A progressive approach on inactivation of bacteria using silver–titania nanoparticles
Jingbo Louise
Liu
a,
Zhiping
Luo
b and
Sajid
Bashir
*acd
aDepartment of Chemistry, Texas A&M University-Kingsville, MSC 161, 700 University Blvd., Kingsville, TX 78363, USA. E-mail: KFJLL00@tamuk.edu; Fax: +001-361-593-3597; Tel: +001-361-593-2914
bMicroscopy and Imaging Center and Materials Science and Engineering Program, Texas A&M University-College Station, ILSB, 2257 TAMU, College Station, TX 77843, USA. E-mail: luo@mic.tamu.edu; Fax: +1-979-847-8933; Tel: +1-979-862-2883
cChemical Biology Research Group and Texas A&M University-Kingsville, MSC 161, 700 University Blvd., Kingsville, TX 78363, USA. E-mail: br9@tamuk.edu; Fax: +1-361-593-3597; Tel: +1-361-593-4253
dAdvanced Light Sources, The Lawrence Berkeley National Laboratory, One Cyclotron Road, Berkeley, CA 94720, USA. E-mail: SBashir@lbnl.gov; Fax: +1-361-593-3597; Tel: +1-361-228-3147
First published on 26th September 2012
Abstract
A silver inserted metal oxide (Ag–TiO2) has been demonstrated to be an effective biocidal agent against prokaryotic microbes found in water. Transmission electron microscopy and electron energy loss spectroscopy indicated cellular damage after co-incubation with the nanocomposite, showing concomitant leakage of ions, which are critical for cell survival.
Introduction
Recently, Dr J. Liu and her colleagues discovered a series of antimicrobial agents, defined as third-wave, showing high potency at inactivating bacteria. These ‘waves’ include commonly-used chemicals (1st wave), nanoparticles (2nd wave) and metal–organic frameworks (3rd wave), which will provide a ‘shield’ for the protection of human health and ability to clean water. The term ‘wave’ relates to historical introduction in the field of disinfection. The ‘first-wave’ relates to the introduction of bleach, antibiotics or other bulk chemicals, the ‘second’ wave relates to the use of nanoparticles of various forms, either in colloid or structured forms and the ‘third’ wave related to constrained motifs, such as metal–organic frameworks, which have not been previously explored as putative disinfectants. This research particularly demonstrates the application of nanoparticles (NPs), namely silver (Ag) and silver decorated titania (Ag–TiO2) NPs.Silver is one of the oldest known metals, used in a variety of fields, including clothing—as silver damask used by Queen Cleopatra VII, treatment of eye infection,1 antisepsis2 and up to four dozen silver “tonics” in the 1800s as homeopathic rejuvenates for a number of “maladies”.3 When it was shown that silver salts had antibacterial properties,4 particularly in burn patients4 in the form of silver nitrate against Pseudomonas aeruginosa,5–7 its usage increased but was tempered by its high cost, limiting its widespread adaptation as a “first choice” disinfectant. The use of bulk silver in the form of cerium silver sulfadiazine demonstrated efficacy against Gram-negative microbes, including treatment for burns, which continues to this day. This broad range of antimicrobial activity from silver colloids8,9 has been attributed to a number of molecular phenomena, such as the denaturation of proteins through the covalent modification of specific amino acids,10–12 cell wall binding,13–15 and disruption of the membrane potential.16 Although no mechanisms seem to be dominate in microbial inactivation,17 the favoured mechanism is membrane lipid peroxidation by the superoxide anion generated by silver in water.18–20 Any use of silver used for water purification is limited by the requirement of long incubation times and high doses of silver, and the use of ultraviolet radiation as a pre-treatment step.21–23 Recent advances in disinfection science have utilized two-dimensional structured colloid arrays24 which have proved to be effective against both Gram-negative and positive prokaryotes.25 The originality of this study lies in using the scanning transmission electron microscopy-electron energy-loss spectroscopy (STEM-EELS) system to quantitatively analyze the elemental composition distribution in the damaged bacteria, which enables mechanistic study on bactericidal performance of these NPs. This research illustrates the NP fabrication, structural characterization, and antimicrobial application.
Discussion
To better understand the mechanism(s) by which nanomaterials in colloid format exhibit disinfection, it is important to control the forces which keep nanocolloids from concatenating to the bulk form in order to design more efficient materials as antimicrobial surfaces or systems.26 The Derjaguin, Landau, Verwey and Overbeek (DLVO) model proposes solute stability in a solvent is related to the sum potential energy function VPE. The VPE itself is a composite value and is related to the energy of attractive (VA), repulsive (VR) and solvent interactions (VS), as shown in eqn (1):| VPE = VA + VR + VS | (1) |
| VA = −C/(12πD2) | (2) |
VR = 2πεrξ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 exp(−κD) 2 exp(−κD) | (3) |
The total potential, ignoring Brownian motion, is mainly determined by attractive forces (e.g. van der Waals) and repulsive (e.g. Coulombic) forces due to the charged double layer. In other words, two particles cannot collide and coalesce due to repulsive forces being greater than the energy barrier, resulting in dispersion and a stable colloid. Conversely, if the particle attractive forces are greater than the energy barrier, flocculation or coagulation will occur as a consequence of the double layer and corresponding magnitude of the total potential. The double layer is formed as a result of the NPs being placed in an aqueous environment, which in turn yields a charged boundary layer between the charged surface and aqueous environment. The electrical double layer therefore can attract free ions in the aqueous via coulombic and thermal attractive forces, netting an electro-kinetic potential for each body, as defined by eqn (1) and (2). The electrokinetic potential (or Zeta potential (ζ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ) for colloidal systems) is thus the electric potential between the double layer at the slipping plane and bulk, reflecting the potential difference between the stationary layer of fluid around the solubilized solute and the dispersion medium. From a practical point of view, particles with smaller diameters exhibit a large absolute negative zeta potential. In our study, the time dependence of the zeta potential over the course of measurement was not observed, which indicated the nanomaterial colloid was stable. The large negative zeta potential (Fig. 1a) would promote repulsion between the nanoparticles, which in turn would minimize particle agglomeration.27 The generated titania was measured by X-ray powder diffraction and well indexed with standard TiO2. The crystalline phase corresponded to anatase (PDF 01-086-1157, lattice constant 3.7852 × 9.5139 Å, 90°) shown in Fig. 1b.
) for colloidal systems) is thus the electric potential between the double layer at the slipping plane and bulk, reflecting the potential difference between the stationary layer of fluid around the solubilized solute and the dispersion medium. From a practical point of view, particles with smaller diameters exhibit a large absolute negative zeta potential. In our study, the time dependence of the zeta potential over the course of measurement was not observed, which indicated the nanomaterial colloid was stable. The large negative zeta potential (Fig. 1a) would promote repulsion between the nanoparticles, which in turn would minimize particle agglomeration.27 The generated titania was measured by X-ray powder diffraction and well indexed with standard TiO2. The crystalline phase corresponded to anatase (PDF 01-086-1157, lattice constant 3.7852 × 9.5139 Å, 90°) shown in Fig. 1b.
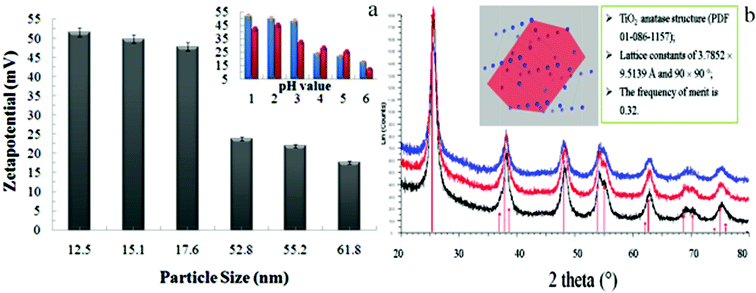 | ||
| Fig. 1 Relationship between the particle size (a) or pH (a, insert) and the zeta potential. Reproduced by permission of The Royal Society of Chemistry),28 and (b) X-ray powder diffraction data of the titania nanoparticles. | ||
Our data supports the expected trends, namely that a smaller diameter yields a greater zeta potential and decreasing the acidity also leads to a smaller zeta potential. The two bars, red and blue, represent nanoparticles reduced with citrate or ascorbic acid respectively, and indicate invariance towards the type of reducing agents used. Therefore, negative zeta potential values are indicative of colloidal stability against flocculation (reversible aggregation) or coagulation (irreversible aggregation). The colloids can be further stabilised through the incorporation of sugars in polar media or the incorporation of amphiphilic lipids, proteins or detergents in oil–water suspensions through segregation of the oil–water interface, resulting in reduced interfacial tension and a stabilized emulsion.
An alternative procedure for de-aggregation takes advantage of steric stabilization through the incorporation of long-chain (amphiphilic) molecules that are attached to the particle through chemisorption (e.g., a long-chain fatty acid) or adsorption (polymers). These molecules can stabilize in a non-aqueous environment over wider particle concentration due to the mechanical nature of the stabilization. Lastly, non-covalent interactions play an important role in engineered nanomaterials in that they can allow spontaneous self-assembly. Since these forces are weaker than covalent forces and include van der Waals forces, hydrophobic, hydrophilic, ionic, and dipole interactions—including coordination bonds in ligands and complexes, and hydrogen bonding. These forces, such as hydrophobic interactions, lend to a reduction in entropy since the enthalpy for water/solute un-mixing is greater than the entropy increase due to localized ordering, thereby yielding a negative Gibbs free energy change for micellization.
In this study, nanomaterials were prepared by the bottom-up wet-chemistry method to control and architectualize the particle size and size distribution. The Ag-decorated TiO2 nanoparticles were achieved through a novel facile colloidal chemistry method previously described.28 Briefly, sixteen formulations were attempted (four reducing agents (ascorbic acid (C6H8O6), sodium citrate (C6H5ONa3), sodium borohydride (NaBH4) and dimethylamine borane (DMAB, C2H10NB) were used) and four reducing-agent-to-metal ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 reducing agent
4 reducing agent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) silver) were tried to optimize the final composition, keeping the molar ratio of Ag
silver) were tried to optimize the final composition, keeping the molar ratio of Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti fixed at 1
Ti fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. In addition, a hot (60 °C) solution of 3% Arabic gum (AG) was added and mixed for 30 min. The Ag-containing solution was cooled to ambient temperature. To the reduced Ag solution, the Ti-dispersion was added incrementally and vigorously agitated. Finally, the binary suspension was vacuum filtrated and dried at 120 °C for 4 h, giving rise to highly polycrystalline particles.
20. In addition, a hot (60 °C) solution of 3% Arabic gum (AG) was added and mixed for 30 min. The Ag-containing solution was cooled to ambient temperature. To the reduced Ag solution, the Ti-dispersion was added incrementally and vigorously agitated. Finally, the binary suspension was vacuum filtrated and dried at 120 °C for 4 h, giving rise to highly polycrystalline particles.
The morphological analyses of bacteria (the controls and damaged ones upon addition of the Ag and Ag–TiO2 nano-disinfectants) were conducted using a transmission electron microscopy (TEM) (FEI Company, Tecnai F20-G2 Hillsboro, OR), equipped with Gatan Image Filter for energy electron loss spectroscopy (EELS). The HRTEM images were taken at a direct magnification of 6 × 105 times with the point resolution of 0.27 nm. The TEM and STEM images (shown in Fig. 2) indicated that the nanoparticles have attached to the membrane of bacteria. The bacteria controls for both E. coli and S. aureus (inset image) were found to be intact under the high vacuum. Due to the selection of low electron dose illumination, bacteria cells were not subject to severe damage by electron radiation, although they have strong tendency towards ionization damage.
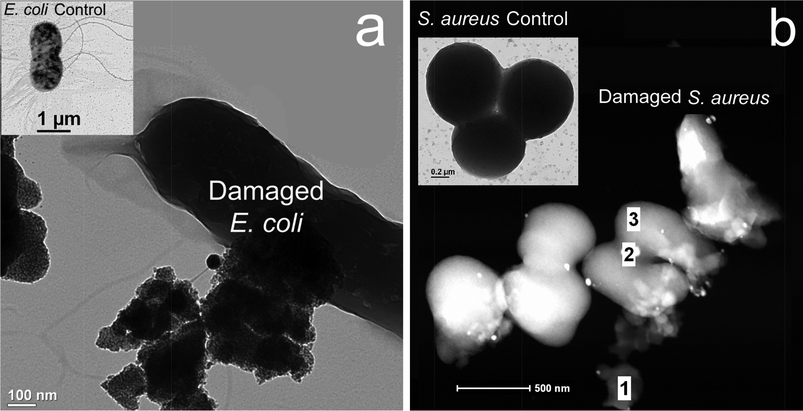 | ||
| Fig. 2 TEM (a) and STEM (b) images of the damaged bacteria interacted with the nanoparticles which are situated on the bacteria surface and remained chemically reactive, resulting in the weakened membrane, a: the E. coli imaging indicated several large agglomerates attached the membrane normal; and b: the S. aureus imaging, indicated a few small nanoparticles (white dots) attached on the membrane surface, with varying degrees of damage (main panel, regions 1, 2 and 3). The TEM images demonstrated that the E. coli (left insert) and S. aureus (right insert) control cells were kept intact. It should be noted that bare TiO2 alone without ultraviolet (UV) irradiation proved ineffective28 compared to Ag–TiO2 under visible light conditions. | ||
The EELS data were acquired in the energy-filtered TEM (EFTEM) mode. The quantitative data provides information about the elemental distribution within a cellular compartment. To avoid massive ionization damage of the bacteria structure under electron radiation, low dose illumination was applied (as mentioned above), while the high-angular dark-field (HAADF) was used for EDS compositional measurement from local areas, with a beam diameter about 1 nm.28 The EELS spectra for both E. coli and S. aureus were acquired before and after incubation with NPs to demonstrate distribution of various heteroatoms within the damaged bacteria. The spectra from the core edges provide the composition from which the alteration of various elements within the bacteria structure can be determined according to the concentration of antimicrobial agents. Metallic atoms occurring in the bacteria system can be also detected by sharp lines from their EELS core edges. The core edge29 and its significance of representative elements are listed in Table 1. EELS mapping of the first/second row of select main block elements enabled cell viability evaluation through the mapping of diagnostic ions. Diagnostic ions such as Cl, K, S and N, Mg, P and O were detected throughout damaged E. coli and S. aureus (Fig. 3), suggesting membrane depolarization and protein/nucleic denaturing. Another explanation to account for C and O is that these atoms are derived from the reducing or dispersing agents. Two observations indicate that the measured C/O are not from any “transfer” of the above agents from nanoparticles to microbes:
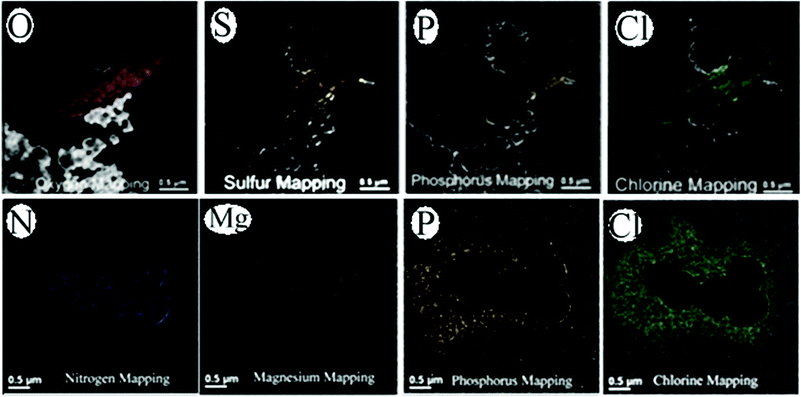 | ||
| Fig. 3 EELS mapping demonstrates changes in the elemental composition after membrane weakening, followed by elemental leakage for E. coli (top) and S. aureus (bottom). These maps also yield information on the role of Ag–TiO2 as an antimicrobial agent. Note: see Table 1 for a rationale on why selected elements (O, S, P, Cl, N and Mg) were used for the demonstration. A color scheme for the atoms and amino acids was developed by Corey and Pauling and was later improved by Koltun and widely used by molecular rendering tools such as Rasmol. A color scheme is applied to the EELS maps based upon the color table at http://www.stanford.edu/group/pandegroup/folding/education/cpk.html. | ||
| Elements | Core edge (eV) | Rationale for elemental selection |
|---|---|---|
| P | L3, 135 | Reveals phosphate groups in nucleic acids, phosphorylated proteins or wall phospholipids/lipoproteins. |
| S | L3, 164 | Indicates the proteins that are rich in cysteine or methionine or a sulphate group attached to carbohydrates. |
| C | K, 284 | These are the major constituents in organic molecules (e.g. glucose) or macromolecules (e.g. oligosaccharides, lipids, proteins or nucleic acids). Note: C and O could also arise from the reducing or dispersing agents. |
| N | K, 399 | |
| O | K, 532 | |
| Ca and Mg | L3, 347 (Ca) | Ca is used as a “second messenger” because chemical or electrical signals from the environment outside the cell are transformed into a Ca signal inside the cell cytoplasm, with Mg as a cofactor for ATP and energy transduction. |
| K, 1305 (Mg) | ||
| K and Cl | L3, 294 (K) | Cl and K are used for cellular homeostasis, while K is also used for membrane polarization and K+ transport. |
| L3, 200 (Cl) | ||
| Fe | L3, 710 | Is a nutrient and is also used as an electron acceptor under anaerobic conditions. |
(1) No incorporation of nanoparticles was observed inside the microbes, although the nanoparticles were adjacent to them. This observation indicates that no transfer of C/O from reducing/dispersing agent to microbes occurred.
(2) The XRD and EELS analyses are not stoichiometric, also suggesting no C or O elements were transferred. Otherwise, if some or all of the carbon (or oxygen) were transferred, a semi-stoichiometric relationship would be expected; and lastly, C and O are not used as diagnostic ions to gauge cell health or to propose a mechanism of inactivation. Viable prokaryotic and eukaryotic cells require ion homeostasis, by storing ions against a concentration gradient. Ions such as Na+, K+ and Ca2+ are used for cellular communication, maintenance of membrane polarization and as cofactors in metabolisms. Disruption of either the membrane structure through the creation of pores, mechanical rupture, or depolarization of the potential results in cellular shutdown due to imbalance. A physical manifestation of this event is leakage of these ions, which was mapped using EELS to provide direct evidence of the interaction of NPs with the bacteria. Therefore the selected ions are ‘molecular thermometers’ through which the health status of the cell can be gauged. When cellular leakage is observed, as in our case, the inference is that the cell is inviable.
The potency of the nanomaterials was evaluated using two model microbes (Escherichia coli, E. coli and Staphylococcus aureus, S. aureus), using previously described protocols.24 Briefly, a number of dilutions of Ag–TiO2 were prepared and co-incubated with E. coli and S. aureus stock containing approximately 1 × 108 colony-forming units (cfu)-per-millilitre (mL). After co-incubation with the Ag–TiO2 at different incubation times, bacterial cultures were then diluted to approximately 100–105 cfu mL−1 and diluted aliquots (100 μL) were spread onto duplicate agar medium plates and incubated at 37 °C for overnight. The viable cells in each category were counted as discrete colony-forming units (CFUs). The viable CFUs were imaged and counted using phase contrast inverted optical microscopy (Olympus CK40 with NAO30 optical assembly Irving, TX and Pixera Penguin CLM digital camera, San Jose, CA). The zeta potential analysis indicates that the particles were uniformly dispersed and stable in an aqueous environment (vide supra). Briefly, the nanocomposite was effective at inactivating 100% of the microbes within 2–8 hours of co-incubation of the microbe and nanocomposite at less than 2.5–10 parts-per-million (ppm) nanocomposite with lower doses observed to be effective at higher co-incubation times (e.g. ≥6 hours, noting that 2.5 ppm was required for 100% microbial inactivation at a co-incubation period of 6 hours for Ag–TiO2 nanocomposite) and higher doses at lower co-incubation times (e.g. ≤2 hours, noting that 10 ppm was required for 100% microbial inactivation at a co-incubation period of 2 hours for Ag–TiO2 nanocomposite). Importantly, our previous research indicated that bare TiO2 alone without ultraviolet (UV) irradiation proved to be ineffective.28
To better understand the bactericidal mechanism, the role of the outer membrane ought to be considered for the interaction between Ag–TiO2 and the microbe. It is an important component in protecting the microbe from a hostile environment. The membrane has a cytoplasmic facing side (inner face) and an extracellular matrix (outer face) side. The former contains lipopolysaccharide bilayer components while the latter contains phospholipids and oligoglycan residues (RPO43−, RCOO−, where ‘R’ represents the lipid or glycan component) which are negatively charged, giving rise to a cell wall which has a negative charge.30 The cell wall functions as a permeability barrier using outer membrane proteins.31 Thus the cell wall allows movement of nutrients and ions by facilitated diffusion, which is an energy demanding process.32 This energy can be supplied by a number of small co-factors: adenosine triphosphates or reducing molecules (reduced from of nicotinamide adenine dinucleotide). The ‘chemical energy carriers’ are recycled through a number of chemical processes, including redox processes via respiratory proteins.33 The facilitative processes usually lead to internal ion accumulation in the cytoplasm and the concomitant release of chlorine or sodium as counter ions to balance the charge.34 It follows that disruption of the associated membrane potential or permeability barrier would allow stored ions to be released, which has been observed in our study for potassium and calcium ions.35 This disruption would take a finite amount of time in microbes with a fully developed cell wall (mature cells) or less time for immature cells which do not have a fully developed cell wall. In our SEM/TEM micrographs we observed cells in different degrees of inactivation, via microbe–nanoparticle interactions, as shown in the TEMs of Fig. 4.
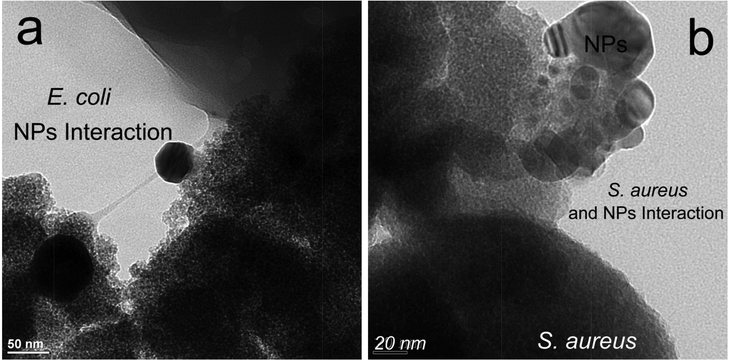 | ||
| Fig. 4 (a) HR TEM of region of interaction between Escherichia coli and 5% Ag/TiO2, showing cytoplasmic membrane peeling (light to dark gray zone); (b) Similar view for Staphylococcus aureus, also showing peeling (light grey regions). It should be noted that bare TiO2 alone without UV irradiation proved to be ineffective28 when compared with Ag–TiO2 under visible light conditions. | ||
In addition, the peptidoglycans in the cytoplasm membrane are composed of phosphatidylglycerol, dodeca to tetradeca saturated lipids, phosphatidylglycerol/ethanolamine phosphatidylglycerol (saturated and monounsaturated), phosphatidylethanolamine (saturated) type phospholipids and phosphorylated glucosamine disaccharide.36 The type of heteroatom in the macromolecules, such as nitrogen (N), sulphur (S), phosphorus (P), and oxygen (O) were observed in the EELS spectra, indicating that the interaction between the Ag–TiO2 and E. coli resulted in inactivation via disruption of the membrane functions such as ion transport and maintenance of the membrane potential, and not by cellular toxicity through cellular intake, since no nanocomposite was observed inside the cell.28 From our findings, a number of general assumptions can be made regarding the mechanisms of inactivation of microbes by Ag–TiO2.
(i) Lipid peroxidation through direct and indirect interaction (via reactive oxygen species (ROS, vide infra), see Fig. 5) appears to be one mode of membrane damage, since a small degree of cytoplasmic to cell wall peeling was observed. This could be accomplished through charge generation by the TiO2 core37 (TiO2 + hν → h+ + e−); recombination (h+ + e− → TiO2 + Δ), and radical generation at the surface38 (H2O + h+ → HO˙ + H+ or OH− + h+ → HO˙) and [TiO2 + e−] + Ag → TiO2 + [Ag + e−].
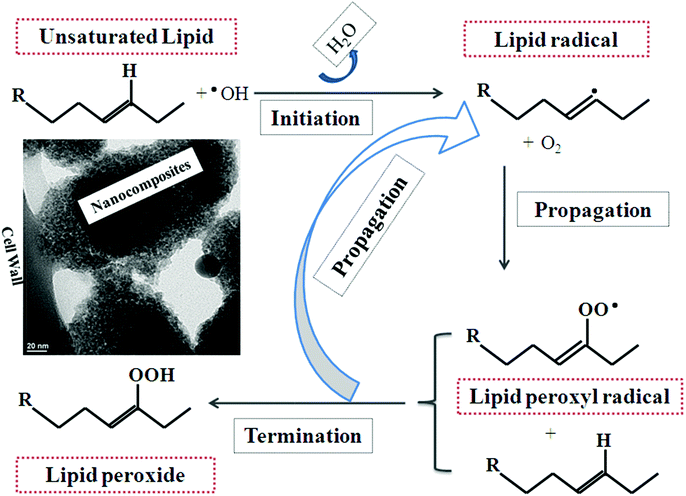 | ||
| Fig. 5 Possible mechanism of lipid peroxidation of the microbe cell wall, leading to cell wall weakening, lysis and inactivation by metal oxide nanoparticle disinfectants. | ||
Once a UV photon or photon of sufficient energy to promote electron transfer is absorbed by the TiO2 in the Ag–TiO2, an electron–hole pair is generated.39 This pair may migrate to the surface and photocatalyse oxidation, such as in membrane lipids.40 The electron–hole pair may also undergo recombination, generating heat in the absence of electron/hole acceptors,41 such as oxygen in biological systems or C60 in model systems.42 Finally, the holes can react with the solvent or surface hydroxyls to form radical species.43 With oxygen as the electron acceptor, superoxide ion generation may occur, including the generation of hydroxyl or peroxyl radical species44 outlined below:
| (O2 + e− → O2˙−, 2O2˙− + 2H+ → 2 ˙OH and O2˙− + H+ → O2H˙). |
The inclusion of silver in the composite is expected to lower the band gap and allow photocatalysis under visible-light conditions45 and possibly increase the catalytic rate of oxidation through by lowering the amount of trapping sites.46 This phenomenon has been observed for other systems such as titania nickel ferrite composites.47 Lastly, since silver is a metal, electron injection from excited TiO2 to facilitate ROS type reactions is also another possible process.48
(ii) Disruption of DNA replication and repair. The TEM micrographs indicated two contrasted regions (light gray and dark gray), which we attribute to DNA conformational changes resulting ultimately in fragmentation. It is known that DNA repair under oxidative stress can result in thymine dimerization which can inhibit DNA replication and repair.49 EELS derived micrographs did yield main block atoms involved in DNA, such as N, S, O, although these atoms are also involved in proteins.29 The likely origin of the phosphorus is from DNA, since the nucleotide contains at least 7–8 P each. In addition, proteins do not contain phosphorus except as phosphorylated derivatives of serine, threonine and, to a lesser degree, tyrosine; histidine is also present if aspartate phosphorylation occurs in prokaryote signalling. Examination of the intensity of the EELS mapping suggests that the origin of the phosphorus is from nucleic acids rather than proteins, whereas sulphur is usually from proteins, since amino acids such as cysteine and methionine contain sulphur as part of their side chains.
(iii) Inhibition of respiratory proteins. We did not observe this effect directly, although it has been postulated that electron transfer complexes or proteins such as coenzyme A (CoA) can transfer an electron to the hole in the valance band of the Ag–TiO2, thereby promoting dimerization of the enzyme and leading to the inhibition of respiration.50 The manner of bacterial inactivation51 through Ag–TiO2 interaction is shown in Fig. 4, strongly indicative of OH˙ assisted ROS damage.52
Conclusions
Silver insertion into titania offers a number of tangible advantages compared with transition metal oxides when applied as nanobiocidal agents. These advantages, relative to titania, are effectiveness under visible light conditions, lower minimum bactericidal concentrations (MBC), lower incorporation of silver (relative to silver nanoparticles) and a broad range of activity. Three concentration-dependant trends are observed: at very high nanoparticle (>50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm) concentrations, cellular lysis is observed—similar to that for bleach—suggesting that lipid oxidation is the major form of inactivation; at intermediate levels (MBC: 2.5 ppm to 20 ppm) inhibition is observed with minimal lysis, suggesting that other mechanisms such as DNA fragmentation, protein and respiration inhibition or disruption of the membrane potential are more prominent in inactivating the microbe than lysis; at ultralow doses (<2.5 ppm) a bacteriostatic-like phenomenon is observed, suggesting some membrane damage inhibiting growth, but not extensive enough to cause cellular inactivation. A plausible mechanism of inactivation is offered to explain the observed events. Lastly, other advantages common with transition metal oxides are retained, such as long-term persistence and 100% metal recycling. Collectively, this approach can be applied where traditional disinfectant approaches are ineffective such as with antibiotic resistant bacteria, high sunlight where bleach is rapidly oxidized and has many potential benefits to the community in terms of a microbe-free water supply.
000 ppm) concentrations, cellular lysis is observed—similar to that for bleach—suggesting that lipid oxidation is the major form of inactivation; at intermediate levels (MBC: 2.5 ppm to 20 ppm) inhibition is observed with minimal lysis, suggesting that other mechanisms such as DNA fragmentation, protein and respiration inhibition or disruption of the membrane potential are more prominent in inactivating the microbe than lysis; at ultralow doses (<2.5 ppm) a bacteriostatic-like phenomenon is observed, suggesting some membrane damage inhibiting growth, but not extensive enough to cause cellular inactivation. A plausible mechanism of inactivation is offered to explain the observed events. Lastly, other advantages common with transition metal oxides are retained, such as long-term persistence and 100% metal recycling. Collectively, this approach can be applied where traditional disinfectant approaches are ineffective such as with antibiotic resistant bacteria, high sunlight where bleach is rapidly oxidized and has many potential benefits to the community in terms of a microbe-free water supply.
Authors’ contributions
Synthesis and formulation of nanoparticles were carried out by Dr Liu based on Dr Medina-Ramirez's invention; the graphical images and editing of the second draft was also undertaken by Dr Liu. The entire electron microscope components were carried out by Dr Luo; EELS data analysis was completed by Dr Bashir, who wrote the first and edited the submitted manuscript. He also oversaw the submission process.Acknowledgements
Authors acknowledge the support from the TAMUK University Research Award (160366-00002) and the National Science Foundation, Major Research Instrumentation (CBET-0821370). Pilot work has been supported by Dr M. Gonzalez-Garcia for access to optical microscope and Dr Perez-Ballestero in instructing Mr Chamakura to conduct biological evaluation in the Department of Chemistry, TAMUK; ref. 24 including the R. Welch Foundation (Department Grant for Chemistry, AC 006).Notes and references
- R. A. Goyer and M. P. Waalkes, in Casarett and Doull's Toxicology: The Basic Science of Poisons, ed. C. D. Klassen, McGraw-Hill, New York, 7th edn., 2007, ch. 23, pp. 931–980 Search PubMed.
- R. H. Demling and L. DiSanti, Wounds, 2001, 13, A5 Search PubMed.
- R. A. Wigley and R. R. Brooks, in Noble Metals and Biological Systems Their Role in Medicine Mineral Exploration and the Environment, ed. R. R. Brooks, CRC Press, Boca Raton FL, 1st edn, 1992, ch. 9, pp. 277–279 Search PubMed.
- L. L. Dupuis, N. H. Shear and R. M. Zucker, J. Am. Acad. Dermatol., 1985, 12, 1112 CrossRef CAS.
- S. M. Fakhry, J. Alexander and D. Smith, J. Burn Care Rehabil., 1995, 16, 86 CrossRef CAS.
- S. Hoffmann, Scand. J. Plast. Reconstr. Surg. Hand Surg., 1984, 18, 119 CrossRef CAS.
- H. J. Klasen, Burns, 2000, 26, 131 CrossRef CAS.
- F. W. Fuller, M. Parrish and F. C. Nance, J. Burn Care Rehabil., 1994, 15, 213 CrossRef CAS.
- J. M. Hamilton-Miller, S. Shah and C. Smith, Chemotherapy, 1983, 39, 405 CrossRef.
- C. F. Cooper and W. C. Jolly, Water Resour. Res., 1970, 6, 98 CrossRef.
- H. G. Petering, Pharmucol. Ther., 1976, 1, 127 CAS.
- H. Kourai, Y. Manabe and Y. Yamada, J. Antibact Antifung Agents, 1994, 22, 595 CAS.
- R. M. Slawson, H. Lee and J. T. Trevors, Biol. Met., 1990, 3, 151 CrossRef CAS.
- R. B. Thurman and C. P. Gerba, Critical Reviews in Environmental Control, 1989, 18, 295 CrossRef.
- P. A. Goddard and T. A. Bull, Appl. Microbiol. Biotechnol., 1989, 31, 314 CAS.
- U. Klueh, V. Wagner, S. Kelly, A. Johnson and J. D. Bryers, J. Biomed. Mater. Res., 2000, 53, 621 CrossRef CAS.
- K. Yoshida, M. Tanagawa, S. Matsumoto, T. Yamada and M. Atsuta, Eur. J. Oral Sci., 1999, 107, 290 CAS.
- N. Vlachopoulos, P. Liska, J. Augustynski and M. Graetzel, J. Am. Chem. Soc., 1988, 110, 1216 CrossRef CAS.
- H. Wei, D. Ratchford, X. Q. Li, H. X. Xu and C. K. Shih, Nano Lett., 2009, 9, 4168 CrossRef CAS.
- S. Link and M. A. El-Sayed, J. Phys. Chem. B, 1999, 103, 4212 CrossRef CAS.
- J. Homola, S. S. Yee and G. Gauglitz, Sens. Actuators, B, 1999, 54, 3 CrossRef.
- S. D. Standridge, G. C. Schatz and J. T. Hupp, J. Am. Chem. Soc., 2009, 131, 8407 CrossRef CAS.
- A. Henglein, Chem. Rev., 1989, 89, 186 CrossRef.
- K. Chamakura, R. Perez-Ballestero, Z. Luo, S. Bashir and J. Liu, Colloids Surf., B, 2011, 84, 88 CrossRef CAS.
- J. Dahl, B. Maddux and J. Hutchison, Chem. Rev., 2007, 107, 2228 CrossRef CAS.
- A. Revil and P. A. Pezard, J. Geophys. Res., 1999, 104, 20021 CrossRef CAS.
- I. Medina-Ramirez, S. Bashir, Z. Luo and J. Liu, Colloids Surf., B, 2009, 73, 185 CrossRef CAS.
- I. Medina-Ramirez, Z. Luo, S. Bashir, R. Mernaugh and J. Liu, Dalton Trans., 2011, 40, 1047 CAS.
- D. Shindo and T. Oikawa, in Analytical Electron Microscopy for Materials Science, ed. D. Shindo and T. Oikawa, Springer-Verlag, Tokyo, 1st edn, 2002, ch. 3 & 6, pp. 43–81 & 139–141 Search PubMed.
- M. J. Osborn, Annu. Rev. Biochem., 1969, 30, 501 CrossRef.
- S. Lugtenberg and L. van Alphen, Biochim. Biophys. Acta, Rev. Biomembr., 1983, 737, 51 CrossRef.
- D. J. Scheffers and M. G. Pinho, Microbiol. Mol. Biol. Rev., 2005, 69, 585 CrossRef CAS.
- D. G. Thanassi, C. Stathopoulos, A. Karkal and H. Li, Mol. Membr. Biol., 2005, 22, 63 CrossRef CAS.
- S. P. Denyer and G. S. A. B. Stewart, Int. Biodeterior. Biodegrad., 1998, 41, 261 CrossRef CAS.
- J. Y. Maillard, J. Appl. Microbiol., 2002, 92, 16S CrossRef.
- K. H. Schleifer and O. Kandlker, Bacteriol. Rev., 1972, 36, 407 CAS.
- J. Liu, I. Medina-Ramirez, S. Bashir and R. Mernaugh, Green Chemistry Derived Nanocomposite of Silver-modified Titania used for Disinfectant, Nanotech clean-tech and techconnect world, Anaheim, CA, June 24, 2010 CrossRef CAS; D. Bahnemann, A. Henglein, J. Lilie and L. Spanhel, J. Phys. Chem., 1984, 88, 709 CrossRef CAS.
- J. S. Tsuji, A. D. Maynard, P. C. Howard, J. T. James, C. W. Lam, D. B. Warheit and A. B. Santamaria, Toxicol. Sci., 2006, 89, 42 CrossRef CAS.
- S. M. Oh, S. S. Kim, J. E. Lee, T. Ishigaki and D. W. Park, Thin Solid Films, 2003, 435, 252 CrossRef CAS.
- P. C. Maness, S. Smolinski, D. M. Blake, Z. H. Wolfrum and W. A. Jacoby, Appl. Environ. Microbiol., 1999, 65, 4094 CAS.
- W. Choi, A. Termin and M. R. Hoffmann, J. Phys. Chem., 1994, 98, 13669 CrossRef.
- P. V. Kamat, M. Gevaert and K. Vinodgopal, J. Phys. Chem. B, 1997, 101, 4422 CrossRef CAS.
- K. Sunada, T. Watanabe and K. Hashimoto, J. Photochem. Photobiol., A, 2003, 156, 227 CrossRef CAS.
- G. R. Bamwenda and H. Arakawa, Appl. Catal., A, 2001, 210, 181 CrossRef CAS.
- K. Iketani, K. Hirota, O. Yamaguchi, R. D. Sun and M. Toki, Mater. Sci. Eng., B, 2004, 108, 187 CrossRef.
- M. Zhou, J. Yu, B. Cheng and H. Yu, Mater. Chem. Phys., 2005, 93, 159 CrossRef CAS.
- M. Cho, H. Chung, W. Choi and J. Yoon, Water Res., 2004, 38, 1069 CrossRef CAS.
- S. Rana, J. Rawat, M. M. Sorensson and R. D. K. Misra, Acta Biomater., 2006, 2, 421 CrossRef CAS.
- G. D. Harris, V. A. Dean, L. S. Darwin and M. S. Curtis, Water Res., 1987, 21, 687 CrossRef CAS.
- T. Matsunaga, R. Tomoda, T. Nakajima and T. Komine, Appl. Environ. Microbiol., 1988, 54, 1330 CAS.
- M. R. Elahifard, S. Rahimnejad, S. Haghighi and M. R. Gholami, J. Am. Chem. Soc., 2007, 191, 9552 CrossRef.
- O. K. Dalrymple, E. Stefanakos, M. A. Trotz and D. Y. Goswami, Appl. Catal., B, 2010, 98, 27 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
