A tissue engineering approach based on the use of bioceramics for bone repair
Antonio J.
Salinas
ab,
Pedro
Esbrit
cd and
María
Vallet-Regí
*ab
aDepartamento de Química Inorgánica y Bioinorgánica, Facultad de Farmacia, Universidad Complutense de Madrid, Madrid, Spain. E-mail: vallet@farm.ucm.es; Tel: +34 91 394 1861; Fax: +34 91 394 1786
bNetworking Research Center on Bioengineering, Biomaterials and Nanomedicine (CIBER-BBN), Madrid, Spain
cLaboratorio de Metabolismo Mineral y Óseo, Instituto de Investigación Sanitaria (IIS)-Fundación Jiménez Díaz, Madrid, Spain
dRed Temática de Investigación Cooperativa en Envejecimiento y Fragilidad (RETICEF), Instituto de Salud Carlos III, Madrid, Spain
First published on 1st October 2012
Abstract
Biomimetics takes advantage of natural strategies for the solution of technological problems, including the proper design of biomaterials. Living bone exhibits a hierarchical porosity with both giant and nanometric pores which must be reproduced for the design of biomaterials for hard tissue repair. Bioactive and degradable bioceramics are a good alternative for the manufacture of scaffolds. Tissue engineering approaches to improve bone regeneration include strategies supporting endogenous osteoblast adhesion, proliferation (osteoconduction), osteoinduction by growth factors, and osteoprogenitors. Understanding the natural ossification mechanisms and the role of biomolecules involved in this process is a requirement for the design of bone tissue scaffolds. Mesoporous bioactive ceramics, namely mesoporous silica and templated glasses with nanometric pores to host growth factors, conformed into 3D scaffolds with micrometric porosity by rapid prototyping, are a good option for bone regeneration. In this regard, biomolecules such as well characterized bone morphogenetic proteins and others under current research, such as osteostatin and osteoprogenitors, are promising strategies in bone tissue engineering applications. Future developments in biomaterials will come in both micro- and nano- scales, and molecular and cell biology approaches will provide suitable solutions to the demanding needs of these compounds.
 Antonio J. Salinas | Antonio Salinas graduated in Chemistry at the Universidad Complutense de Madrid (UCM) and PhD at the same university in 1992. He is Associate Professor of Inorganic Chemistry and member of the Biomedical Research Networking Center on Bioengineering, Biomaterials and Nanomedicine, CIBER-BBN. He was visiting Professor at the Universities of Florida, Aveiro and Paris. Dr. Salinas has published more than 100 articles and books chapters most of them in the field of bioceramics and participated in 22 research projects. He has more than 1,500 world-wide citations (ISI Web of Knowledge). Dr. Salinas is Corresponding Academician of the Spanish Royal National Academy of Pharmacy. |
 Pedro Esbrit | Pedro Esbrit graduated in Chemistry at Universidad Complutense, and received his Ph.D. in Biochemistry from Universidad Autónoma, in Madrid, Spain. He was a post-doc Fulbright fellow at Barnes and Jewish Hospitals (St. Louis, MO), and visiting researcher at Veterans Administration Hospital (West Haven, CT). He currently holds an Associate Chief position as Head of Bone and Mineral Metabolism Laboratory at Instituto de Investigación Sanitaria-Fundación Jiménez Díaz, Madrid. Dr. Esbrit has published >100 papers and several books in the field of bone and mineral metabolism, particularly focusing on the actions of PTHrP, in which he is one of the 10-top experts according to BiomedExperts. |
 María Vallet-Regí | María Vallet-Regí was born in Las Palmas, Spain. She studied Chemistry at the Universidad Complutense de Madrid (Spain) and received her PhD at the same university in 1974. She is full professor of Inorganic Chemistry and head of the research group Smart Biomaterials in the Department of Inorganic and Bioinorganic Chemistry of the Faculty of Pharmacy at Universidad Complutense de Madrid. Prof. Vallet-Regí has written more than 600 articles and several books. She was the most cited Spanish scientist according to ISI Web of Knowledge, in the field of Materials Science in these past decades. She is Fellow of Biomaterials Science and Engineering at the International College of Fellows of Biomaterials Science and Engineering (ICF-BSE), Numbered Fellow of the Spanish Royal Academy of Engineering and the Royal National Academy of Pharmacy. She has received the Prix Franco-Espagnol 2000 from Societé Française de Chimie, the Spanish Royal Society of Chemistry (RSEQ) award in Inorganic Chemistry 2008, the Spanish National Research Award in Engineering 2008, FEIQUE Research award 2011 and the RSEQ Research Award and Gold Medal 2011. |
1. State of the art: the process of bone regeneration and repair. How nature works
Bone regeneration after a fracture and the healing of small bone defects are very effective processes. However, a challenge arises with an increasing number of fractures associated with poor healing through various causes such as osteoporosis, fractures involving bone sites of poor vascularity or large bone tissue loss.1 In this regard, the number of fractures related to osteoporosis has doubled in the last decade, in part related to the significant increase in life span in Western societies.2 Osteoporosis occurs frequently in women after menopause due to the loss of estrogen, but bone loss is also known to occur for both men and women with aging. In fact, the increasing number of “non-union” fractures in the aging population is a significant clinical problem. A number of other situations related to osteopenia/osteoporosis, namely diabetes mellitus and glucocorticoid treatment, are known to affect optimal skeletal healing after trauma or osteotomy.3–8 This represents a major challenge to our health systems, and determines that bone regeneration following maxillo-facial and orthopaedic surgery is an important clinical issue in regenerative medicine. Therefore, therapeutic strategies to improve bone healing in these circumstances are of utmost importance. Therapies derived from a better knowledge of the cellular and molecular events in bone healing together with state-of-the-art biomaterials are being developed as bone tissue engineering strategies to improve bone repair.1.1 Bone: a model to imitate
Nature is an important source of inspiration in the development of new implant materials. In this sense, the design of these biomaterials relies on imitating the structure and function of biological systems (see Fig. 1). The biomimetic approach is based on the fact that after billions of years of evolution, nature has learned which systems are the most appropriate in terms of optimal properties with minimal resources.9,10 Thus, biomimetics take advantage of these natural strategies for the solution of specific problems in all branches of materials science and engineering. | ||
| Fig. 1 Nature is an ideal inspiration to solve technological problems associated with implant materials. | ||
Bone is formed by a series of biomineralization processes, a sequence of physicochemical reactions that give rise to organic–inorganic nanocomposites with exceptional mechanical properties that would be impossible to reach with pure materials.10 This tissue is made up of a cellular component and an extracellular matrix consisting of an organic phase, mainly type 1 collagen, and an inorganic ceramic phase of calcium-deficient carbonated hydroxyapatite (CHA) nanocrystals.11 As observed in Fig. 2, the main characteristics of biological apatites are: variable composition, nanometric crystal size, calcium deficiency, the presence of carbonate groups and structural disorder. Another important feature of the apatite structure is the ability to accommodate different ions in its three ionic sublattices (calcium, phosphate and hydroxy).11 These apatite nanocrystals grow at ordered mineralization sites of collagen molecules in bone.
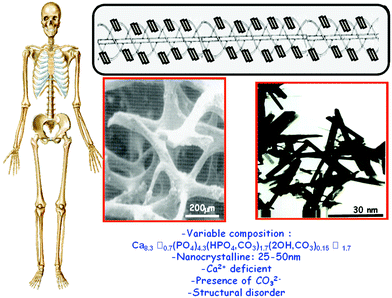 | ||
| Fig. 2 Top: Schematic image of CHA nanocrystals reinforcing the triple helix of collagen. Middle: Cancellous bone observed by scanning electron microscopy and CHA nanocrystals by transmission electron microscopy. Bottom: Main features of biological apatites. | ||
More than 200 bone pieces with different lengths and shapes constitute the human skeleton.12 However, all of them present a hierarchical structure that ranges from the collagen-CHA nanocomposite, the lamellae, lacunae and osteons to the macroscopic material. Another important characteristic of bone is its porosity. In this sense, bone tissue with less than 20% porosity is known as cortical (or compact) bone, whose most important function is to provide mechanical support (strength and stiffness). On the other hand, cancellous (trabecular or spongy) bone can contain up to 90% of porosity. This type of bone provides a large surface area which determines the key role of the skeleton in ion homeostasis and haematopoiesis. Furthermore, pores with different sizes, from one to several hundred micrometers are present in bone; those below 1 μm in diameter are responsible for bioactivities involving protein interactions. This hierarchical porosity must be reproduced in the design of new biomaterials for regeneration and repair of hard tissues.13
There is general agreement on both scaffolds required for osteoconduction and osteogenic growth factors as key elements for applications in bone repair and regeneration.14 The increasing importance of the latter factors, mainly bone morphogenic proteins and other growth factors, is illustrated in Fig. 3 showing the participation of different products in the US Orthopedic Market in 2009.15 These agents represent 20% of this global market, with an expected growth of 30–35% each year in the next five years. This growth is 3-fold higher than that expected for the US global market for orthopedic biomaterials.
 | ||
| Fig. 3 Economic impact of different products in US Orthopedic Biomaterials market in 2009 (Adapted from ref. 15). “Others” category includes the big markets for sealants, hemostats and anti-adhesion agents. | ||
1.2 Cell and molecular mechanisms underlying the process of bone healing
Bone repair—a key process for resolution of orthopedic trauma which causes bone disjunction—is a unique process involving the interplay of complex cellular and molecular events to generate new bone instead of a fibrous scar which is the final outcome in other connective tissues. In contrast to bone remodeling in adults, which requires a relatively long period to complete at random sites (called “bone remodeling units”) throughout the skeleton, bone repair occurs in a compressed time frame and in a precise location. The latter basically recapitulates normal bone formation during embryogenesis, except for some events such as inflammation, the limited number of osteoprogenitors available and the key influence of mechanical stimuli that greatly influence bone healing in adults.8 At the bone injury site, mesenchymal cells interact with inflammatory cells in an orderly fashion by yet poorly understood mechanisms.16 Recent evidence also suggests that osteoclasts as well as osteoprogenitors might have an active role in the early response to injury, as occurs in the bone remodeling units in the bone surface.17,18New bone formation is initiated by the condensation of mesenchymal stem cells (MSCs), which then leads to either the formation of a cartilage template in the bone marrow (endochondral ossification) or direct differentiation into osteoblasts at the periostium (intramembranous or appositional ossification). The former ossification mechanism predominates in most cases of fracture healing, but rigid fixation of the injury causes primary bone apposition as the major repair mechanism.8,19 The general pattern of endochondral ossification after fracture includes several chronological phases (Fig. 4).
 | ||
| Fig. 4 Phases of endochondral ossification in fracture healing. The well microvascularized periostium is shown, consisting of an outer fibrous layer and the inner cambium which is rich in osteoprogenitors. Immediately after injury, hematoma formation and inflammation take place. Osteoprogenitors are then recruited from the periosteum to differentiate into chondrocytes or osteoblastic cells (depending on the oxygen supply). New bone is thus starting to be formed at the borders of the injury site. Simultaneously, a callus mostly made up of hypertrophic cartilage develops and begins to revascularize. Eventually, osteoclast-mediated remodeling of the newly formed bone leads to the restoration of structural bone integrity (modified from the Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, 7th Edition. http://www.asbmrprimer.org). | ||
Immediately following trauma, vascular endothelium integrity is lost, giving rise to disruption of the blood supply and hematoma formation. This is accompanied by an inflammatory response, related to the production of pro-inflammatory cytokines—namely tumour necrosis factor-α, interleukin-1 (IL-1), and IL-6—from aggregated platelets, which have chemotactic activity towards lymphocytes, monocytes-macrophages and fibroblasts (inflammatory cells), and also endothelial cells.20 Macrophages can also produce and secrete the aforementioned cytokines and various growth factors, platelet-derived growth factor, fibroblast growth factors, insulin-like growth factors, the transforming growth factor-β superfamily, including bone morphogenetic proteins (BMPs) and vascular endotelial growth factor (VEGF), which induce angiogenesis and bone formation from recruited periosteal progenitors, as well as extracellular matrix synthesis.21–23
Neovascularization of injured tissue is key to successful bone repair, providing oxygen, facilitating metabolic waste removal, and delivering progenitor cells of haematopoietic origin.8,24 Whereas mechanical stability preserves vascular integrity and promotes osteogenesis, instability disrupts microvessels producing hypoxic conditions that favor chondrogenesis, as occurs in the central avascular region of the callus.25 This determines that stabilized fractures have less cartilage and a reduced callus volume. VEGF, which is expressed under the control of hypoxia-inducible factor (HIF)-1α under low oxygen tension, is a prototype osteogenic–angiogenic factor that can expand the osteoprogenitor pool by interaction with the endothelium.26 Osteogenic factors such as BMPs and parathyroid hormone (PTH)-related protein (PTHrP) stimulate the expression of VEGF by osteoblastic cells.27,28 The critical role of this factor in bone repair has been well exemplified in mice with conditional overexpression of HIF1α in mature osteoblasts (using the osteocalcin promoter) undergoing distraction osteogenesis to stimulate bone regeneration, showing a robust angiogenic response closely related to new bone formation.1 The key final step to correct the bone defect consists of newly formed woven (or disorganized) bone replacement by lamellar bone through the activity of osteoclasts and osteoblasts (bone remodeling).
Current evidence also points to the role of osteoclastogenesis and osteoclast-mediated bone resorption during both early and late stages of bone repair. Osteoprogenitors may activate osteoclast-mediated resorption of damaged bone, while osteoclasts themselves can modulate osteoblast activity and thus contribute to bone formation.19,29 This evidence can explain the reported deleterious effects of bone resorption inhibitors, namely bisphosphonates, on callus formation and remodeling.18,30
The proliferation and differentiation of osteoblasts from pluripotent MSCs is a complex process under systemic and local control. Many of the regulatory factors underlying this control constitute a matter of investigation as putative targets or therapies to stimulate bone repair.8,31 Of interest, these factors appear to act through common intracellular signaling pathways—namely mitogen-activated protein kinase (MAPK) and Wnt/β-catenin pathways8—to induce proliferation of osteoprogenitors and activation of Runx2, a key transcription factor for osteoblast differentiation.32 The fact that MAPK signaling acts as an important mediator of pro-inflammatory cytokines determines that its therapeutic manipulation might be restrained to the early phase in bone repair, which involves inflammation.33 The canonical Wnt/β-catenin pathway has an important role in bone formation, and recently, its impact on bone regeneration has also been examined in mice. Thus, systemic administration of an adenovirus expressing an inhibitor of this pathway into transgenic mice was found to arrest the program of tissue regeneration in the mouse tibia.34 On the other hand, it has been suggested that pre-treatment of bone marrow cells with Wnt/β-catenin pathway activators may increase their osteogenic capacity;35 a particularly relevant manoeuvre in conditions associated with a low number of osteoprogenitors (e.g., aging and diabetes mellitus).
A reduction in the number of osteoprogenitors as well as a decreased proliferative and differentiation capacity of pre-osteoblasts to osteoblasts, associated with an overall remodeling capacity decline, correlates with old age.36–38 Alterations in osteoblast function related to aging might be accounted for at least in part by increased oxidative stress and inflammation status.39,40 Several recent studies have explored the putative age-related alterations in the temporal pattern of gene expression during bone regeneration in rats and humans.18,41 In old rats, the machinery of bone healing based on the gene profile seems to be conserved with age despite the delay in new bone formation to bridge the fracture gap.41 In the callus after hip fracture in elder human subjects, the expression of inflammation-related genes is highest at the earlier phase, and it shifts towards bone remodeling genes later on after fracture.18 Interestingly, expression of Sost—the sclerostin-encoding gene, a key modulator of bone remodeling—was rapidly reduced in the callus, suggesting that this would allow osteoblasts to escape from its inhibitory effect to promote bone formation.18 These observations provide new insights for specific interventions targeting excessive inflammation and Sost to promote bone regeneration in elder subjects.42,43
2. Bioceramics to promote bone regeneration. From the raw materials to scaffolds
Synthetic materials used for bone repair can be divided into: ceramics, polymers, metals and composites where at least two classes of materials are combined together.44 Ceramics have a structure which mimics that of natural bone and thus were considered attractive materials in this respect.45,46 This category of materials includes oxides, phosphates, carbonates, nitrides, carbides, carbons and glasses. Those designed to interact with living tissues are called bioceramics,46,47 and they are the subject of this review.The so-called bioinert bioceramics elicit a foreign body reaction which gives rise to a non-adherent fibrous capsule isolated from surrounding bone, which accounts for the aseptic loss of many implants.48 For this reason, research efforts at the end of the past century were devoted to biodegradable and bioactive bioceramics, sometimes denoted as second generation bioceramics. Biodegradable bioceramics are designed to fulfil specific functions for a given time period, helping in the self-repair processes of the living organism, and are subsequently resorbed. The critical aspect in the design of these bioceramics is their relatively fast degradation rate, with the loss of their mechanical properties, compared to the slower new bone formation process. Bioactive bioceramics can react with physiological fluids, but only at surface level, giving rise to a sequence of surface reactions producing a strong bond between the material and bone.47 Several calcium phosphates such as tricalcium phosphates (alpha or beta polymorphs) or calcium sulphates are good examples of biodegradable bioceramics. Likewise, well known bioactive ceramics are hydroxyapatite (HA) and certain compositions of glasses and glass-ceramics. Bioactive and biodegradable bioceramics are clinically used as bone fillers, bone cements or for coating metallic implants.47Fig. 5 shows representative bioceramics of each category. Because the in vivo reactivity of these materials depends on its surface area, one single material such as HA can be classified in different categories depending on its particle size.
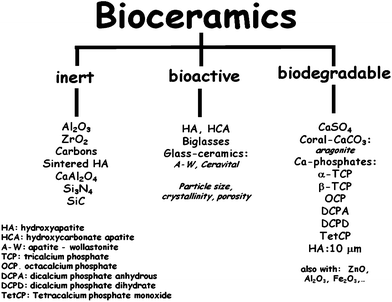 | ||
| Fig. 5 Main bioceramics classified as a function of its reactivity with living tissues. | ||
Present research in bioceramics is based mainly on biological criteria since it pursues bone tissue “regeneration” rather than its mere “replacement”. Thus, current efforts are devoted towards the synthesis of bioceramics—so-called third generation bioceramics49—which are able to induce a cell response leading to osteogenesis.50 In this regard, research has mainly focused on porous resorbable or bioactive bioceramics that are used to manufacture porous scaffolds able to host cells and bioactive agents (growth factors, hormones, peptides). The signals triggered by these biomolecules should promote bone formation. Moreover, advanced bioceramics such as ordered mesoporous silica-based materials or organically modified bioceramics are now under investigation in this respect.51–53
2.1 Second generation bioceramics
Most widely used second generation bioceramics are calcium salts—phosphates and sulphates—and silica (SiO2)-based bioceramics which are extensively used in orthopaedics and dentistry because of their biocompatibility and capacity to promote osteointegration. Many of these bioceramics are also osteoconductive, meaning their ability to provide the appropriate surface to support osteoblast adhesion and proliferation and therefore, bone formation.50 Furthermore, the presence of silicon in the structure of bioactive bioceramics appears to confer them osteoinduction features.49 However, a major drawback of calcium phosphates and silica-based bioceramics is their poor mechanical strength, namely brittleness and low resistance to fatigue. They exhibit excellent features for filling small bone defects, but their brittleness is a drawback for repairing large bone defects. This is especially so in highly porous bioceramics and scaffolds with high porosity percentages and pores larger than 100 μm in diameter. Consequently, for bone substitution and repair, these bioceramics, are mainly used as fillers in non-load bearing applications or as coatings of metallic prostheses, but also can be proposed for the manufacture of scaffolds, as will be described in the following section.Next, the major features of some calcium phosphates including synthetic apatites, biphasic mixtures, bone cements and coatings, and silica-based materials including bioglasses, mesoporous ordered silica-based materials and templated glasses will be described. Of all the synthetic apatites, HA [Ca10(PO4)6(OH)2], is the most widely studied for its structural and chemical similarities with the inorganic component of bone.54,55 HA is biocompatible, bioactive and osteoconductive. To mimic biological apatites, synthetic apatites must present nanometric particle size and contain 4–8 wt% of CO32− ions.56,57 HA structure can accept the inclusion of many ions, such as Na+, K+, Mg2+, Sr2+, Cl−, F−, HPO42−, etc.57 This can be exploited in the design of HA with specific surface structure and electric charge in order to improve the material behaviour in biological environments. In addition, the presence of certain elements (i.e., strontium, zinc or silicate) that can be released in the regenerating bone environment might facilitate the process of bone healing. Carbonate and strontium ions also facilitate apatite dissolution and implant resorption. Silicate ions increase the mechanical strength of apatite and accelerate its bioactive response. Thus, the synthesis of substituted apatites is a current research trend.
The most popular biphasic mixtures of calcium phosphates are based on HA and β-tricalcium phosphate, β-Ca3(PO4)2, (β-TCP) mixtures that evolve to CHA under physiological conditions.58 The dissolution of resorbable β-TCP acts as a source for new bone by the increase of Ca2+ and PO43− ion concentration in the local environment, simultaneously creating extra porosity that favours a suitable in vivo behaviour of the material. This biphasic material can be injected, used as a coating or as bulk in bone replacement. Other biphasic mixtures are under investigation including calcium phosphates, bioactive glasses, and calcium sulphates, etc.
Bone cements based on calcium salts, mainly phosphates, carbonates or sulphates, present excellent biocompatibility and bone-repair properties.59,60 Most of the injectable calcium phosphate-based cements evolve to calcium phosphate apatite during the setting reaction. These cements harden in situ and can be slowly resorbed. During this gradual process, the bone grows and replaces the cement. Research is under way to shorten the curing time and improve the mechanical toughness.
Coatings of calcium phosphates over metallic substrates have been used to overcome the main limitation of calcium phosphates as bioceramics: their poor mechanical properties. Common substrates are titanium alloys, Ti6Al4V and commercially pure Ti. Calcium phosphate coatings are usually produced by plasma spray because of the rapid deposition rate at a relatively low cost.61,62 However, this method presents several drawbacks: (i) the presence of highly soluble amorphous calcium phosphate in the coatings produce resorption problems; (ii) the poor adherence of the bioceramic-substrate; (iii) the instability of HA at high temperatures; and (iv) the phase transition of titanium at high temperatures. Nowadays, alternative techniques to obtain calcium phosphate coatings are under investigation including physical vapor deposition, chemical vapor deposition, magnetron sputtering, electrophoretic deposition, pulsed laser deposition and dip coating. Some of these techniques permit higher control of the coating thickness and crystallinity or can take place at low temperatures, an interesting advantage compared with plasma spray. In Fig. 6, different uses of calcium phosphate bioceramics are presented.
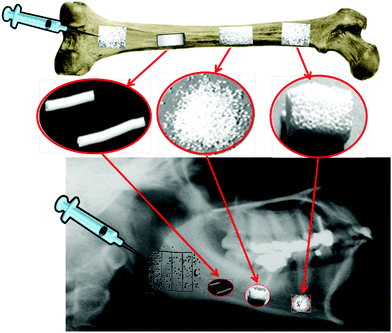 | ||
| Fig. 6 Different uses of calcium phosphate bioceramics. | ||
For silica-containing bioceramics, bioglasses were the first synthetic materials for which bioactivity was described.63 They contain silica as the network former together with other metallic oxides as modifiers. Among them, Bioglass® 45S5 (45% SiO2 24.5% NaO2, 24.5% CaO and 6% P2O5), obtained by traditional “quenching-of-a-melt” method, is the best characterized and used bioceramic of this group.63
In the 1990s, the synthesis of porous bioactive bioglasses by the sol–gel method began.64–67 This technique requires lower temperatures, which facilitates the possible incorporation of biologically active molecules or living cells into the bioglasses. These bioglasses exhibited high surface areas and porosity as well as an abundance of surface silanol (Si–OH) groups, which greatly improves their bioactive response. Nowadays, the most promising application of bioactive bioglasses is in bone tissue engineering as 3D scaffolds in association with osteogenic agents, as will be detailed later. These bioactive matrices present osteoconduction and osteoinduction.
In recent years, silica-based ordered mesoporous bioceramics attracted interest as templates for drug delivery applications.68 These mesoporous materials have the same chemical composition, namely silica, and are denoted by different acronyms, e.g., MCM-41, SBA-15, MCM-48, among others. Mesoporous silica-based bioceramics exhibit a high surface area (ca. 1000 m2 g−1), high pore volume (ca. 1 cm3 g−1), narrow pore size distribution in the mesopore region (2–50 nm), and a pore channel system homogeneously organized in 2D or 3D arrangements. The surface of these materials covered with silanol groups should govern their interaction with the host tissue, although the kinetics of the nanoapatite layer formation is slow.69 Therefore, in the last few years a great interest was devoted towards the so-called templated glasses,70 which exhibit analogous textural properties to mesoporous materials but a greater bioactivity response that made them very attractive in bone regeneration technologies.
2.2 3D bioceramic scaffolds for bone tissue engineering
In bone tissue engineering, synthetic scaffolds should fulfil various requirements—they must provide mechanical stability as well as an appropriate environment for new bone formation; e.g., they must favour cell attachment as well as cell growth and differentiation.71–75 Bioceramics can be manufactured as scaffolds in this regard. The in vivo behaviour of such scaffolds depends on both the intrinsic properties of the bioceramic and specific features resulting from their fabrication method.76–78One advantage of bioceramic scaffolds lies in the fact that they usually have hierarchical porosity with highly interconnected pores analogous to bone. Pores can be classified in three categories depending on the role they play. Those with diameters in the range 150 to 400 μm and larger are needed for cell colonization and vascularisation.79 Another group of pores under 20 μm is necessary for capillary ingrowths and cell-matrix interactions. In the case of scaffolds made of mesoporous ordered silica-based bioceramics or templated glasses, pores of a few nanometers are present, which allow the inclusion of osteogenic factors to decorate the scaffold. This confers a very important added value to the resulting scaffold: the capacity of hosting drugs for locally treating different bone morbidities and pathologies, such as bone infection, osteoporosis or cancer. The scaffold processing method should thus preserve the original mesoporosity combined with macroporosity. Fig. 7 shows the different pore sizes required for several physiological functions of bone tissue.
 | ||
| Fig. 7 Porosity features involved in the design of scaffolds for bone tissue engineering. | ||
Several conformation methods are available to obtain scaffolds at room temperature, a manoeuvre that permits the inclusion of different biomolecules in the material. An appropriate and useful method to produce scaffolds as bone implants is rapid prototyping (RP) 3D printing,80,81 which makes possible the design of the correct scaffold structure based on tomography information of the bone tissue to repair. Then, with a computer-assisted design, suitable scaffolds with the appropriate shape and porosity can be obtained for different clinical requirements. Generally, biodegradable or bioactive bioceramics are used for this purpose; a bioceramic paste is prepared to load in the injector and to mould the scaffold with a previously designed macroporosity. A combination of different methods, such as sol–gel chemistry, double polymer templating and RP, has lead to 3D mesoporous bioglass-based scaffolds exhibiting hierarchical pore networks, with giant (30–1000 μm), macro- (10–30 μm) and meso- (5 nm) porosity.
An attractive strategy in bone tissue engineering has been the incorporation of osteogenic factors with the aim to improve the osteoinductive properties of the scaffolds (Fig. 8). Incorporation of these factors relies on conventional impregnation techniques using manufactured scaffolds.82 The type of pore and exposed (inner and outer) surface, and the quality of the loading solution (including pH) are all parameters that highly affect the adsorption capacity of the bioceramics scaffolds.83 However, this capacity can be improved by previous chemical functionalization of scaffold mesopores with different organic groups tailored to interact with a particular factor of interest.53 This approach allows fine control over the drug load and a predictable release pattern.83,84
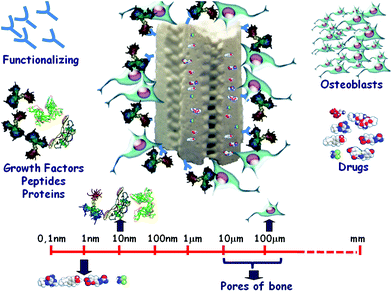 | ||
| Fig. 8 Bone regeneration and local drug delivery by mesoporous-based 3D scaffolds. | ||
The efficacy of local administration of osteoinductive agents, e.g., peptides and growth factors, may be improved by their covalent grafting to the scaffolds, which determines a sustained exposure in the bone microenvironment.85,86 These osteoinductive agents would act as attracting signals for bone cells.
3. Symbiosis between nature and materials
A traditional approach in tissue engineering strategies involves the implantation of different types of osteogenic cells seeded onto suitable templates, including bioceramics. Cells of the osteoblastic lineage with different differentiation status can be used to drive bone tissue regeneration.87,88 In addition, different sources of osteoprogenitors have been tested for their capacity to regenerate bone (reviewed in ref. 31 and 89). MSCs—defined as adherent multipotent cells capable of differentiating into osteogenic cells90—have distinctive and interesting features such as immunosuppressive, cell protective, and angiogenic activities.91 Current data support the notion that MSCs are indeed pericytes residing on blood vessels; in this context, an insult causing vasculature disruption would make these cells available to help regenerate the resulting damaged tissue.92,93 MSCs are relatively abundant in the bone marrow from which they can be easily isolated and expanded in cultures for use in different bone tissue engineering schemes. The major drawback of MSCs from various origins, however, lies in their limited quantity and/or differentiation capacity;94,95 these hurdles are presently a subject of intense investigation through different approaches.96,97 In addition, elucidating the true role of the host contribution to bone tissue regeneration is paramount to optimize tissue engineering approaches. Of interest in this line, MSCs and more committed cells of the osteoblast lineage appear to have a different capability to recruit host endothelial cells and, therefore, to increase vascularization.93,97Osteopromotion can be achieved by using factors which enhance the healing process. Thus, a common strategy to promote bone healing relies on the use of osteogenic factors which are either directly implanted as recombinant proteins or embedded in a suitable carrier such as bioceramics previous to implantation.98,99 Osteoinduction properties are conferred to these biomaterials by growth factors which can induce differentiation of MSCs along the bone repair process.31 BMP-2 and BMP-7 in particular, have been widely used in animal models and also in randomized clinical trials for healing fractures and stimulating spine fusion. BMP-2 has proven essential for MSCs recruitment and expansion; it is the only United States Food and Drug Administration (FDA)-approved molecular therapy for use in the management of fracture repair and spine non unions.100 In fact, BMP-2 represented 18% of the US orthopaedic biomaterials market in 2009 (See Fig. 3). However, some concerns have recently been raised due to the poor outcomes of BMP-2 administration in some cases.101,102
Recently, our group has started to explore the advantage of the small peptide osteostatin, consisting of the 107–111 domain (Thr-Arg-Ser-Ala-Trp) of PTHrP, to promote bone healing. Fig. 9 shows the comparison of some biological characteristics of osteostatin and BMP-2. As can be seen, local application of osteostatin is expected to bring significant advantages with respect to BMP-2. We have recently demonstrated that osteostatin loading onto silica-based ceramics conferred osteogenic features to these materials, including stimulation of osteoblastic cell growth and differentiation.103,104 Moreover, osteostatin has recently been proven to increase the osteogenic efficacy of fibroblast growth factor-2 as coated onto Si-doped hydroxyapatite by enhancing its angiogenic potential in vitro.105 These data are consistent with the recently described anabolic features of the native peptide, PTHrP107–139, independent of its concomitant inhibitory effect on bone resorption.106,107 Attractive properties of osteostatin lie in the fact that it can affect bone cells at <nM concentrations and its structure is not likely to be degraded by proteolysis. This prompted us to further investigate whether osteostatin-coated bioceramics might be able to improve osteointegration and accelerate bone repair after a cavitary defect in the femoral epiphysis of healthy rabbits. This in vivo model, in which trabecular bone damage predominates, has been proven to be suitable for testing biomaterials. Histological analysis revealed the absence of significant inflammation or bone resorption within the time of study (4 and 8 weeks) after implantation.104 At 8 weeks, microcomputerized tomography (μCT) analysis showed that osteostatin-loaded biomaterials were highly osteoconductive and osteoinductive by promoting infiltration of proliferating osteogenic precursor cells and connective tissue formation (Fig. 10). In addition, these bioceramics induced revascularization of the defect, related to an increased immunostaining for VEGF in the healing bone tissue.104 More recently, these biomaterials were also found to improve the early stage of bone healing in the same bone fracture model in osteoporotic rabbits.108 Thus, these osteostatin-loaded bioceramics appear to be an attractive approach for bone tissue engineering applications even in the setting of osteopenia.
 | ||
| Fig. 9 A comparison between bioactivities of osteostatin and BMP-2. | ||
 | ||
| Fig. 10 3D reconstruction microtomography images of mesoporous silica bioceramics with SBA 15 structure, unloaded and loaded with osteostatin before being implanted for 8 weeks in a cavitary defect model performed in the rabbit femur. | ||
4. Future perspectives
Second generation bioceramics including those bioactive and resorbable bioceramics are being used today as implants for bone regeneration and repair in Orthopedics and Dental applications. Third generation bioceramics, those driving bone regeneration in various bone tissue engineering schemes, are now under intense research in the hope of significant developments in the near future.A representative picture of the current research interests in biomaterials can be obtained from the program of the Nine World Biomaterials Congress that took place in Chengdu (China) from the 1st to the 5th of June 2012. The central topic of the Congress was: “Innovative Biomaterials and Crossing Frontiers in Biomaterials and Regenerative Medicine”. Over 3,000 communications were classified in 87 Symposia that were divided into 167 scientific sessions. The main topic was “scaffolds”, which was included in 12 sessions, followed by “cell-materials interactions”, which were discussed in 8 sessions. In addition, the following topics were present in 6 sessions: “nanomaterials, gene delivery and hydrogels”. Other 5 sessions were devoted to “tissue inducing biomaterials, biomimetics, and molecular imaging”, while four more sessions dealt with “bioinorganics and bioceramics, surface modifications, hybrid materials and polymer design”. These 12 topics represented almost 50% of all scientific sessions of the Congress. No doubt that they will be major topics in the field of Biomaterials in the next years. But the question arises as to how biomaterials research will evolve? Interesting advances in this regard were presented in specific sessions such as “growth factor delivery” or “bone tissue engineering”.
Regarding the growth factor delivery topic, various complementary approaches are now envisioned to promote bone healing. As a promising approach, the off-label use of PTH1–34 as intermittent subcutaneous injection—an approved anabolic therapy in osteoporotic patients109—has recently been proposed to promote bone regeneration.110 This type of administration of PTH1–34 increased the rate of callus formation and its mechanical properties.110-112 The use of a degradable matrix with plasmid coding for PTH and BMP-4 as implants has also proven to be efficient to fill a femoral gap in rats.113 A pulsatile release system, consisting of PTH-loaded alginate disks and several polyanhydride isolation layers alternately stacked within the device, has also been developed.114 This biocompatible system, as well as a matrix-bound engineered active fragment of human PTH1–34, whose development is associated with matrix remodeling,115 hold promise for the controlled delivery of PTH locally into a bone injury site. Attempts have also been made to manipulate the controlled release of various osteotropic agents with variable kinetics with the aim of achieving an optimal bone healing response.116
The use of superparamagnetic mesoporous silica particles as part of “nanogate” systems with a potential “on/off” factor release is also under investigation.117
A consistent notion throughout all the plenary lectures in the aforementioned Congress was that biomaterials science is moving into the nanometric arena. Combined with smart molecular and cell biology strategies, they will provide a solution to many of the current orthopedic demands.
Acknowledgements
Funding for the authors came from grants by Comisión Interministerial de Ciencia y Tecnología (CICYT, Spain) (MAT2008-736 and CSO2010-11384-E), Comunidad Autónoma de Madrid (CAM; S2009/MAT-1472), Instituto de Salud Carlos III (RETICEF RD06/0013/1002) and Fundación de Investigación Médica Mutua Madrileña.References
- C. Wann, S. R. Gilbert, Y. Wang, X. Cao, X. Shen, G. Ramaswamy, K. A. Jacobsen, Z. S. Alaq, A. W. Eberhard, L. C. Gerstenfeld, T. A. Einhorn, L. Deng and T. L. Clemens, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 686 CrossRef.
- The bone and joint decade. http://www.boneandjointdecade.org. 2005.
- R. Gruber, H. Koch, B. A. Doll, F. Tegtmeier, T. A. Einhorn and J. O. Hollinger, Exp. Gerontol., 2006, 41, 1080 CrossRef.
- P. Peichl, L. A. Holzer, R. Maier and G. Holzer, J. Bone Joint Surg. Am., 2011, 93, 1583 CrossRef.
- E. C. Gaetti-Jardim, J. F. Santiago-Junior, M. C. Goiato, E. P. Pellizer, O. Magro-Filho and E. G. Jardim Jr, J. Craniofac. Surg., 2011, 22, 1111 CrossRef.
- L. Fernández de Castro, D. Lozano, S. Dapía, S. Portal-Núñez, J. R. Caeiro, E. Gómez-Barrena and P. Esbrit, Tissue Eng. Part A, 2010, 16, 1157 CrossRef.
- A. Gandhi, H. A. Beam, J. P. O'Connor, J. R. Parsons and S. S. Lin, Bone, 2005, 37, 482 CrossRef CAS.
- F. Deschaseaux, L. Sensébé and D. Heymann, Trends Mol. Med., 2009, 15, 417 CrossRef CAS.
- Y. Bar-Cohen, in Biomimetics Nature-Based Innovation, ed. Y. Bar-Cohen, Taylor and Francis (CRC Press), Boca Raton, FL, 2011. p. 14 Search PubMed.
- L. T. Kuhn, D. J. Fink and A. H. Heuer, in Biomimetic Materials Chemistry, ed. S. Mann, Wiley-VCH, United Kingdom, 1996, p. 41 Search PubMed.
- M. Vallet-Regí and D. Arcos, Biomimetic Nanoceramics in Clinical Use: From Materials to Applications, Royal Society of Chemistry, Cambridge, 2008 Search PubMed.
- D. G. Steele and C. A. Bramblett, The Anatomy and Biology of the Human Skeleton, Texas A&M University Press, 1988, p. 10 Search PubMed.
- W. Frieb and J. Werner, in Handbook of Porous Solids, ed. F. Shüth, S. Kienneth, W. Sing and W. Kamps, Wiley-VCH, Weinheim, Germany, 2000, p. 2923 Search PubMed.
- M. Schieker, H. Seitz, I. Drosse, S. Seitz and W. Mutschler, Eur. J. Trauma, 2006, 32, 114 CrossRef.
- P. Driscoll 2009, http://mediligence.com/blog/2009/01/30/orthopedic-biomaterials-market-growth-strongest-in-us/.
- L. Claes, S. Recknagel and A. Ignatius, Nat. Rev. Rheumatol., 2012, 8, 133 CrossRef CAS.
- H. Schell, J. Lienau, D. R. Epari, P. Seebeck, C. Exner, S. Muchow, H. Bragulla, N. P. Haas and G. N. Duda, Bone, 2006, 38, 547 CrossRef.
- J. Caetano-Lopes, A. Lopes, A. Rodrigues, D. Fernandes, I. P. Perpétuo, T. Monjardino, R. Lucas, J. Monteiro, Y. T. Konttinen, H. Canhão and J. E. Fonseca, PLoS One, 2011, 6, e16947 CAS.
- F. Tortelli, T. Tasso, F. Loiacono and R. Cancedda, Biomaterials, 2010, 31, 242 CrossRef CAS.
- T. Kon, T. J. Cho, T. Aizawa, M. Yamazaki, N. Nooh, D. Graves, L. C. Gerstenfeld and T. A. Einhorn, J. Bone Miner. Res., 2001, 16, 1004 CrossRef CAS.
- H. C. Pape, R. Marcucio, C. Humphrey, C. Colnot, M. Knobe and E. J. Harvey, J. Orthop. Trauma, 2010, 24, 522 CrossRef.
- C. M. Champagne, J. Takebe, S. Offenbacher and L. F. Cooper, Bone, 2002, 30, 26 CrossRef CAS.
- W. Lehmann, C. M. Edgar, K. Wang, T. J. Cho, G. L. Barnes, S. Kakar, D. T. Graves, J. M. Rueger, L. C. Gerstenfeld and T. A. Einhorn, Bone, 2005, 36, 300 CrossRef CAS.
- S. Portal-Núñez, D. Lozano and P. Esbrit, Histol. Histopathol., 2012, 27, 559 Search PubMed.
- J. D. Boerckel, B. A. Uhrig, N. J. Willett, N. Huebsch and R. E. Guldberg, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, E674 CrossRef CAS.
- M. Grellier, N. Ferreira-Tojais, C. Bourget, R. Bareille, F. Guillemot and J. Amédée, J. Cell. Biochem., 2009, 106, 390 CrossRef CAS.
- S. Akeel, A. El-Awady, K. Hussein, M. El-Refaey, M. Elsalanty, M. Sharawy and M. Al-Shabrawey, Arch. Oral Biol., 2012, 57, 445 CrossRef CAS.
- P. Esbrit, M. V. Alvarez-Arroyo, F. de Miguel, O. Martín, M. E. Martínez and C. Caramelo, J. Am. Soc. Nephrol., 2000, 11, 1085 CAS.
- L. Pederson, M. Ruan, J. J. Westendorf, S. Khosla and M. J. Oursler, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 20764 CrossRef CAS.
- E. Shimizu, J. Tamasi and N. C. Partridge, J. Dent. Res., 2012, 91, 268 CrossRef CAS.
- K. Arvidson, B. M. Abdallah, L. A. Applegate, N. Baldini, E. Cenni, E. Gómez-Barrena, D. Granchi, M. Kassem, Y. T. Konttinen, K. Mustafa, D. P. Pioletti, T. Sillat and A. Finne-Wistrand, J. Cell. Mol. Med., 2011, 15, 718 CrossRef CAS.
- B. L. Vaes, P. Ducy, A. M. Sijbers, J. M. Hendriks, E. P. van Someren, N. G. de Jong, E. R. van den Heuvel, W. Olijve, E. J. van Zoelen and K. J. Dechering, Bone, 2006, 39, 724 CrossRef CAS.
- F. H. Zhou, B. K. Foster, X. F. Zhou, A. J. Cowin and C. J. Xian, J. Bone Miner. Res., 2006, 21, 1075 CrossRef CAS.
- J-B. Kim, P. Leucht, K. Lam, C. Luppen, D. T. Berge, R. Nusse and J. A. Helms, J. Bone Miner. Res., 2007, 22, 1913 CrossRef CAS.
- U. Krause, S. Harris, A. Green, J. Ylostalo, S. Zeitouni, N. Lee and C. A. Gregory, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 4147 CrossRef CAS.
- P. J. Roholl, E. Blauw, C. Zurcher, J. A. Dormans and H. M. Theuns, J. Bone Miner. Res., 1994, 9, 355 CrossRef CAS.
- M. E. Martínez, M. T. del Campo, S. Medina, M. Sánchez, M. J. Sánchez-Cabezudo, P. Esbrit, P. Martínez, I. Moreno, A. Rodrigo, M. V. Garcés and L. Munuera, Calcif. Tissue Int., 1999, 64, 280 CrossRef.
- P. Martínez, I. Moreno, F. De Miguel, V. Vila, P. Esbrit and M. E. Martínez, Bone, 2001, 29, 35 CrossRef.
- M. Almeida, L. Han, M. Martín-Millán, C. A. O'Brien and S. C. Manolagas, J. Biol. Chem., 2007, 282, 27298 CrossRef CAS.
- S. Salvioli, M. Capri, S. Valensin, P. Tieri, D. Monti, E. Ottaviani and C. Franceschi, Curr. Pharm. Des., 2006, 12, 3161 CrossRef CAS.
- R. A. Meyer Jr, B. R. Desai, D. E. Heiner, J. Fiechtl, S. Porter and M. H. Meyer, J. Orthop. Res., 2006, 24, 1933 CrossRef.
- E. C. Wahl, J. Aronson, L. Liu, J. L. Fowlkes, K. M. Thrailkill, R. C. Bunn, R. A. Skinner, M. J. Miller, G. E. Cockrell, L. M. Clark, Y. Ou, C. M. Isales, T. M. Badger, M. J. Ronis, J. Sims and C. K. Lumpkin Jr, J. Bone Miner. Res., 2010, 25, 114 CrossRef CAS.
- M. S. Ominsky, C. Li, X. Li, H. L. Tan, E. Lee, M. Barrero, F. J. Asuncion, D. Dwyer, C. Y. Han, F. Vlasseros, R. Samadfam, J. Jolette, S. Y. Smith, M. Stolina, D. L. Lacey, W. S. Simonet, C. Paszty, G. Li and H. Z. Ke, J. Bone Miner. Res., 2011, 26, 1012 CrossRef CAS.
- A. S. Hoffman, in Biomaterials Science, ed. B. D. Ratner, A. S. Hoffman, F. J. Schoen and J. E. Lemons, Elsevier Academic Press, Amsterdam, 2004. p. 67 Search PubMed.
- M. Vallet-Regí and E. Ruiz-Hernández, Adv. Mater., 2011, 23, 5177 CrossRef.
- M. Vallet-Regi and A. J. Salinas, in Bone Repair Biomaterials, ed. J. A. Planell, S. M. Best, D. Lacroix and A. Merolli, CRC Press Boca Raton, 2009, p. 194 Search PubMed.
- L. L. Hench, J. Am. Ceram. Soc., 1998, 81, 1705 CrossRef CAS.
- D. G. Castner and B. D. Ratner, Surf. Sci., 2002, 500, 28 CrossRef CAS.
- L. L. Hench and J. M. Polak, Science, 2002, 295, 1014–1017 CrossRef CAS.
- T. Albrektsson and C. Johansson, Eur. Spine J., 2001, 10(Suppl 2), S96 Search PubMed.
- A. J. Salinas and M. Vallet-Regí, Z. Anorg. Allg. Chem., 2007, 1762 CrossRef CAS.
- M. Vallet-Regí, I. Izquierdo-Barba and M. Colilla, Philos. Transact. A Math. Phys. Eng. Sci., 2012, 370, 1400 CrossRef.
- M. Vallet-Regí, M. Colilla and B. González, Chem. Soc. Rev., 2011, 40, 596 RSC.
- J. Juhasz and S. Best, J. Mater. Sci., 2012, 47, 610 CrossRef CAS.
- J. C. Elliott, Structure and Chemistry of the Apatites and Other Calcium Orthophosphates, Elsevier, London, 1994 Search PubMed.
- J. C. Elliott, J. Appl. Crystallogr., 1980, 13, 618 CrossRef CAS.
- R. Z. LeGeros, Calcium phosphates in oral biology and medicine, in Monographs in Oral Science, ed. H. M. Myers, S. Karger, Basel, 1991 Search PubMed.
- J. M. Bouler, R. Z. LeGeros and G. Daculsi, J. Biomed. Mater. Res., 2000, 51, 680 CrossRef CAS.
- L. C. Chow, J. Ceram. Soc. Jpn., 1991, 99, 954 CrossRef CAS.
- M. P. Ginebra, F. C. M. Driessens and J. A. Planell, Biomaterials, 2004, 25, 3453 CrossRef CAS.
- L. M. Sun, C. C. Berndt, K. A. Gross and A. Kucuk, J. Biomed. Mater. Res., 2001, 58, 570 CrossRef CAS.
- L. L. Yan, Y. Leng and L. T. Weng, Biomaterials, 2003, 24, 2585 CrossRef CAS.
- L. L. Hench, R. J. Splinter, W. C. Allen and T. K. Greenlee, J. Biomed. Mater. Res., 1971, 2, 117 CrossRef.
- R. Li, A. E. Clark and L. L. Hench, J. Appl. Biomater., 1991, 2, 231 CrossRef CAS.
- M. M. Pereira, A. E. Clark and L. L. Hench, J. Biomed. Mater. Res., 1994, 28, 693 CrossRef CAS.
- A. J. Salinas, M. Vallet-Regí and I. Izquierdo-Barba, J. Sol-Gel Sci. Technol., 2001, 21, 13 CrossRef CAS.
- M. Vallet-Regí, C. V. Ragel and A. J. Salinas, Eur. J. Inorg. Chem., 2003, 1029 CrossRef.
- M. Vallet-Regí, A. Rámila, R. P. del Real and J. Pérez-Pariente, Chem. Mater., 2001, 13, 308 CrossRef.
- M. Vallet-Regí, M. L. Ruiz-González, I. Izquierdo-Barba and J. M. González-Calbet, J. Mater. Chem., 2006, 16, 26 RSC.
- A. López-Noriega, D. Arcos, I. Izquierdo-Barba, Y. Sakamoto, O. Terasaki and M. Vallet-Regí, Chem. Mater., 2006, 18, 3137 CrossRef.
- J. R. Jones and L. L. Hench, Curr. Opin. Solid State Mat. Sci., 2003, 7, 301 CrossRef CAS.
- J. R. Jones, J. Eur. Ceram. Soc., 2009, 29, 1275 CrossRef CAS.
- J. R. Porter, T. T. Ruckh and K. C. Popat, Biotechnol. Prog., 2009, 25, 1539 CAS.
- F. O'Brien, Mater. Today, 2011, 14, 88 CrossRef CAS.
- B. M. Willie, A. Petersen, K. Schmidt-Bleek, A. Cipitria, M. Mehta, P. Strube, J. Lienau, B. Wildemann, P. Fratzl and G. Duda, Soft Matter, 2010, 6, 4976 RSC.
- Q. Fu, E. Saiz, M. N. Rahaman and A. P. Tomsia, Mater. Sci. Eng., C., 2011, 31, 1245 CrossRef CAS.
- A. J. Wagoner Johnson and B. A. Herschler, Acta Biomater., 2011, 7, 16 CrossRef CAS.
- D. M. Yunos, O. Bretcanu and A. R. Bocaccini, J. Mater. Sci., 2008, 43, 4433 CrossRef.
- O. Gauthier, J. M. Bouler, E. Aguado, P. Pilet and G. Daculsi, Biomaterials, 1998, 19, 133 CrossRef CAS.
- S. F. Yang, K. F. Leong, Z. H. Du and C. K. Chua, Tissue Eng., 2002, 8, 1 CrossRef CAS.
- A. García, I. Izquierdo-Barba, M. Colilla, C. López de Laorden and M. Vallet-Regí, Acta Biomater., 2011, 7, 1265 CrossRef.
- W. Habraken, J. G. C. Wolke and J. A. Jansen, Adv. Drug. Deliv. Rev., 2007, 59, 234 CrossRef CAS.
- M. Vallet-Regí, F. Balas and D. Arcos, Angew. Chem., Int. Ed., 2007, 46, 7548 CrossRef.
- M. Vallet-Regí, Chem.–Eur. J., 2006, 12, 1 CrossRef.
- M. Manzano, D. Lozano, D. Arcos, S. Portal-Núñez, C. L. Orden, P. Esbrit and M. Vallet-Regí, Acta Biomater., 2011, 7, 3555 CrossRef CAS.
- A. Baeza, I. Izquierdo-Barba and M. Vallet-Regí, Acta Biomater., 2010, 6, 743 CrossRef CAS.
- M. I. Santos, R. E. Unger, R. A. Sousa, R. L. Reis and C. J. Kirkpatrick, Biomaterials, 2009, 15, 2373 Search PubMed.
- A. Arthur, A. Zannettino and S. Gronthos, J. Cell. Physiol., 2009, 218, 237 CrossRef CAS.
- S. Ohba, F. Yano and U-i. Cheng, BoneKEy, 2009, 6, 405 Search PubMed.
- L. Da Silva Meirelles, A. I. Caplan and N. B. Nardi, Stem Cells, 2008, 26, 2287 CrossRef.
- A. I. Caplan and J. E. Dennis, J. Cell. Biochem., 2006, 98, 1076 CrossRef CAS.
- P. Bianco, P. G. Robey and P. J. Simmons, Cell. Stem Cell, 2008, 2, 313 CrossRef CAS.
- J. M. Sorrell, M. A. Baber and A. I. Caplan, Tissue Eng. Part A, 2009, 15, 1751 CrossRef CAS.
- S. Shi, S. Gronthos, S. Chen, A. Reddi, C. M. Counter, P. G. Robey and C. Y. Wang, Nat. Biotechnol., 2002, 20, 587 CrossRef CAS.
- L. Duplomb, M. Dagouassat, P. Jourdon and D. Heymann, Stem Cells, 2007, 25, 544 CrossRef CAS.
- Y. Chen, V. Bloemen, S. Impens, M. Mohecen, F. P. Luyten and J. Schrooten, Tissue Eng. Part C Methods, 2011, 17, 1211 CrossRef CAS.
- A. I. Caplan, Tissue Eng. Part B., 2009, 15, 195 CrossRef CAS.
- H. Autefage, F. Briand-Mesange, S. Cazalbou, C. Drouet, D. Fourmy, S. Goncalves, J. P. Salles, C. Combes, P. Swider and C. Rey, J. Biomed. Mater. Res., Part B., 2009, 91B, 706 CrossRef CAS.
- M. Manzano and M. Vallet-Regí, Prog. Solid State Chem., 2012, 40, 17 CrossRef CAS.
- S. Govender, C. Csimma, H. K. Genant, A. Valentin-Opran, Y. Amit, R. Arbel, H. Aro, D. Atar, M. Bishay, M. G. Börner, P. Chiron, P. Choong, J. Cinats, B. Courtenay, R. Feibel, B. Geulette, C. Gravel, N. Haas, M. Raschke, E. Hammacher, D. van der Velde, S. Oevre, L. Nordsletten, A. Patel, A. Pohl, W. Rennie, P. Reynders, P. M. Rommens, J. Rondia, W. C. Rossouw, P. J. Daneel, S. Ruff, A. Rüter, S. Santavirta, T. A. Schildhauer, C. Gekle, R. Schnettler, D. Segal, H. Seiler, R. B. Snowdowne, J. Stapert, G. Taglang, R. Verdonk, L. Vogels, A. Weckbach, A. Wentzensen and T. Wisniewski, J. Bone Joint Surg. Am., 2002, 84, 2123 CrossRef.
- L. B. Shields, G. H. Raque, S. D. Glassman, M. Campbell, T. Vitaz, J. Harping and C. B. Shields, Spine, 2006, 31, 542 CrossRef.
- H. T. Aro, S. Govender, A. D. Patel, P. Hernigou, A. P. de Gregorio, G. I. Popescu, J. D. Goleen, J. Christensen and A. Valentin, J. Bone Joint Surg. Am., 2011, 93, 801 CrossRef.
- D. Lozano, M. Manzano, J. C. Doadrio, A. J. Salinas, M. Vallet-Regí, E. Gómez-Barrena and P. Esbrit, Acta Biomater., 2010, 6, 797 CrossRef CAS.
- C. G. Trejo, D. Lozano, M. Manzano, J. C. Doadrio, A. J. Salinas, S. Dapía, E. Gómez-Barrena, M. Vallet-Regí, N. García-Honduvilla, J. Buján and P. Esbrit, Biomaterials, 2010, 31, 8564 CrossRef CAS.
- D. Lozano, M. J. Feito, S. Portal-Núñez, R. M. Lozano, M. C. Matesanz, M. C. Serrano, M. Vallet-Regí, M. T. Portolés and P. Esbrit, Acta Biomater., 2012, 8, 2770 CrossRef CAS.
- D. Lozano, L. Fernández-de-Castro, S. Portal-Núñez, A. López-Herradón, S. Dapía, E. Gómez-Barrena and P. Esbrit, Br. J. Pharmacol., 2011, 162, 1424 CrossRef CAS.
- L. F. de Castro, D. Lozano, S. Portal-Núñez, M. Maycas, M. De la Fuente, J. R. Caeiro and P. Esbrit, J. Cell Physiol., 2012, 227, 1752 CrossRef.
- D. Lozano, C. G. Trejo, E. Gómez-Barrena, M. Manzano, J. C. Doadrio, A. J. Salinas, M. Vallet-Regí, N. García-Honduvilla, P. Esbrit and J. Buján, Acta Biomater., 2012, 8, 2317 CrossRef CAS.
- R. L. Jilka, Bone, 2007, 40, 1434 CrossRef CAS.
- M. Takahata, H. A. Awad, R. J. O'Keefe, S. V. Bukata and E. M. Schwarz, Cell Tissue Res., 2012, 347, 545 CrossRef CAS.
- G. L. Barnes, S. Kakar, S. Vora, E. F. Morgan, L. C. Gerstenfeld and T. A. Einhorn, J. Bone Joint Surg. Am., 2008, 90(Suppl 1), 120 CrossRef.
- T. T. Andreassen, C. Fledelius, C. Ejersted and H. Oxlund, Acta Orthop. Scand., 2001, 72, 304 CrossRef CAS.
- K. Kamo, N. Miyakoshi, Y. Kasukawa, K. Nozaka, H. Sasaki and Y. Shimada, J. Bone Miner. Metab., 2010, 28, 634 CrossRef CAS.
- J. Fang, Y-Y. Zhu, E. Smiley, J. Bonadio, J. P. Rouleau, S. A. Goldstein, L. K. McCauley, B. L. Davidson and B. J. Roessler, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 5753 CrossRef CAS.
- I. Arrigí, S. Mark, M. Alvisi, B. von Rechenberg, J. A. Hubbell and J. C. Schense, Biomaterials, 2009, 30, 1763 CrossRef.
- J. H. Jeon and D. A. Puleo, Biomaterials, 2008, 29, 3591 CrossRef CAS.
- E. Ruiz-Hernández, A. Baeza and M. Vallet-Regí, ACS Nano., 2011, 5, 1259 CrossRef.
| This journal is © The Royal Society of Chemistry 2013 |
