A molecular hydrogel of a camptothecin derivative†
Zhijian
Song
a,
Hanxia
Liu
a,
Jie
Shen
*b and
Xuemei
Chen
*a
aSchool of Chemical and Materials Engineering, Hubei Institute of Technology, Huangshi 435003, P. R. China. E-mail: mixue.zi@163.com
bTianJin First Center Hospital, Tianjin 300192, P. R. China. E-mail: shenjie_vip@126.com
First published on 3rd December 2012
Abstract
We report on the first example of self-assembled nanotubes of a camptothecin derivative in a molecular hydrogel.
Camptothecin (CPT) is an anti-cancer drug which inhibits the DNA enzyme topoisomerase I (topo I) and induces apoptosis of rapidly dividing cells (e.g., cancer cells).1 However, several shortcomings hinder its practical application in clinics (e.g. low water solubility and adverse side effects from its carboxylate form). Therefore, nanosized vehicles such as liposomes, capsules formed by DNA and block co-polymers, and some inorganic materials are used as carriers of hydrophobic anti-cancer drugs including CPT to enhance their water solubility and stability, target cancer tissues via the tumors' enhanced permeability and retention (EPR) effect or the targeting effect of folic acid, and prolong its circulation in blood.2 For example, the Wang group have designed a dual pH-sensitive nanoparticle for a highly selective delivery of the anti-cancer drug to cancer cells including cancer stem cells.3 Besides these successful examples, spherical carriers formed by the self-assembly of CPT derivatives have recently been demonstrated as promising self-delivery systems because of their very high drug loading capacity and no premature burst releases.4 For instance, the Murdoch group and the Li group have reported conjugates of CPT with polyethylene glycol (PEG) that can spontaneously self-assemble into spherical nanostructures, thereby greatly enhancing the chemical stability and anti-tumor efficacy of CPT.5 These observations, combined with other self-assembled nanostructures of therapeutic agents,6,7 not only opened up a new research area for the development of self-delivery systems of therapeutic agents, but also would lead to novel delivery forms of therapeutic agents for practical applications. In this study, we report on another example of a self-assembled system of a CPT derivative. Moreover, this is the first example of self-assembled nanotubes of a CPT derivative.
We designed CPT-G-Succ-FFYGE-ss-EEE (1) as a precursor of a self-assembled molecule of CPT-G-Succ-FFYGE-s (2) (see Scheme 1). The principles of our design are based on Yang et al.’s results7–9 and are described as follows: (1) the self-assembling ability of FF and FFY had been demonstrated and they had been widely used for the construction of self-assembled molecules;10 (2) tri-glutamic acids (EEE) could enhance the solubility of 1 in aqueous solutions and could be cleaved via disulfide bond reduction and (3) the first E on CPT-G-Succ-FFYGE-s adjusted the balance between the hydrophobicity and hydrophilicity and helped to stabilize the resulting self-assembled structures of 2. Upon treatment of the reductant of glutathione (GSH), compound 1 could be converted to 2via disulfide bond reduction, which might be able to self-assemble into a kind of nanostructure.8,11
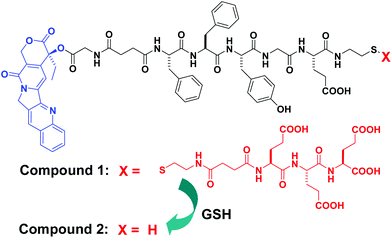 | ||
| Scheme 1 Chemical structures of the precursor (1) of a self-assembled CPT derivative (blue: camptothecin (CPT), black: linker, and red: hydrophilic part of the EEE derivative to improve the solubility of 1) and schematic illustration of the conversion from 1 to 2 by glutathione (GSH). | ||
The synthesis of 1 is easy and straightforward. The peptide of FFYGE-ss-EEE was obtained via a solid phase peptide synthesis by using Fmoc-amino acids and Fmoc-CS.8 It was then used to couple with an N-hydroxysuccinimide (NHS) active ester of the CPT derivative (compound 5, Scheme S1†) in a moderate yield (∼75% after HPLC purification). After the successful synthesis of 1, its ability to self-assemble was evaluated by treatment with 2 equiv. GSH. Compound 1 was highly soluble in phosphate buffer saline (PBS) solutions (pH = 7.4) with a solubility of >5.0 wt%. Upon the addition of 2.0 equiv. GSH, the clear PBS solution containing 1.0 wt% 1 transformed to a hydrogel after about 20 minutes (inserted image in Fig. 1), which clearly indicated the self-assembling ability of 1 by GSH. The hydrogel was stable for more than 3 months at room temperature (22–25 °C).
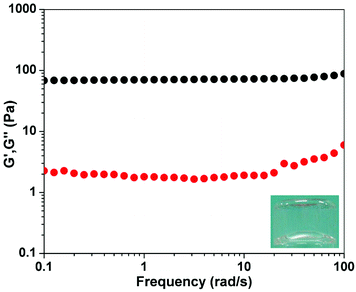 | ||
| Fig. 1 Dynamic frequency sweep of the gel from the solution of 1 (1.0 wt%) at a time of 2 h (strain value = 1%) (insert: optical image of the gel). | ||
The HPLC traces of the solution of 1 with 2 equiv. GSH were used to determine the kinetics of conversion. As shown in Fig. S12 and Table S1,† the single peak centered at about 6.3 min indicated the pure form of 1. Upon the addition of 2 equiv. GSH, about 77% of 1 was converted to 2 and a dimer of 2 with a ratio of about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 after 5 min. More than 98% of 1 had been converted after 20 min, suggesting the rapid reduction of the disulfide bond on 1. Compound 2, formed by the reduction of 1, rapidly converted to its dimer form through the oxidation of 2 by oxygen or the attack of 2 to 1. More than 90% of 2 had changed to the dimer form after 18 hours at room temperature in air. The conversion was also monitored by fluorescence (Fig. S12†). The emission peak from the solution of 1 showed a continuous red-shift, from 427 nm to 438 nm, and its intensity decreased gradually in the first 30 min after the addition of GSH. The red shift and intensity decrease of the emission peak, in combination with the HPLC traces in Fig. S11,† suggested the occurrence of the self-assembly of CPT derivatives, which were frequently observed in other self-assembled systems of small molecules. The emission peak was centered lower than 440 nm (Fig. S12†), indicating the existence of the lactone form of CPT (the emission peak of the carboxylate form of CPT would be centered at >450 nm).12
2 after 5 min. More than 98% of 1 had been converted after 20 min, suggesting the rapid reduction of the disulfide bond on 1. Compound 2, formed by the reduction of 1, rapidly converted to its dimer form through the oxidation of 2 by oxygen or the attack of 2 to 1. More than 90% of 2 had changed to the dimer form after 18 hours at room temperature in air. The conversion was also monitored by fluorescence (Fig. S12†). The emission peak from the solution of 1 showed a continuous red-shift, from 427 nm to 438 nm, and its intensity decreased gradually in the first 30 min after the addition of GSH. The red shift and intensity decrease of the emission peak, in combination with the HPLC traces in Fig. S11,† suggested the occurrence of the self-assembly of CPT derivatives, which were frequently observed in other self-assembled systems of small molecules. The emission peak was centered lower than 440 nm (Fig. S12†), indicating the existence of the lactone form of CPT (the emission peak of the carboxylate form of CPT would be centered at >450 nm).12
We performed rheological measurements with the mode of dynamic frequency sweep to characterize the mechanical properties of the gel at a time of 2 h. As shown in Fig. 1, both G′ (elasticity) and G′′ (viscosity) exhibited weak frequency dependencies in the range 0.1–100 rad s−1. The value of G′ was at least 10 times bigger than that of G′′ at the same frequency value. These results indicated the formation of a true gel.13
We then used transmission electronic microscopy (TEM) to characterize the self-assembled structures in the sample in Fig. 1 (insert) at different time points (Fig. 2A–C). Fig. 2A showed the self-assembled structures formed by adding 2 equiv. GSH to a PBS solution of 1 (1.0 wt%) at a time of 5 minutes, where we observed amorphous helical ribbons with diameters ranging from 50 to 100 nm. Since more and more 1 was converted to 2 and more and more 2 was oxidized to its dimer form, the helical ribbons further grew into crystalline nanotubes with diameters 50–80 nm after 48 hours (Fig. 2C and Fig. S17†). As shown in Fig. 2B and Fig. S16,† the nanotubes were formed by a twisting of the helical ribbons (indicated by arrows), which was similar to the observations reported by the Oda group.14 We hypothesized that the reason for the thin tapes observed in the sample at a time of 2 h (Fig. 2B and Fig. S16†) rolling into nanotubes was probably due to the formation of the dimer of 2 with a different chemical structure and self-assembly property.
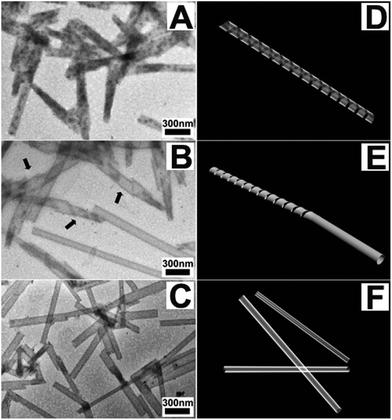 | ||
| Fig. 2 TEM images of (A) the solution of 1 (1.0 wt%) 5 minutes after the addition of 2 equiv. GSH, (B) the gel at a time of 2 hours from the solution of (A), and (C) the gel at a time of 48 hours (arrows in B indicate the entanglement of helical ribbons and images of D, E and F illustrate the formation process of the nanotubes). | ||
The hydrogel could gradually release the original CPT via ester bond hydrolysis at a rate of about 1.58 μg mL−1 per hour in the first 24 hours (Fig. 3). About 6% of the CPT was released during the 24 hour experimental period (119 μg mL−1 or 339 μM), suggesting that it might be used as a long-acting delivery system. The Yang group has recently reported that a taxol hydrogel could be locally applied to inhibit tumor growth and prevent metastasis,15 and our hydrogel might also be injected into tumors after its formation. Usually, CPT in physiological conditions is in an equilibrium between the lactone form and the carboxylate form. However, its carboxylate form will lead to toxicity, thereby hindering its clinical applications.16 Similar to the results reported by Yang and coworkers that more than 99% of the 10-hydroxy camptothecin (HCPT) released from their hydrogel of the HCPT-dexamethasone derivative was in its lactone form,8 the CPT released from our gel was also in its lactone form.17 Therefore, our results indicated that self-assembled forms (i.e. nanotubes) of CPT could help stabilize the lactone form of CPT, which would be beneficial for its practical applications.
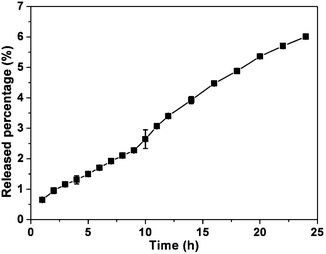 | ||
| Fig. 3 Accumulating percentage of CPT released from the gel. | ||
We also evaluated the activity of the compounds by treating different cells including the normal cell of 3T3 cells and cancer cells of HeLa, HepG2, MD231, and A549 cells at different concentrations (Table 1). After 48 hours incubation with the cells, compound 1 and the gel (freeze-dried gel after 48 h) exhibited comparable IC50 values for HeLa, MD231, and A549 cells to CPT itself. Compound 1 and the gel possessed much lower IC50 values against HepG2 cells than CPT itself—the IC50 values were 7.00 and 2.63 nM for 1 and the gelators in the gel, respectively, while the value of CPT was 35.77 nM. For normal cells of 3T3 cells, compound 1 and the gel showed similar IC50 values to CPT. These observations suggested the great potential for our hydrogel system to be used for the treatment of cancers, especially for liver cancer.
In summary, we have developed a molecular hydrogel of a CPT derivative. To the best of our knowledge, this is the first example of a CPT derivative that can self-assemble into nanotubes. The compounds in gels exhibited a similar activity against different cancer cells to CPT. The gel can constantly release the original CPT via ester bond hydrolysis. Overall, this is another example of a molecular hydrogel of CPT derivatives, which has the potential to be developed into a carrier-free delivery system for cancer therapy. The in vivo stability and activity of CPT in gels need to be evaluated in the future.
We acknowledge financial support from NSFC (51173060).
Notes and references
- W. D. Kingsbury, J. C. Boehm, D. R. Jakas, K. G. Holden, S. M. Hecht, G. Gallagher, M. J. Caranfa, F. L. McCabe, L. F. Faucette and R. K. Johnson, J. Med. Chem., 1991, 34, 98 CrossRef CAS; C. J. Thomas, N. J. Rahier and S. M. Hecht, Bioorg. Med. Chem., 2004, 12, 1585 CrossRef.
- N. G. Sahoo, H. Bao, Y. Pan, M. Pal, M. Kakran, H. K. F. Cheng, L. Li and L. P. Tan, Chem. Commun., 2011, 47, 5235 RSC; W. Wu, R. Li, X. Bian, Z. Zhu, D. Ding, X. Li, Z. Jia, X. Jiang and Y. Hu, ACS Nano, 2009, 3, 2740 CrossRef CAS; Z. Liu, J. T. Robinson, X. Sun and H. Dai, J. Am. Chem. Soc., 2008, 130, 10876 CrossRef.
- J Z. Du, X. J. Du, C. Q. Mao and J. Wang, J. Am. Chem. Soc., 2011, 133, 17560 CrossRef CAS; M. H. Xiong, Y. Bao, X. Z. Yang, Y. C. Wang, B. L. Sun and J. Wang, J. Am. Chem. Soc., 2012, 134, 4355 CrossRef.
- B.-S. Lee, A. K. Nalla, I. R. Stock, T. C. Shear, K. L. Black and J. S. Yu, Bioorg. Med. Chem. Lett., 2010, 20, 5262 CrossRef CAS.
- Y. Shen, E. Jin, B. Zhang, C. J. Murphy, M. Sui, J. Zhao, J. Wang, J. Tang, M. Fan, E. Van Kirk and W. J. Murdoch, J. Am. Chem. Soc., 2010, 132, 4259 CrossRef CAS; X. Q. Li, H. Y. Wen, H. Q. Dong, W. M. Xue, G. M. Pauletti, X. J. Cai, W. J. Xia, D. L. Shi and Y. Y. Li, Chem. Commun., 2011, 47, 8647 RSC.
- F. Zhao, M. L. Ma and B. Xu, Chem. Soc. Rev., 2009, 38, 883 RSC; H. Wang, C. Yang, L. Wang, D. Kong, Y. Zhang and Z. Yang, Chem. Commun., 2011, 47, 4439 RSC; A. Altunbas, S. J. Lee, S. A. Rajasekaran, J. P. Schneider and D. J. Pochan, Biomaterials, 2011, 32, 5906 CrossRef CAS; P. K. Vemula, J. Li and G. John, J. Am. Chem. Soc., 2006, 128, 8932 CrossRef; P. K. Vemula, G. A. Cruikshank, J. M. Karp and G. John, Biomaterials, 2009, 30, 383 CrossRef; S. Soukasene, D. J. Toft, T. J. Moyer, H. M. Lu, H. K. Lee, S. M. Standley, V. L. Cryns and S. I. Stupp, ACS Nano, 2011, 5, 9113 CrossRef.
- H. Wang and Z. Yang, Soft Matter, 2012, 8, 2344 RSC.
- L. N. Mao, H. M. Wang, M. Tan, L. L. Ou, D. L. Kong and Z. M. Yang, Chem. Commun., 2012, 48, 395 RSC.
- H. M. Wang, L. N. Lv, G. Y. Xu, C. B. Yang, J. T. Sun and Z. M. Yang, J. Mater. Chem., 2012, 22, 16933 RSC.
- M. Reches and E. Gazit, Science, 2003, 300, 625 CrossRef CAS; Y. Zhang, Y. Kuang, Y. A. Gao and B. Xu, Langmuir, 2011, 27, 529 CrossRef; Y. Gao, F. Zhao, Q. G. Wang, Y. Zhang and B. Xu, Chem. Soc. Rev., 2010, 39, 3425 RSC; A. M. Smith, R. J. Williams, C. Tang, P. Coppo, R. F. Collins, M. L. Turner, A. Saiani and R. V. Ulijn, Adv. Mater., 2008, 20, 37 CrossRef; C. Tang, A. M. Smith, R. F. Collins, R. V. Ulijn and A. Saiani, Langmuir, 2009, 25, 9447 CrossRef; H. M. Wang, C. H. Ren, Z. J. Song, L. Wang, X. M. Chen and Z. M. Yang, Nanotechnology, 2010, 21 Search PubMed; H. M. Wang, C. H. Yang, M. Tan, L. Wang, D. L. Kong and Z. M. Yang, Soft Matter, 2011, 7, 3897 RSC.
- D. Li, H. Wang, D. Kong and Z. Yang, Nanoscale, 2012, 4, 3047 RSC; C. Yang, D. Li, Z. Liu, G. Hong, J. Zhang, D. Kong and Z. Yang, J. Phys. Chem. B, 2012, 116, 633 CrossRef CAS; C. H. Ren, Z. J. Song, W. T. Zheng, X. M. Chen, L. Wang, D. L. Kong and Z. M. Yang, Chem. Commun., 2011, 47, 1619 RSC.
- I. Chourpa, J. M. Millot, G. D. Sockalingum, J. F. Riou and M. Manfait, Biochim. Biophys. Acta, Gen. Subj., 1998, 1379, 353 CrossRef CAS.
- L. Chen, K. Morris, A. Laybourn, D. Elias, M. R. Hicks, A. Rodger, L. Serpell and D. J. Adams, Langmuir, 2010, 26, 5232 CrossRef CAS.
- R. Oda, I. Huc, M. Schmutz, S. J. Candau and F. C. MacKintosh, Nature, 1999, 399, 566 CrossRef CAS; L. A. Estroff and A. D. Hamilton, Chem. Rev., 2004, 104, 1201 CrossRef.
- H. M. Wang, J. Wei, C. B. Yang, H. Y. Zhao, D. X. Li, Z. N. Yin and Z. M. Yang, Biomaterials, 2012, 33, 5848 CrossRef CAS.
- H. G. Lerchen, J. Baumgarten, K. von dem Bruch, T. E. Lehmann, M. Sperzel, G. Kempka and H. H. Fiebig, J. Med. Chem., 2001, 44, 4186 CrossRef CAS; A. Shenderova, T. G. Burke and S. P. Schwendeman, Pharm. Res., 1999, 16, 241 CrossRef.
- See the ESI†.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of compounds, HPLC traces, emission spectra, and inhibition congress curves. See DOI: 10.1039/c2bm00110a |
| This journal is © The Royal Society of Chemistry 2013 |
