrosc. Lett., 1998, 31(7), 1529–1535 Search PubMed.
- G. Daoust and M. Leclerc, Macromolecules, 1991, 24(455), 459 Search PubMed.
- G. Kossmehl and M. Niemitz, Synth. Met., 2012, 41(3), 1065–1071 Search PubMed.
- M. Lampin, C. Warocquier, C. Legris, M. Degrange and M. F. Sigot-Luizard, J. Biomed. Mater. Res., 1997, 36(1), 99–108 CrossRef CAS.
- A. Ranella, M. Barberoglou, S. Bakogianni, C. Fotakis and E. Stratakis, Acta Biomater., 2010, 6(7), 2711–2720 CrossRef CAS.
- A. B. Fulton, J. Prives, S. R. Farmer and S. Penman, J. Cell Biol., 1981, 91(1), 103–112 Search PubMed.
- M. A. Griffin, S. Sen, H. L. Sweeney and D. E. Discher, J. Cell Sci., 2004, 117(Pt 24), 5855–5863 Search PubMed.
- S. J. Nowak, P. C. Nahirney, A. K. Hadjantonakis and M. K. Baylies, J. Cell Sci., 2009, 122(Pt 18), 3282–3293 Search PubMed.
- S. Kim, K. Shilagardi, S. Zhang, S. N. Hong, K. L. Sens, J. Bo, G. A. Gonzalez and E. H. Chen, Dev. Cell, 2007, 12(4), 571–586 Search PubMed.
- H. Schweikl, R. Muller, C. Englert, K. A. Hiller, R. Kujat, M. Nerlich and G. Schmalz, J. Mater. Sci.: Mater. Med., 2007, 18(10), 1895–1905 CrossRef CAS.
- S. Balloni, E. M. Calvi, F. Damiani, G. Bistoni, M. Calvitti, P. Locci, E. Becchetti and L. Marinucci, Int. J. Oral Maxillofac. Implants, 2009, 24(4), 627–635 Search PubMed.
- J. Lin, Y. Cai, X. Wang, B. Ding, J. Yu and M. Wang, Nanoscale, 2011, 3(3), 1258–1262 RSC.
- D. P. Dowling, I. S. Miller, M. Ardhaoui and W. M. Gallagher, J. Biomater. Appl., 2011, 26(3), 327–347 Search PubMed.
- M. N. Sela, L. Badihi, G. Rosen, D. Steinberg and D. Kohavi, Clin. Oral Implants Res., 2007, 18(5), 630–638 CrossRef.
- P. J. Molino, M. J. Higgins, P. C. Innis, R. M. Kapsa and G. G. Wallace, Langmuir, 2012, 28(22), 8433–8445 Search PubMed.
- B. R. Prasad, M. A. Brook, T. Smith, S. Zhao, Y. Chen, H. Sheardown, R. D'Souza and Y. Rochev, Colloids Surf., B, 2010, 78(2), 237–242 CrossRef CAS.
- B. Baharloo, M. Textor and D. M. Brunette, J. Biomed. Mater. Res., Part A, 2005, 74(1), 12–22 Search PubMed.
- D. D. Deligianni, N. Katsala, S. Ladas, D. Sotiropoulou, J. Amedee and Y. F. Missirlis, Biomaterials, 2001, 22(11), 1241–1251 CrossRef CAS.
- X. Hu, S. H. Park, E. S. Gil, X. X. Xia, A. S. Weiss and D. L. Kaplan, Biomaterials, 2011, 32(34), 8979–8989 CrossRef CAS.
- J. Roncali, Chem. Rev., 1992, 92, 711–738 CrossRef CAS.
- M. J. Higgins, W. Grosse, K. Wagner, P. Molino and G. G. Wallace, J. Phys. Chem. B, 2011, 115(13), 3371–3378 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3bm60059a |
| ‡ Present address: NICTA, Department of Electrical and Electronic Engineering, University of Melbourne, Victoria, 3010, Australia. |
| This journal is © The Royal Society of Chemistry 2013 |