Understanding the role of nano-topography on the surface of a bone-implant
Alexey
Klymov
,
Ljupcho
Prodanov
,
Edwin
Lamers
,
John A
Jansen
and
X Frank
Walboomers
*
Radboud University Nijmegen Medical Centre, Department of Biomaterials, P.O. Box 9101, 6500HB Nijmegen, The Netherlands. E-mail: f.walboomers@dent.umcn.nl; Fax: +31243614657; Tel: +31243614086
First published on 26th September 2012
Abstract
Bone-implant material development is proceeding at a high pace, and has shifted from straightforward biomaterial testing to more advanced cell-targeted approaches for surface modification and design. It has been long known that cells can recognize and respond to topographical features by changing their morphology and behavior. The progress in surface analytical devices, as well as in techniques for production of topographical features on the nanometer scale allow for the characterization of natural tissues and the reproduction of biomimetic nanofeatures in material surfaces. In this review some of the most common surface-characterization and surface-manufacturing techniques will be addressed and results from in vitro and in vivo studies will be presented. Knowledge on biomaterial nanotopography can be exploited for active stimulation and control of cellular behavior like attachment, migration, spreading, gene expression, proliferation, differentiation and secretion of matrix components.
Introduction
During the lifetime of an organism tissue-damage can occur due to reasons like accidents, disease or aging. When lost tissues cannot be restored by natural regeneration processes, biomaterials can be used to (at least partially) replace the natural function. The term “biomaterial” is often used as the synonym for synthetic or non-living materials that, due to their physical and/or chemical properties, resemble natural tissues and can be introduced into a biological system.Nowadays implant treatment has become routine, but still more advanced related regenerative medicine approaches remain challenging in clinics. The steadily increasing average age of patient-populations, and risk factors like diabetes, high blood pressure, osteoporosis and smoking habits can result in poor device performance.1–6 Interestingly, also younger patients from healthy and risk groups show significantly higher failure rates than older patients,7–9 which could be explained by the higher mechanical loads during the daily life activities. Moreover, revision surgery of a failed medical device usually is ever more challenging. These facts show that there is still the need for improvement of the implants and implant materials used today. Therefore the number of implantable biomaterials used for tissue regeneration of bone is growing at a high pace. Materials frequently used in clinics are metals, ceramics and polymers, which are systematically tested and improved to optimize their properties. Features like strength, hardness and wear resistance are desirable, whereas adverse reactions like abundant inflammatory responses or toxicity should not occur.
However, in the ambition to produce biomaterials, principles and rules of natural tissue formation are often overlooked or simply ignored. Bringing a biomaterial into a living system, does not only disturb the biologically equilibrated state of the organism, but also confronts this system with a material of foreign structure and (bio-) chemical properties. Studying the natural mechanisms of tissue formation and regeneration on the one hand, and cellular interaction with that tissues on the other hand, will not only increase fundamental understanding of organogenesis, but will also allow utilization of this knowledge for efficient and intelligent tissue-reconstruction applications.
The rather complex process of tissue formation and organization in humans is mainly driven by the interaction of single cells with their environment. During the one billion years after the transition from single-cell organisms to multicellular living systems, efficient features have developed that allow cell-control on an almost single-cell level. These control mechanisms make it possible for a single fertilized oocyte to develop into a systematically organized multibillion-cell organism, which is able to maintain, reshape and regenerate many of its tissues. The three important coordination systems within an organism are: (1) cell signaling using cytokines and hormones, (2) direct interactions between cells, and (3) interactions between cells with non-cellular tissues.
The ability of single cell organisms to sense a gradient of chemical compounds and to follow or to avoid the highest concentration evolved very early. The process, that is called chemotaxis, allowed the organisms to find sources of nutrients, or to stay away from toxic compounds. The main mechanism behind it is the recognition of compounds (ligands) by a specific receptor expressed on the cellular membrane. Ligand-binding to the receptor activates an intracellular signal transition process, which allows regulation of transporters, gene expression and cell-migration. Information about the concentrations of nutrients, growth factors, cytokines and hormones is sensed and integrated by cells, resulting in adaptation of cellular behavior. The process can be controlled on different levels but mainly depends on ligand concentrations and receptor expression levels. However, cells are able to sense, but also produce and release ligands, thereby allowing signaling processes that for instance are crucial during regeneration and inflammation processes. Following that, principle mesenchymal stem cells (MSCs) have been shown to follow the concentration gradient of 9 out of 26 chosen growth factors, of which the platelet-derived growth factor (PDGF) showed the strongest effect.10 Combinations of different growth factors have been shown to synergistically increase the cellular response, or to block each other and thereby to decrease the migration. Interestingly, thrombin could attract MSCs but not fibroblasts, explained by different sub-sets of expressed receptors. These findings show that cells possess a fine-tuning system, which allows them to react to different situations very specifically. It is possible that the PDGF that is strongly released after bone-injury will stimulate chemo-attraction of MSCs,11 which will migrate to the place of injury and differentiate into bone-forming cells under the stimulation by additional factors.12
Second, cell–cell recognition and interaction is crucial for a proper development and function of a multicellular organism. We can distinguish between stable and transient cell–cell interactions. Stable interactions are provided by tight- and gap-junctions, which allow the formation and organization of organ tissues. Transient cell–cell interactions are based on the interaction of cell-surface adhesion proteins and recognition of transmembrane and glycoprotein motifs on the extracellular site of one cell by receptors on the membrane of another cell. Transient bindings are crucial for processes like recognition of cells during immunological processes and migration. During bone remodeling cell–cell interactions strictly control the phases of periodic bone resorption and bone formation. The interaction between osteoclast-precusor cells and osteoblasts induces the formation of bone-resorbing osteoclast cells, while bidirectional signaling between osteoclasts and osteoblast precursors initiates osteoblast differentiation.13
Finally, cellular receptors play also an important role in cellular interaction with the non-cellular environment such as the matrix of connective tissue, bone and cartilage. Protein patterns from the connective tissue, like the three amino acid arginine–glycine–aspartic acid (RGD) motif, are directly recognized by receptors. Recent research has shown that cells are also capable of sensing mechanical features like elasticity,14 size15 and topography16 on the culturing surfaces, leading to changes in their behavior. By utilizing this knowledge cells can be directed to execute the required performance, such as the differentiation of MSCs to bone-producing osteoblast(-like) cells. For instance, when culturing the progenitor MSCs on a rigid matrix-material,14 surfaces that allow cell spreading instead of restricting the cell size15 or surfaces featuring disordered nano-scale pillars instead of ordered16 the cells will differentiate into the osteogenic lineage. These properties could allow a rapid repopulation on the implant surface with osteogenic cells, thereby inducing osteogenesis.
Although some implantable biomaterials that feature protein motifs or release chemo-attractants and growth factors to stimulate cellular behavior have been studied, in this review we will mainly focus on the interaction of cells with nano-topographical features of implants, how these can be manufactured and characterized.
Characterization of surfaces
A plausible starting point for biomaterial-implant design is the observation of local conditions and environment in the living organism. Most tissues will feature a hierarchical organization ranging from macroscopic scales to sizes in the nanometer-range. Such strongly standardized and systematic arrangement of certain tissues can provide unique and pivotal clues about tissue–cell interactions. For example, collagen in the extracellular matrix (ECM) that is packed in dense parallel nano-sized fibers, which not only provide strong mechanical properties,17 but seem also crucial for cell-migration.18 This knowledge can be applied in biomaterials-design, for instance as production of surfaces having nano-grooved structures can partly mimic collagen-like topography.19 Moreover, the topography can be varied thereby providing a fine-tuning mechanism to manipulate cellular behavior. However, one crucial part when reproducing such advanced surface features is the characterization of living tissues as well as biomaterials allowing a goal-oriented design and optimization of implants. In this part, the most frequently used methods for analysis will be presented, which can be subdivided in microscopic, physical and chemical analysis.Of course, routine microscopic techniques can be used for determination of tissue-properties like surface macro- and micro-topographical structure size and feature distribution. Morphology of single cells and cellular organelles can be obtained using the same techniques giving more insight in the dimensions of cellular-movement mechanisms, membrane structures and how these can be influenced by biomaterial topography. Conventional optical microscope techniques could be a useful and easy to use method for surface analysis. However, due to the nature of visible light-waves, the maximal resolution of the microscopes is limited to about 200 nm20 and thus unsuitable for more detailed characterization of nano-metric features, which by definition are below 100 nm.21 Lately, the development of new generation lenses22 and several fluorescence based techniques23–26 could reduce the theoretical diffraction limit to dimensions of only few nanometers. Although so far not being used for surface analysis, these so called super-resolution microscopes might become a helpful tool in that area of research.
Nowadays one of the most extensively used techniques for surface-imaging is electron microscopy (EM) that utilizes a dense electron beam to scan the probe of interest. Electrons interact with substrate-surface atoms and can penetrate, be absorbed or reflected by the material. Scanning electron microscopes (SEM) produce contrast images with a resolution of less than 2 nm by detection of reflected electrons.27 One disadvantage of SEM is the need for sputtering of conductive material and placement of the specimen into a vacuum chamber, making a trustful analysis of nanostructure biological samples challenging. This problem can be resolved by the use of the environmental SEM technique, which works following the same principles but without the need for electrical conductivity and vacuum environment27 reaching image resolutions that are comparable with that of SEM.28 Also, the transmission electron microscope utilizes electrons for image acquisition, but contrary to SEM detects the electrons that are penetrating the sample, and allows resolutions down to single angstroms.29
Although SEM and TEM provide excellent resolution dimensions, the obtained quantitative information is limited to the longitudinal axis. This disadvantage can be circumvented by the use of the atomic force microscope (AFM), which combines mechanical interaction and imaging.30 Although a disadvantage of AFM is a slow scanning process, it is outweighed by the fact that measurements can be done with living tissues, almost without preparation and at a resolution of atomic sizes.31
A structure perfectly rebuilt from a biomaterial does not necessary mean that it will behave in the same manner as the real tissue it is mimicking during in vitro or in vivo experiments or at a later time-point during clinical applications. Adverse physical properties can interfere and bias the interaction of the substrate with living cells and tissues. For this reason factors like roughness, stiffness, energy and charge should also be determined, compared and eventually optimized.
Roughness analysis of surface topographies has become a routine method in material design.32–34 One of the reasons is the observation that cellular function strongly depends on the grade of roughness,35,36 which has also been confirmed by in vivo experiments.37,38 Roughness can be analyzed by profilometric techniques, which can be subdivided into mechanical contact and optical measurements. Contact methods are based on physical interaction of a stylus or a cantilever with the surface topography. Stylus profilometers and AFM are examples that due to small dimensions of the interacting tip can sense roughness features in the nanometer range. The non-contact optical profilometers make use of a beam of light that is reflected by the surface and picked up by a detector. On a completely plane surface, the angle of entry would result in a predicted same dimensional angle of reflection. However, when hitting a rough surface, the light detection would occur with a non-predicted angle of reflection, which will be relative to the grade of roughness. No need for physical interaction with the surface is a big advantage of the optical techniques, since it is faster and more cost-effective. However, because of the diffraction limit of light the lateral resolution is less accurate. Therefore similar methods like X-ray reflectometry, which is based on reflection of X-rays from the surface, can be used for roughness measurements at the nanometer scale.
Another important physical characterization method is the determination of surface-wettability, usually assessed by contact-angle measurements. High surface tension will result in a low contact angle, and low surface tension in a high contact angle between the edge of a liquid-drop and the surface it is placed on (Fig. 1). However, measuring of wettability on nano-topographic or even porous materials remains challenging, since the measured angle will reflect not only the surface energy, but also the topographic features. Advancing and receding contact angle tests can be performed to increase the sensitivity of this approach for patterned surfaces. Another disadvantage of the test is that it can only give an average value of the surface-area underneath the droplet and thereby is not suitable to study single isolated nano-sized features. Nevertheless, differences in wettability seem not only to have an influence on liquid interaction with the surface, but have also been shown to result in changes in cellular behavior. When increasing the wettability of titanium discs by argon plasma treatment, Duske et al. showed fibroblast spreading was significantly higher on treated compared to non-treated samples.39
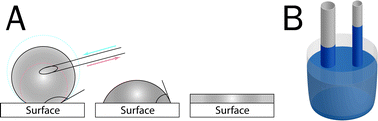 | ||
| Fig. 1 Wettability and capillary action. (A) Wettability, the property of a surface to interact with liquids, can be defined by placement of a liquid drop on the surface and measuring their contact angle. Low wettability will result in a high contact angle (left), high wettability in a low contact angle (right) between the edge of a liquid-drop and the surface. Advancing and receding contact angle tests can be performed to increase the sensitivity of this approach. (B) Capillary action can be visualized by dipping a thin glass tube into water, which instantly will begin to rise. The height of the water column is relative to the diameter of the tube. | ||
For some purposes, like the above mentioned mimicking of collagen fibers in vitro by using nano-grooved structures, capillary forces should also be taken into account. These for instance can be observed when dipping a thin tube into water, which instantly will begin to rise (Fig. 1B). Anisotropic wetting measurements, consisting of a combined wettability test and liquid dispersion measurements,40 can be performed to allow relative estimations of the capillary forces.
Cells are also able to sense and react to surface stiffness or elasticity41 as has been shown by the interesting finding made by Engler et al. when culturing MSCs on surfaces having different elasticity.14 While MSCs cultured on soft surfaces differentiated into neuron-like cells, culturing on intermediate and rigid materials drove the cells into entering the myogenic and osteogenic differentiation-pathways respectively. AFM interaction with the surface and the micro-indentation (MI) technique can be used for surface stiffness characterization. Since the force of the AFM cantilever that is affecting the surface is adjustable, the counterforce of the surface can be interpreted as the surface stiffness. For MI, a diamond stylus of predefined geometry is pressed against the surface with a predefined force, the data of which are integrated and used for stiffness value calculations. The same methods can also be used to define elastic properties of cellular membranes and how these are affected by surface topographies.
AFM can also be used to evaluate the adhesion forces of a nano-topographic surface at a high resolution and precision.42 The measured adhesion force is often the sum of various separate forces like the Van der Waals forces, electrostatic forces, capillary forces and chemical-bond forces. For the measurement the cantilever is brought into contact with and removed from the surface, while the forces that act on the cantilever during both motions are recorded. Depending on the experimental setup the material of the cantilever as well as the functionalization of the cantilever-surface can be performed. Chemical groups, proteins, or even cells can be attached to the cantilever tip, thereby allowing the measurement of cell-surface adhesion interactions.43 However, when measuring surfaces with nano-topographies the relative roughness has to be taken into account for which the calculations should be corrected.44
As implant-materials are confronted with a wet local environment full of charged ions, proteins and cells when brought into a patient, it is of high importance to take the zeta potential (Fig. 2) into account when designing and testing a biomaterial.45 Charged particles in a suspension medium are surrounded by two layers of ions. The inner “fixed” or Stern layer consists of strongly bound ions, the outer “diffuse” layer of loosely bound ions. During motion and interactions of the particle, the outer ions begin to move, thereby leading to a shear effect between the two layers. The arising electrokinetic potential—the zeta potential—can influence the behavior of these particles and has been shown to be utilized by evolution for natural behavior of living systems.46 The surface zeta potential can be calculated from streaming potential experiments and allows estimations of available functional groups and thereby also of the overall charge of the surface.47 Similar to the wetting experiments, zeta potential measurements can only give information about the average potential of the measured surface.
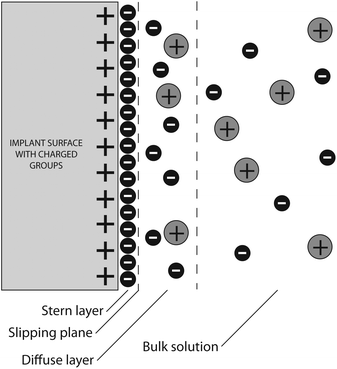 | ||
| Fig. 2 Schematic overview of ion-distribution at the surface of positively charged implanted biomaterial originating in zeta potential. Polarized particles in the suspension medium are surrounded by two layers of ions. The inner “fixed” or Stern layer consists of strongly bound, and the outer “diffuse” layer of loosely bound ions. During motion and interactions of the particle, the outer ions begin to move, thereby leading to a shear effect between the two layers inducing the development of an electrokinetic potential. | ||
Chemical characterization can be used to obtain spatial and quantitative information about the composition of surface elements. The underlying theoretical background of many characterization techniques is the fact that every element has its own specific and unique atomic structure, which allows element-differentiation based on radiation produced by atoms as a result of electron ejection. Energy-dispersive X-ray spectroscopy (EDS) makes use of electron-beams that, when hitting and ejecting an electron from an atom, will produce X-rays. Since the same process occurs as a byproduct during electron microscopy, EDS is often combined with (S)EM, allowing parallel analysis and mapping of visual and chemical properties of a material in the range of only few nanometers. X-Ray photoelectron spectroscopy (XPS) uses X-ray-beams instead of electrons. However, it is following the same basic principles of element-characteristic measurements of energy spectra that are produced by electrons ejected by X-rays. The rather poor XPS resolution of several micrometers can be improved with X-ray photoelectron emission microscopy allowing resolutions down to 30 nm.48,49 Exploitation of ion beams can also be used for characterization of the chemical surface composition in secondary ion mass spectrometry. Also here goes a similar principle. When an ion hits an electron, it can generate secondary electrons that are detected by a mass spectrometer. The lateral resolution of this technique goes down to about 50 nm.50
The advantage of the Raman spectroscopy technique compared to the previously described characterization methods is the possibility to obtain not only the atom composition, but also molecular structures of the surface-compounds. This approach is based on the interaction of monochromatic photons with molecular electrons. Similar to fluorescence, the photon will interact with one of the molecules’ electrons thereby exciting it to a higher energy state. Upon relaxation of the molecule, the electron will return to a lower energy state, while releasing the remaining energy in form of a photon. Contrary to fluorescence where the emitted photon always has less energy than the exciting photon, Raman scattered electrons can either remain at the same energy state, loose, or gain energy thereby shifting in wavelength. The resulting spectrum of the emitted light, however, molecule-specific and can therefore be used to make conclusions about the surface chemistry. Additional to molecule structures, Raman spectroscopy can be used for determination of crystal structures.
X-Ray diffraction (XRD) techniques utilize X-rays that are scattered by surfaces under certain conditions. Therefore the sample needs to be scanned by, or rotated in front of the X-ray-beam. Information about scattered angle, wavelength or the polarization of the scattered X-rays can be used to determine the crystal structure, texture and orientation, crystal phases, lattice parameters, but also chemical compositions of the surface material at the nanometer level.
Manufacturing of nano-patterned surfaces
Characteristic features of the tissue structure ideally can be replicated for in vitro or in vivo experiments. Most of the basic principles used for production of surface topographies are the same as (or at least have evolved from) techniques that were used for already more than 50 years for the semiconductor industry. In 1965, Gordon Moore stated that the number of transistors on integrated circuits is doubling almost every two years.51 This statement has proven to be true and is nowadays known under the term of Moore's Law. However, to keep such pace it was necessary to miniaturize all produced materials, leading to an immense investment and drive of streamlining development-pathways allowing fast and parallel production of microchips at a cheap price. In particular, the focus on cost-effectiveness has allowed these techniques to step into life-science research.A huge number of new techniques for nano-topography production have been invented so far and can be used, separately or in combination, allowing control over the generation of specific surface features. Description of all of these methods and combinations would go far beyond the scope of this review and only the common used methods will be described. Approaches can be subdivided into production of rough or non-organised surface structures, like chemical etching, machining, polymer demixing, self-organization, laser ablation/deposition and electrospinning, production of semi-ordered structures by anodic oxidation and production of organized textures produced by writing and replicating techniques.
Depending on the scientific question to be answered, not every experimental setup is based on ordered nano-topography. The production of non-ordered surface-features is usually less elaborate and less expensive than ordered-surface production techniques, nevertheless allowing research on nano-patterned surfaces. The most straightforward method is chemical etching as it is applicable for practically all solid materials using acids, bases or other aggressive chemicals like peroxides. Such agents will remove parts of the used material thereby introducing random topographic features. By variation of chemicals, their concentration and the time of exposure the amount of etched away material can be regulated, thereby allowing certain control over the dimensions of the produced features that can reach dimensions down to the size of few nanometers52 and probably beyond that. Another easy to perform technique is machining, which is a mechanical introduction of scratches resembling grooves into the surface of a solid material. The size of the grooves is dependent on the force and size of the scratch inducing aperture. A third inexpensive straight forward method is polymer demixing. This phenomenon is based on phase separation of different immiscible polymer blends, like the combination of polystyrene and poly(4-bromostyrene), when mixing and using them for spin casting on silicon-wafers. The dimensions of the thus created topographies like pits, island and ribbons can be controlled by varying the polymer ratio, concentrations, solving agent, humidity and the speed of spinning, being at sizes of about 10 nm.53 Self-organization is a “bottom-up” process, meaning that single (nano-) particles aggregate to form higher order structures under the influence of thermodynamic forces. Despite the fact that self-organization again cannot be used for the production of ordered textures, fine-tuning of the process can induce the formation of semi-ordered rough structures in the nano-scale range.54 Although the above described methods can be used to obtain surface features in the nano-scale range, usually also formation of structures of higher-order sizes will occur.
Laser ablation and deposition are easy-to-implement and cost effective techniques that can be exploited to change surface properties and to produce nano-size topographic features. Therefore, laser ablation makes use of irradiation of various solid material-surfaces by a high-energy pulsed laser system. The photon energy is absorbed by electrons on the material-surface, thereby inducing material heating and evaporation or formation of plasma. By adjustment of the laser-wavelength, pulse repetition and dwell time it is possible to fine-tune the dimensions of the ablated material. The ablated material can be further transferred to other materials and surfaces during the process of pulsed laser deposition, where it can form roughness- or pillar-like structures. Both techniques have been shown to be able to produce features of sub-micron and nano-sized (down to 16 nm–20 nm) dimensions.55,56
Another method to produce nano-fibers is the electrospinning method using various polymer materials. A polymer is mixed with a solvent to form a viscous solution (also melting can be performed to avoid solvent incorporation into the end-product), which is extruded from a needle thereby forming a droplet at an electrode. The droplet can be electrically charged leading to the formation of a cone structure arising due to an electrical field. Increase of the electrical field will result in the ejection of a polymer-jet from the cone into the direction of a grounded collector, where the fiber will randomly form a mesh-like membrane structure. By optimizing factors like polymer properties, viscosity of the solution, conductivity, electrical properties, distance between needle and collector, humidity and temperature it is possible to fine-tune the resulting product and to obtain fibers with average diameters below 100 nm.57 Adjusting the method of collecting through a water bath allows for scaffolds of near infinite thickness.58
Anodic oxidation can be used to self-organize nano-tubes on titanium- or various titanium alloy- surfaces.59,60 The substrate is brought into a fluoride-based electrolyte-filled two-electrode electrochemical cell and connected to an anode, while platinum is used as the cathode. An increase of anodizing voltages induces a topography change of the titanium surface from porous, over particulate to nano-tube featuring at voltages higher than 10 V (reviewed by61). Nano-tube properties like pore diameter, wall thickness and tube height can be fine-tuned by changing the anodizing voltage, electrolyte composition and time of anodizing and feature dimensions in the range of few nanometers (diameter 22 nm–110 nm; wall thickness 7 nm–34 nm; height 200 nm–6000 nm).61
Writing techniques form “top-down” processes, in which direct removal of bulk material can be used for production of (semi-)ordered nano-topography surfaces. Photolithography is the progenitor of many used nano-manufacturing techniques all following the same principles (Fig. 3 left). The fundamental principle for the lithography approach is the fact that some materials can be influenced to change their properties by interaction with energy from light sources. Upon exposition to light, positive resist materials change their physical state and become soluble when in contact with a developer solution. Negative resist-materials behave in the opposite way and cross-link, becoming insoluble after illumination. Dependent on the experimental setup, a wafer with a silicon-dioxide surface is coated with either a positive or negative photoresist. On top of the resist a chrome-layer photomask is placed, into which a desired pattern is reproduced. When illuminated, light hitting the metal will be reflected, whereas passing light will interact with the resist. Subsequently the mask is removed and the wafer is exposed to developer solution. The removed photoresist will expose the silicon oxide layer on the wafer that subsequently is etched away. In the last step, the remaining resist is stripped away leaving the wafer with the positive or negative silicon oxide replicate of the photomask. The wafer can be used afterwards as a template for the reproduction of nano-topographic structures into polymers (Fig. 3 right), allowing a theoretically unlimited production of replicates with only one single master-wafer.
Photolithography can also be used to generate patterns without using a mask. However, comparable to microscopy, the resolution of patterns thus created is strongly dependent on the wavelength of the used light source. Mercury lamps have been used for a long time because of their strong near-ultraviolet (UV) peaks that could be isolated and used for the production of patterns in the sub-micrometer range.62 With the development of deep UV-light excimer lasers the use of lower wavelength light sources has pushed the resolution limit down to dimensions below 100 nm. Other examples of radiation sources that can be exploited for lithographical patterning are ions, X-rays and electrons.
One of the commonly used lithography-based techniques in life-science research is electron beam lithography (EBL) (Fig. 4 left), which has been used for the generation of pits, pillars and grooves in several studies.16,63–69 An electron-sensitive polymer resist, coated on a substrate, is exposed to a high-energy electron beam thereby allowing precise and maskless patterning. Although rather elaborate, timely and thus expensive, EBL allows software-controlled generation of isolated features down to a resolution of 5 nm on flat surfaces.70 However, due to electron scattering, patterning of larger surface areas reduces the accuracy to resolutions between 30 and 40 nm.70
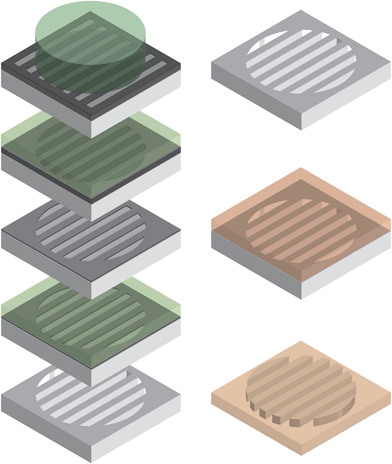 | ||
| Fig. 3 Photolithography production of grooved patterns and replication. Left: photolithography is based on the fact that resist material that has been exposed to light becomes soluble in development solutions. A physical mask can be used to reproduce a pattern into the resist by shielding it from light. The non-protected regions will dissolve after contact with the developer solution, thereby uncovering the underneath material, which is usually silicon. Etching and the final removing of the photoresist will remain the replicated (nano-)pattern within the substrate. Right: the patterned silicon replicate can be used as a master for reproduction of the pattern into different polymeric materials. Replication allows a cost-effective production of practically unlimited (nano-)patterned copies of one single master. | ||
Another maskless lithographic technique to create periodic nano-structures is laser interference lithography. Additional advantages of LIL are no need for finalizing processes like etching or photoresist development, production of nano-topographies with sizes less than 30 nm71 on surface-materials like ceramics, metals and polymers and areas up to square meters. The patterns are produced directly onto the surface by an interference of two or more coherent light beams from one laser source. Often one laser-beam is divided into two that are recombined at the surface plane resulting in grooved structures.72 However, use of additional laser-beams can be exploited to produce other types of topographies. While three-beam interference can be used for hexagonal dot or hole formations,73 applying four beams will result in rectangular patterns.71,74
When not interested in strongly ordered textures, colloidal lithography (Fig. 4 right) can produce features of about 20 nm75–77 cost effectively and over large areas. Instead of a pre-produced rigid mask, colloidal lithography is making use of randomly dispersed colloid nano-particles. Using particles of the same dimensions allows production of surfaces having features of identical shape and height. One additional dimension of order can be controlled by the adjustment of the electrostatic particle–particle interaction during the adsorbing process, resulting in a specific distance of separation.77 After the particles have been immobilized on the surface they and the surface material surrounding them can be etched away by using ion-beams to produce nanocolumns78 or by film evaporation for nano-pit production.62
In vitro studies
Experiments showing that cells can respond to, and change their behavior dependent on the underlying surface have been performed since more than a century ago. When culturing frog embryonic cells in 1911 Ross Harrison noticed that cells showed differences in attachment, migration and morphology on different topographies.79 That process was confirmed by Paul Weiss, who coined the term “contact guidance”80 and by Curtis and Varde, who could prove that the cellular response indeed directly resulted from the experience of surface topography.81 Later it was proposed that the alignment to micro-grooved patterns that was observed by Rovensky et al.82 could be varied by manipulation of substrate-characteristics83 and included the interaction between cellular focal adhesions and the surface.84 The development of micro- and nano-fabrication techniques led to an increase in experimental setups using micro- (reviewed by85–88) and nano-patterns for in vitro experiments.The next chapter will focus on the nano-topography induced changes and control of cellular behavior, which can be measured on different levels. Since direct interaction between cells and surface are crucial for cellular function89 factors like kinetics and force of cellular adhesion to a surface, migration, deposition of ECM-proteins and minerals, changes in gene expression, proliferation and differentiation can all be obtained and related to the experienced topography. The most commonly used topographic features are different grades of stochastic roughness, (semi-)ordered tubes, pits and pillars, and highly organized grooves.
Various experiments have shown that surface-roughness can influence cell behavior like adhesion, migration, proliferation and differentiation. Surface nano-roughness introduced by acid-etching usually is reported to have positive influence on cell activity as for example has been shown by Takeuchi et al., who found that when cultured on dual acid-etched titanium surface with a roughness average (Ra) of 110 nm rat bone-marrow derived osteoblast differentiation increased compared to machined titanium samples (Ra = 49 nm).90 Similar observations have been made by de Oliveira et al. on acid-etched titanium surfaces when using calvaria derived osteogenic cells.91,92 Contrary to this, rat periosteal cell-differentiation into osteoblasts, which could be seen on machined titanium surfaces (Ra = 49 nm), was inhibited on acid etched surfaces (Ra = 183 nm), while chondrocyte specific genes were activated.93 The proliferation of periosteal cells was measured to be increased on machined surfaces compared to the acid etched substrates. However, the aspect of nano-roughness being the determining factor for specific effects on cellular behavior remains controversial, since the roughness-generating methods generate features not exclusively having dimensions in the nano-range. Moreover, when culturing human osteoblast-like cells on ordered and non-ordered surfaces with similar surface roughness Ball et al. observed that ordered patterns induced higher metabolic activity and alignment than non-ordered features.94 This observation shows that cells do recognize patterns and necessitates studies on (semi-)organized topographies to elucidate the underlying phenomena of cellular response.
It has been shown that acid etching can be used to generate a surface that in combination with TiO2-sputtering can self-assemble to form nodule-like structures of similar sizes down to dimensions of about 100 nm.95,96 Using these methods Kobo et al. have studied rat osteoblast behavior on surfaces with micro-pits, which either featured nano-nodules (diameter = 100 nm, 300 nm, 500 nm) or had no nodular features.96 The group found osteoblast function was stimulated by the nano-nodules, which on the other hand had no effect on fibroblast function. The 300 nm nodule pattern showed the most prominent effect concerning cell attachment, differentiation and proliferation. Also, the biomechanical testing using a rat femural model showed the advantage of the nano-nodules on the implant surface. Forces needed to push-in the implant after 2 weeks of healing were 3 times higher with the 300 nm structures than the force that was needed for the micro-pit only implants. Regarding the easy to use method and the possible benefit that could be achieved in clinics by adapting the surface production method, it would be interesting to evaluate the topography in more detail in an in vivo model.
Straightforward production of nanotubes by anodic oxidation improves hydrophilic properties of titanium-surfaces97 and shows a semi-ordered nano-sized pattern,60 which has been applied for in vitro studies. When culturing rat MSCs on titanium oxide nanotubes (pore size 80 nm; depth 400 nm) Popat et al. observed higher adhesion, proliferation and viability during the first 7 days of culture compared to non-oxidized titanium surfaces.98 Moreover, cells cultured on the patterned surface demonstrated higher alkaline phosphatase (ALP) activity and 50% more matrix mineralization. Similar results have been found by Yao et al. when observing osteoblast behavior after 3 weeks of culturing99 and Yu et al. after 2 and 3 weeks of pre-osteoblast culturing.100 Likewise, chondrocyte functions like adhesion, synthesis of collagen, ALP activity and mineralization could be increased by nanotubes (pore 70–80 nm: depth 100–200 nm).101 In combination with electrical stimulation, Ercan and Webster were even able to increase osteoblast long-term functions like ALP synthesis, collagen type I synthesis and mineral deposition on nanotube surfaces (pore 40–60 nm; depth 80–120 nm).102 The last result shows that providing combinations of cues to cells can result in a synergistic improvement of desired cellular functions. Nevertheless, it should be taken into account that the same combinations could also increase unwanted effects. However, the positive effect of nano-tubes on the behavior of bone forming cells and the rather easy to perform production method also on non-planar surfaces could be utilized for implant production.
When not specifically interested in the material itself, but more in the physical interaction between topographic features and cells, polymer-based techniques can be used for large array studies. When comparing osteoblast adhesion properties on poly-L-lactic acid (PLLA)/polystyrene demixed thin film blends that resulted in different nano-topographic features, Lim et al. demonstrated nano-islands (height 9–21 nm; area 0.01–0.06 μm2) having a stronger adhesion-stimulation than nano-pits having comparable dimensions (depth 3–29 nm; area 0.01–0.18 μm2) and smooth surfaces.103 The same group found osteoblast-proliferation and differentiation increased on shorter islands (height 11 nm) compared to higher islands (height 38–85 nm) produced by demixing of polybromostyrene and polystyrene.104 Cell adhesion was stronger on smaller islands, which could be seen by a prominent focal adhesion formation and cytoskeleton formation. Similar results have been obtained by Dalby et al. who performed several polystyrene–polybromostyrene demixed nano-island based studies, showing that fibroblasts and endothelial cells adhered stronger to and formed greater focal adhesions on shorter islands (13 nm) than on higher islands (33–45 nm).53,65,105–108 On the other hand higher islands (height 160 nm; diameter 100 nm; centre-to-centre spacing 230 nm) that have been produced by colloidal lithography have been shown to decrease adhesion and proliferation of fibroblasts54,87,88 and osteoblasts65 when compared to flat controls. Although cells react differently to the height of nano-islands, the density of the features seems to have less influence. When evaluating the adhesion, proliferation and growth of osteoblasts and macrophages cultured on 110 nm high islands Rice et al. have found no significant differences that have been induced by different nano-island densities (3–43%).109 Interestingly, not only migration, proliferation and differentiation can be affected by nano-islands. When studying mouse osteoblast cells on nano-islands with dimensions between 11 and 38 nm, Hansen et al. demonstrated increased cell stiffness when nano-topography was introduced.110 Also, mechanosensitivity can be influenced by nano-patterns as has been shown by Salvi et al., who have studied mechanosensitivity of hMSCs on nano-islands (height 10–80 nm).111 They observed a stronger intracellular calcium increase as a response to fluid flow on smaller islands compared to cells cultured on higher islands and proposed that topography, synergistically with mechanical cues, could increase downstream signaling and thereby proliferation and differentiation.
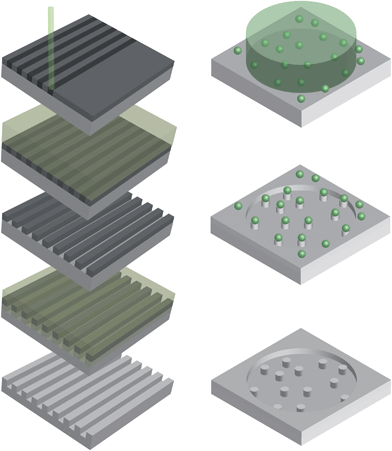 | ||
| Fig. 4 Lithography based nano-topography manufacturing methods. Left: electron beam lithography makes use of a high-energy electron beam to expose an electron-sensitive resist layer, thereby allowing a maskless introduction of nano-dimensional features into a substrate. The exposed area can be removed during development and the underlying area is etched away. After removing the non-exposed resist, the substrate surface will contain nano-sized features. Right: colloidal lithography makes use of randomly dispersed nano-sized colloid particles that serve as a physical barrier against ion bombardment of the substrate-surface, which will etch away the particles and the particle-surrounding area. After removing the remnants of the particles by a lift-off process the nano-sized features will remain in the substrate-surface. | ||
The height/depth of nano-features seems to be a crucial factor during the initial phase of cell–surface interaction. Cells cultured on nano-pits, which are more uniform in size than nano-islands, also show a diminished response. Several experiments have been performed by the group around Curtis et al.,64 Dalby et al.16,65,68,69 and Biggs et al.63,66,67 on nano-pit structures (often in combination with nano-pillars), which have been produced by electron beam lithography in combination with dry etch techniques on silicon surfaces. By optimization of the EBL technique an area of 1 cm2 could be produced within 1 hour and replicated into polymers such as poly(methyl methacrylate) (PMMA), polycaprolactone or polycarbonate. When cultured on nano-pits (diameter 37–120 nm; centre-to-centre spacing 100–300 nm; high 160 nm) cell spreading of fibroblasts was significantly decreased on patterned substrates compared to flat control substrates.112 Moreover, proliferation occurred at a higher pace on the control flat surface. Similarly, when culturing human fibroblasts on PMMA nano-pillars produced by colloidal lithography (diameter 100 nm; center-to-center spacing 230 nm; height 160 nm) reduced adhesion, a less organized cytoskeleton and decreased growth was observed.113 A strong formation of filopodia could be seen on the pitted surfaces and direct interaction with the substrates occurred on 75 and 120 nm pits.112 Nano-pit recognition by cells seems to be a size-specific event with a lower limit of about 35 nm as has been shown for fibroblasts.65,112 Also, osteoblasts cultured on ordered nano-pit patterns displayed decreased adhesion ability compared to smooth control surfaces.63,66,67 Interestingly, osteoblast spreading increased when the pit-pattern became more random, although still being less when compared to plane controls66 and differentiation-specific gene and protein expression was generally induced on nano-patterned surfaces in MSCs and osteoblasts.16,66
Nano-grooved structures can be produced by various techniques and in different forms. Several factors of these topographic features can have nano-sized dimensions, such as the pitch, the height of the ridge and the depth of the groove. The grooves themselves can be rectangular, V-shaped, truncated V-shaped or U-shaped, which is often defined by the limitations of the production mechanisms. When mammalian cells are cultured on nano-grooves, it has been shown that cells do not penetrate grooves narrower than 2 μm or deeper than 500 nm, but in contrast primarily bind to the ridges.114,115 However, a study of contact-guidance induced morphological behavior on nano-grooved (width 20–1000 nm and depth 5–350 nm) surfaces showed that fibroblasts could react and aligned to groove sizes down to 100 nm in width and 35 nm in depth.116 Alignment to nano-grooves (Fig. 5) has also been found for stem cells,117 smooth muscle cells,118 epithelial cells,119 endothelial cells,120 kidney cells121 and fibroblasts.116,122 Limitations of topography-induced orientation-change on nano-grooves having dimensions beneath 100 nm have also been reported by other groups and seem to be rather cell-type specific.19,119
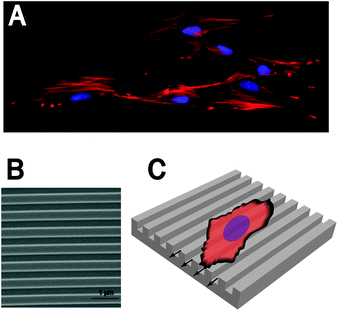 | ||
| Fig. 5 Cellular response to (nano-)grooved surfaces. (A) When cultured on nano-grooved surfaces cells feature elongated spindle-like morphologies. (B) SEM obtained image of a 500 nm pitch grooved polystyrene surface. (C) The cellular elongation occurs parallel to the groove direction. | ||
Lamers et al. have systematically studied the nano-scale topography influence on osteoblast behavior.19 Therefore different nano-grooved patterns (pitch sizes between 40 and 2000 nm; depth 10–360 nm) have been created by EBL and LIL on silicon wafers and reproduced in cell culture plastic polystyrene (PS). Cell alignment to the grooves was evaluated and showed a significant response on grooves with dimensions down to 75 nm in width and 33 nm in depth. Interestingly, the cell-driven generated calcium phosphate deposition was found to be aligned to even smaller nano-patterns. Vinculin staining analysis showed that focal adhesions mainly resided on top of the ridges. Moreover, gene expression analysis demonstrated that osteoblast-specific genes were activated on the patterned samples, when compared to smooth controls. In a different experimental setup the group has found that also the ratio between the ridge and the groove of nano-grooved patterns can influence morphology and migration of osteoblasts.123 It was discovered that grooves having a groove![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ridge ratio of 1
ridge ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (especially 400 nm pitch) have induced the highest migration speed and were correlating with short focal adhesions.
3 (especially 400 nm pitch) have induced the highest migration speed and were correlating with short focal adhesions.
Yang et al. have investigated the influence of nano-grooved silicon surfaces, which were produced by EBL and dry etching, on morphology of osteoblast-like cells.124 All of them used different nano-grooved patterns (ridge![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) groove 90–500 nm in width and 300 nm in depth) featured increased cell-spreading after 4 hours and cell-alignment after 24 hours of culturing compared to flat surfaces. Furthermore, the measured cell-spreading area was decreased on all nano-patterned surfaces. Interestingly, the nuclei also showed an elongated, groove-aligned morphology, which was also confirmed by other studies on nano-grooved textures.125 The rearrangement of intracellular structures has been found also for other organelles using different substrate topographies and could be an important factor leading to observation of changes in gene expression, as reviewed by Dalby.126,127
groove 90–500 nm in width and 300 nm in depth) featured increased cell-spreading after 4 hours and cell-alignment after 24 hours of culturing compared to flat surfaces. Furthermore, the measured cell-spreading area was decreased on all nano-patterned surfaces. Interestingly, the nuclei also showed an elongated, groove-aligned morphology, which was also confirmed by other studies on nano-grooved textures.125 The rearrangement of intracellular structures has been found also for other organelles using different substrate topographies and could be an important factor leading to observation of changes in gene expression, as reviewed by Dalby.126,127
An example for the ability of nano-grooves to synergistically increase cell function has been described by You et al.128 Human mesenchymal stem (hMSCs) cells have been cultured in osteogenic medium for 8 days on nano-grooved (pitch sizes between 200 and 1200 nm; groove width between 150 and 600 nm) patterns that were produced by UV-assisted capillary force lithography. A significantly higher osteogenic marker expression was measured on the 150 nm grooved pattern compared to cells cultured on a planar surface and grooves wider than 150 nm. In a similar experiment, hMSCs were cultured on nano-grooves (pitch 700 nm–20 μm; width 350 nm–10 μm; depth 250 nm) produced by nano-imprinting and soft lithography techniques on polydimethylsiloxane (PDMS).125 Interestingly, the experiment also showed that the process of trans-differentiation into neuron-like cells in neuronal induction media could be significantly increased by the nano-grooved pattern. Moreover, Lee et al. were able to show that nano-grooved patterns can induce differentiation even in absence of differentiation medium.117 When cultured on grooves (ridge![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) groove width 350 nm; depth 500 nm) human embryonic stem cells (hESCs) increased the expression of neuronal differentiation markers and decreased expression of markers of other lineages.
groove width 350 nm; depth 500 nm) human embryonic stem cells (hESCs) increased the expression of neuronal differentiation markers and decreased expression of markers of other lineages.
Interestingly, when combining topographic cues from nano-grooved (width 300 nm; depth 60 nm) silicon surfaces and mechanical loading cues, which were simulated by periodic stretching of the material parallel to the grooves, the alignment of osteoblast-like cells to the grooves could be overruled and resulted in anti-parallel alignment.129 Moreover, in combination the two cues resulted in synergistic up-regulation of the genes involved in ECM generation and osteoblast differentiation. The development of such multi-factorial models can further aid in understanding the effectiveness of surface patterning and developing biomaterial surfaces with enhanced efficacy towards translation.
Considerable effort over the last decade was focused on the evaluation of behavior of primary-interest cells responding to differently patterned surfaces. Nevertheless, some groups have also studied also the interactions of materials with “fouling” components, such as various plasma proteins. Materials with different chemical properties have been investigated several times and have often been shown to have little influence on cell behavior, which could possibly be explained by adherence of ECM proteins that mask surface groups.89,130 For this reason, surface chemical cues could be of high importance only during the first moments after implantation and in so far usually seem important to cell behavior as they could specifically attract different subsets of proteins. Once adsorbed to the implant surface, the adhered proteins are the main interaction links between the biomaterials and cells.131 The amount of fibronectin for example has been found to be an determining factor for cell-attachment.132 However, nano-topographic features have been shown to interact with the cells during cell adhesion only limited in serum-free medium, but resulted in increased adhesion in combination with serum proteins.104,130 This is an interesting finding, which allows speculations about surface design not for cells, but more prominently for proteins as primary targets as has been studied by MacDonald et al.133
Wettability and charge of the surface will primarily determine the hydrophilic or hydrophobic properties and thus the overall charge of the proteins that will bind in first instance to the implant. For example it has been shown that albumin can block cell–implant interaction by binding strongly to hydrophobic surfaces so that it cannot be replaced by other ECM proteins.134 On the other hand, when binding to hydrophilic groups, adsorbed albumin can indeed be replaced by ECM proteins.134 These consequently will dictate cellular adhesion and migration processes. In particular, roughening has been shown to influence surface properties,135 usually resulting in a higher wettability,104,136 but its effect on protein adsorption remains controversial. Cai et al., were not able to show significant differences in amounts of adsorbed albumin and fibrinogen between titanium films displaying different degrees of nano-roughness.137 However, experiments by other groups with nano-features have shown a roughness-dependent protein adhesion. Sela et al. have studied the adsorption of plasma proteins on titanium surfaces modified using machining, acid-etching and acid-etching followed by grit blasting.138 It was found that most of the tested proteins preferably bound to surfaces having higher roughness. Similar results showing that roughness has a positive effect on protein adhesion have been obtained on surfaces of various other materials like tantalum,139,140 alumina,141 platinum142 and polymers.143,144 Nevertheless, the fact that some proteins are undergoing conformational change under certain of the above mentioned conditions should also be taken into account, since exposition of the organism unknown epitopes could result in adverse effects not only having an influence on cell–implant interactions, but could also provoke immune responses.
As stated above, after implantation of a biomaterial into a living system, the local environment will act on and interact with the material surface. The first wave of compounds to arrive at the surface is the body fluid mainly consisting of water, proteins and ions. Since an inflammatory response is provoked by the operation, secondary to arrive in high concentrations are white blood cells of the inflammatory system. Especially neutrophils and later macrophages will accumulate at the damaged area in order to protect the tissue from pathogens and by production of cytokines to attract additional immune system cells and to stimulate tissue repair. Cells that arrive last are the ones that the surface topography is often aimed at. These cells are actively producing the tissues and have the power to integrate the implant into the host organism. It would be ideal to consider all of the factors and cell types that get in contact with the surface when designing an implantable bio-material, since the interaction with soluble factors and cells can change the long term behavior of the implant in vivo. The properties of the surface will mainly dictate how and which proteins and ions will bind to it, thereby determining the later interaction with cells. Depending on the topographic features and sizes it is even thinkable that due to protein accumulation cells, which should be influenced by the surface, will encounter a completely different topography when getting in contact with the implant for the first time, which can ultimately lead to a different response than observed from in vitro experiments.
In vivo studies
The first period after implantation of a biomaterial into a living system is crucial for further implant–host development and will determine whether the implant will be integrated successfully into the organism or whether complications will occur. Close proximity to the epithelium can lead to extrusion of the implant, meaning that epithelial cells will form a continuous layer on the border between implant and tissue, ultimately leading to a physical displacement of the implant from the organism. Sometimes it is of advantage to have a “space holder” biomaterial that is destined to be degraded temporally while being replaced by host-material. On the other hand non-degradable biomaterial can evoke the so called “foreign body response”,145 which is often the result of the immune-system failure to remove a harmful compound by macrophages from the organism. After a period of time macrophages fuse in order to form giant cells that will produce chemo-attractants to recruit fibroblasts.146 In turn, fibroblasts will secrete a collagen-layer and fibrous tissue capsule around the material, thereby isolating and also “camouflaging” it from the local environment146 and allowing a physical separation of system-own from system-foreign.Although frequently observed after biomaterial implantation, encapsulation does not necessary mean a drawback, and in fact could even serve as a scaffold for bone regeneration processes like protein adhesion and hydroxyapatite nucleation.147 Nevertheless, generally fibrous encapsulation of bone implants is seen as a negative result, whereas direct contact of newly formed bone on the implant-surface is considered to be a positive outcome.148
So far the ability of implant-surface topography to influence the rate and quality of bone healing has been recognized.149 However, before application of in vitro favorable nano-topographies on implant surfaces can occur for clinical purposes, the biocompatibility, which by definition is the ability of a material to perform with an appropriate host response in a specific application,150 has to be evaluated. The difficulty to make the step from in vitro to in vivo is evident by the fact that fewer animal-studies have been published so far. One underlying reason for this could be the problematic reproduction of the nano-topographies on (concave) surfaces of implantable materials.
Only one single study can be found that uses polymer implants having an ordered nano-pattern surface topography. For this study Giavaresi et al. made use of colloidal lithography and hot embossing for polymer-reproduction of nano-scale pillars (depth 100 nm; width 120 nm) and pits (height 160 nm; width 100 nm).151 Polymers featuring the topographic cues and a control non-structured polymer were subcutaneously introduced into rats and a histological and histomorphometric analysis was performed after 1, 4 and 12 weeks. Generally, nano-pit structures increased the fibrous capsule development and decreased vascular density, while nano-pillars increased fibroblast cellularity of the fibrous capsule and the vascular density.
Several groups have studied the in vivo behavior of polymer bio-composites with roughness nano-topography allowing comparison between the materials.152,153 One interesting finding was made by Wu et al., who found poly(ether ether ketone) (PEEK), which is known to have good biomechanical properties, to perform better when used in combination with nano-TiO2 (n-TiO2).152 Four different substrates were prepared, which were smooth (Ra > 0.1 μm) PEEK and n-TiO2–PEEK, and rough (Ra = 1 μm–2.2 μm) PEEK and n-TiO2/PEEK. Osteoblasts cultured on the n-TiO2–PEEK nanocomposite and PEEK control substrates preferably attached to surface areas where TiO2 was present and showed a stronger attachment capability to the rough surface of the n-TiO2–PEEK nanocomposite. Moreover, in vivo results using a Beagle dog tibia model showed a twice larger bone volume around the rough n-TiO2–PEEK nanocomposite implants compared to the rough PEEK implants. The results show a clear advantage of titanium in vivo and it would be very interesting to compare these implants with pure titanium implants in order to validate the benefit of such composite-products.
In titanium bone implant material, it remains more challenging to produce ordered nano-size features on large surfaces at an affordable price. For this reason all of the studies made use of non-ordered or semi-ordered topographies.
One example for semi-ordered topographies are nano-tubes, which can be produced by chemical anodization. Popat et al. produced nano-tubular titanium oxide surfaces having a pore size of 80 nm and length of 400 nm.98 The substrates were implanted subcutaneously into rats and showed no induction of fibrous scar tissue formation, suggesting that neither the material, nor the topography are inducing adverse immune responses. The authors were concluding that nano-tube architectures on implant-surfaces could promote long-term osseointegration. A later study by Bjursten et al. confirmed this theory when they compared bone bonding capacities between grit-blasted (roughness 6 μm) and by anodization produced nanotube (pore size 80 nm and length 250 nm) titanium dioxide implant surfaces.154 For this purpose, the implants had been implanted into the tibia of rabbits for 4 weeks, after which pull-out testing and histological analysis were performed. Histology clearly showed greater bone–implant contact area and increased bone formation resulting in a nine-fold higher strength that was necessary to pull out the nano-tube implant compared with the grid-blasted implant.
A more systematic study using titanium dioxide nanotubes in vivo has been performed by Wang et al.155 in order to investigate implant with bone interaction. Therefore nanotubes with different diameters (30 nm, 70 nm and 100 nm) produced by anodization and a machined control have been implanted into cranial defects in minipigs. Like in the above described study histology analysis at 4, 5 and 8 weeks after implantation showed an increased bone-to-implant contact for all nano-patterned surfaces compared to the machined control. Also osteogenesis-related gene expression of alkaline phosphatase, osterix, collagen-I and tartrate-resistant acid phosphatase was upregulated in the bone attached to the implant. In particular, the 70 nm implants showed the significantly strongest effect.
Also, interactions between soft tissues and titanium dioxide nanotube implants have been studied. Smith et al. have compared by anodization produced nanotubes (pore size 70 nm and length 250 nm) with titanium dioxide control surfaces (surface roughness less than 1 μm) after implantation into the abdominal wall of rats.156 The group has shown that nanotube implants induced less encapsulation and lower nitric oxide production at the implant surface. The authors propose that these results may occur due to increased catalytic properties of titanium dioxide on the nanotube structure.
Another commonly used technique for the generation of nano-topography on titanium surfaces for in vivo studies is the deposition of discrete calcium phosphate (CaP) or hydroxyapatite (HA) particles thereby increasing the nano-scale roughness. The work of Meirelles et al. compares the effect of nano-hydroxyapatite coated titanium surfaces and nano-titanium particle coated titanium surfaces on early bone response in a rabbit tibia bone model.157 The average surface roughness of the HA-coated implant was with about 22 nm with a feature diameter of about 30 nm slightly higher than the topography of the titanium particle-coated surface (surface roughness 10 nm and feature diameter 24 nm). Although HA is a main component of bone and could be thought to be an excellent biomimetic material,158 the texture did not increase the bone formation compared to the nano-titanium sample.
In two studies Mendes et al. have observed osteoconduction in a rat femur bone model, where they compared nano-CaP crystal coated titanium and titanium alloy surfaces with uncoated implants.159,160 They report that nano-CaP (size 20–100 nm) coating induced a significantly stronger osteoconduction than non-patterned implants and conclude that this effect mainly is induced by the nano-CaP increased surface topography complexity.
Similarly, it has been shown that rod-shaped nano-CaP particle (width 10–20 nm and length 100–200 nm) coated titanium had favorable effects in a rabbit tibia bone model compared to the non-coated control.161 Summed up, the nano-CaP patterned implants enhanced the early osseointegration, since more force was needed to remove the implants from the bone tissue after 2 weeks. Moreover, the surface suppressed inflammatory responses and significantly up-regulated the expression genes involved in bone production.
Some groups have used commercially available implants, which they have modified in order to obtain the desired nano-topographic features. Palmquist et al. have compared the behavior of machined laser-modified with machined non-modified titanium alloy implants.162 While machined surfaces showed semi-ordered valleys and ridges, laser treatment resulted in micro- and nano-sized non-ordered globular features, giving the surface higher roughness and a thicker oxide surface layer. The implants had been shaped in screw-form and implanted into rabbit cortical bone. Analysis 8 weeks after implantation showed that laser-modified implants were strongly anchored to bone on the nano-level resulting in a 270% increase in shear strength during torsion compared to the machined sample. Similar results could be obtained using the same experimental setup after a long term experiment of 6 months.163 In an additional study the same group studied the acute inflammatory response to machined titanium surfaces with and without additional laser treatment modification.164 Implantation occurred subcutaneously in a rat model and resulted in the observation that significantly less inflammatory cells were attracted to the laser-modified surfaces and less pro-inflammatory cytokines have been measured around the surface compared to machined controls. In a recent study, commercial laser-modified implants were evaluated after retrieval at 10 weeks of healing from a human patient.165 Similar to the above described topographic features, the implants showed globular micro- and nano-topography. The analysis of the surface–bone interaction showed strong osseointegration properties on the nano-scale level between the laser-modified titanium surface and human bone.
Conclusion
The great variety of materials used nowadays, feature forms, sizes and cell lines makes it very difficult to integrate the experimental observations for development of a general model. Nevertheless, it is possible to state that topography (in combination with other surface properties and cues) is able to affect cellular behavior on different levels and should be the topic of more systematic research in vitro and in vivo. Interestingly, many of the clinically used implant materials, which have been proven to deliver good performance in patients, feature nano-scale roughness.166 This shows that nano-topographic features have been probably automatically selected during the trial and error process of implant development. Nevertheless, the here described studies showed that the use of ordered and semi-ordered nano-topographies provide additional possibilities for manipulation of bone-growth and could probably be used to further increase the success rate of bone implants. The ability to design surfaces on a nano-metric scale could allow us to actively communicate with single cells by using a common language in the form of topographic features that are already used by our own organisms since the beginning of multi-cellularity.References
- D. Hwang and H. L. Wang, Medical contraindications to implant therapy: part II: relative contraindications, Implant Dent., 2007, 16(1), 13–23 CrossRef.
- D. Hwang and H. L. Wang, Medical contraindications to implant therapy: part I: absolute contraindications, Implant Dent., 2006, 15(4), 353–360 CrossRef.
- F. P. Strietzel, et al., Smoking interferes with the prognosis of dental implant treatment: a systematic review and meta-analysis, J. Clin. Periodontol., 2007, 34(6), 523–544 CrossRef.
- G. Hulleberg, et al., A clinical and radiographic 13-year follow-up study of 138 Charnley hip arthroplasties in patients 50–70 years old: comparison of university hospital data and registry data, Acta Orthop., 2008, 79(5), 609–617 CrossRef.
- K. T. Makela, et al., Total hip arthroplasty for primary osteoarthritis in patients fifty-five years of age or older. An analysis of the Finnish arthroplasty registry, J. Bone Jt. Surg., 2008, 90(10), 2160–2170 CrossRef.
- G. Alsaadi, et al., Impact of local and systemic factors on the incidence of oral implant failures, up to abutment connection, J. Clin. Periodontol., 2007, 34(7), 610–617 CrossRef.
- O. L. Harrysson, O. Robertsson and J. F. Nayfeh, Higher cumulative revision rate of knee arthroplasties in younger patients with osteoarthritis, Clin. Orthop. Relat. Res., 2004,(421), 162–168 CrossRef.
- S. P. Johnsen,
et al., Patient-related predictors of implant failure after primary total hip replacement in the initial, short- and long-terms. A nationwide Danish follow-up study including 36
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 984 patients, J. Bone Jt. Surg., Br. Vol., 2006, 88(10), 1303–1308 CrossRef CAS.
984 patients, J. Bone Jt. Surg., Br. Vol., 2006, 88(10), 1303–1308 CrossRef CAS. - S. D. Ulrich, et al., Total hip arthroplasties: what are the reasons for revision?, Int. Orthop., 2008, 32(5), 597–604 CrossRef.
- Y. Ozaki, et al., Comprehensive analysis of chemotactic factors for bone marrow mesenchymal stem cells, Stem Cells Dev., 2007, 16(1), 119–129 CrossRef CAS.
- A. Tokunaga, et al., PDGF receptor beta is a potent regulator of mesenchymal stromal cell function, J. Bone Miner. Res., 2008, 23(9), 1519–1528 CrossRef CAS.
- R. Bielby, E. Jones and D. McGonagle, The role of mesenchymal stem cells in maintenance and repair of bone, Injury, 2007, 38(Suppl 1), S26–S32 CrossRef.
- K. Matsuo and N. Irie, Osteoclast–osteoblast communication, Arch. Biochem. Biophys., 2008, 473(2), 201–209 CrossRef CAS.
- A. J. Engler, et al., Matrix elasticity directs stem cell lineage specification, Cell, 2006, 126(4), 677–689 CrossRef CAS.
- R. McBeath, et al., Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment, Dev. Cell, 2004, 6(4), 483–495 CrossRef CAS.
- M. J. Dalby, et al., The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder, Nat. Mater., 2007, 6(12), 997–1003 CrossRef CAS.
- M. J. Buehler, Nature designs tough collagen: explaining the nanostructure of collagen fibrils, Proc. Natl. Acad. Sci. U. S. A., 2006, 103(33), 12285–12290 CrossRef CAS.
- F. Grinnell and W. M. Petroll, Cell motility and mechanics in three-dimensional collagen matrices, Annu. Rev. Cell Dev. Biol., 2010, 26, 335–361 CrossRef CAS.
- E. Lamers, et al., The influence of nanoscale grooved substrates on osteoblast behavior and extracellular matrix deposition, Biomaterials, 2010, 31(12), 3307–3316 CrossRef CAS.
- M. Born and E. Wolf, Principles of Optics: Electromagnetic Theory of Propagation, Interference and Diffraction of Light, 7th expanded edn, Cambridge University Press, Cambridge, New York, 1999, xxxiii, 952 p Search PubMed.
- D. Williams, The relationship between biomaterials and nanotechnology, Biomaterials, 2008, 29(12), 1737–1738 CrossRef CAS.
- E. G. van Putten, et al., Scattering lens resolves sub-100 nm structures with visible light, Phys. Rev. Lett., 2011, 106(19), 193905 CrossRef CAS.
- M. Bates, et al., Multicolor super-resolution imaging with photo-switchable fluorescent probes, Science, 2007, 317(5845), 1749–1753 CrossRef CAS.
- T. A. Klar and S. W. Hell, Subdiffraction resolution in far-field fluorescence microscopy, Opt. Lett., 1999, 24(14), 954–956 CrossRef CAS.
- M. J. Rust, M. Bates and X. Zhuang, Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM), Nat. Methods, 2006, 3(10), 793–795 CrossRef CAS.
- E. Betzig, et al., Imaging intracellular fluorescent proteins at nanometer resolution, Science, 2006, 313(5793), 1642–1645 CrossRef CAS.
- G. Michler, Electron Microscopy of Polymers, Springer, 2008 Search PubMed.
- M. Toth, et al., Nanostructure fabrication by ultra-high-resolution environmental scanning electron microscopy, Nano Lett., 2007, 7(2), 525–530 CrossRef CAS.
- R. Erni, et al., Atomic-resolution imaging with a sub-50 pm electron probe, Phys. Rev. Lett., 2009, 102(9), 096101 CrossRef.
- C. A. Siedlecki and R. E. Marchant, Atomic force microscopy for characterization of the biomaterial interface, Biomaterials, 1998, 19(4–5), 441–454 CrossRef CAS.
- J. V. Lauritsen and M. Reichling, Atomic resolution non-contact atomic force microscopy of clean metal oxide surfaces, J. Phys.: Condens. Matter, 2010, 22(26), 263001 CrossRef CAS.
- H. K. Webb, et al., Roughness Parameters for Standard Description of Surface Nanoarchitecture, Scanning, 2012, 34(4), 257–63 CrossRef CAS.
- V. D'Anto, et al., Evaluation of Surface Roughness of Orthodontic Wires by Means of Atomic Force Microscopy., Angle Orthod, 2012 DOI:10.2319/100211-620.1.
- A. Dugal and G. Thakur, Surface analysis of indigenous stainless steel miniplates used in facial fractures, Journal of Maxillofacial and Oral Surgery, 2010, 9(4), 403–406 CrossRef.
- U. Hempel, et al., Response of human bone marrow stromal cells, MG-63, and SaOS-2 to titanium-based dental implant surfaces with different topography and surface energy, Clin. Oral Implants Res., 2011 DOI:10.1111/j.1600-0501.2011.02328.x.
- D. Geblinger, et al., Effects of surface microtopography on the assembly of the osteoclast resorption apparatus, J. R. Soc. Interface, 2012, 9(72), 1599–608 CrossRef.
- A. Ungersböck, O. Pohler and S. M. Perren, Evaluation of the soft tissue interface at titanium implants with different surface treatments: experimental study on rabbits, Biomed. Mater. Eng., 1994, 4(4), 317–325 Search PubMed.
- A. Chakraborty, et al., In vivo bone response and interfacial properties of titanium-alloy implant with different designs in rabbit model with time, Indian Journal of Dental Research, 2011, 22(2), 277–284 CrossRef.
- K. Duske, et al., Atmospheric plasma enhances wettability and cell spreading on dental implant metals, Journal of Clinical Periodontology, 2012, 39(4), 400–407 CrossRef CAS.
- S. Lenhert, et al., Capillary-induced contact guidance, Langmuir, 2007, 23(20), 10216–10223 CrossRef CAS.
- S. Kress, et al., Stem cell differentiation depending on different surfaces, Adv. Biochem. Eng. Biotechnol., 2012, 126, 263–283 CrossRef.
- H. J. Butt, B. Cappella and M. Kappl, Force measurements with the atomic force microscope: Technique, interpretation and applications, Surf. Sci. Rep., 2005, 59(1–6), 1–152 CrossRef CAS.
- E. Lamers, et al., Dynamic cell adhesion and migration on nanoscale grooved substrates, Eur. Cell Mater., 2012, 23, 182–193 CAS ; discussion 193–4.
- E. J. Thoreson, J. Martin and N. A. Burnham, The role of few-asperity contacts in adhesion, J. Colloid Interface Sci., 2006, 298(1), 94–101 CrossRef CAS.
- G. Altankov, K. Richau and T. Groth, The role of surface zeta potential and substratum chemistry for regulation of dermal fibroblasts interaction, Materialwiss. Werkstofftech., 2003, 34(12), 1120–1128 CrossRef CAS.
- K. M. Jan and S. Chien, Role of surface electric charge in red blood cell interactions, J. Gen. Physiol., 1973, 61(5), 638–654 CrossRef CAS.
- A. B. García, et al., Zeta potential as a tool to characterize plasma oxidation of carbon fibers, Journal of Colloid and Interface Science, 1997, 192(2), 363–367 CrossRef.
- J. P. Szaflarski, et al., Constraint-induced aphasia therapy stimulates language recovery in patients with chronic aphasia after ischemic stroke, Med. Sci. Monit., 2008, 14(5), CR243–CR250 Search PubMed.
- M. Stamm, Polymer Surfaces and Interfaces, Springer, 2008 Search PubMed.
- M. L. Kraft, et al., Phase separation of lipid membranes analyzed with high-resolution secondary ion mass spectrometry, Science, 2006, 313(5795), 1948–1951 CrossRef CAS.
- G. E. Moore, Cramming more components onto integrated circuits (Reprinted from Electronics, pg 114–117, April 19, 1965), Proc. IEEE, 1998, 86(1), 82–85 CrossRef.
- Y. W. Fan, et al., Culture of neural cells on silicon wafers with nano-scale surface topograph, J. Neurosci. Methods, 2002, 120(1), 17–23 CrossRef CAS.
- M. J. Dalby, et al., In vitro reaction of endothelial cells to polymer demixed nanotopography, Biomaterials, 2002, 23(14), 2945–2954 CrossRef CAS.
- G. M. Whitesides and B. Grzybowski, Self-assembly at all scales, Science, 2002, 295(5564), 2418–2421 CrossRef CAS.
- A. Tavangar, B. Tan and K. Venkatakrishnan, Synthesis of bio-functionalized three-dimensional titania nanofibrous structures using femtosecond laser ablation, Acta Biomater., 2011, 7(6), 2726–2732 CrossRef CAS.
- M. Fusi, et al., Titanium oxide nanostructured films by reactive pulsed laser deposition, Appl. Surf. Sci., 2009, 255(10), 5334–5337 CrossRef CAS.
- L. Li and Y. L. Hsieh, Chitosan bicomponent nanofibers and nanoporous fibers, Carbohydr. Res., 2006, 341(3), 374–381 CrossRef CAS.
- S. H. Shang, et al., The Effect of Electrospun Fibre Alignment on the Behaviour of Rat Periodontal Ligament Cells, Eur. Cells Mater., 2010, 19, 180–192 CAS.
- V. Zwilling, et al., Structure and physicochemistry of anodic oxide films on titanium and TA6V alloy, Surf. Interface Anal., 1999, 27(7), 629–637 CrossRef CAS.
- D. Gong, et al., Titanium oxide nanotube arrays prepared by anodic oxidation, J. Mater. Res., 2001, 16(12), 3331–3334 CrossRef CAS.
- G. K. Mor, et al., A review on highly ordered, vertically oriented TiO2 nanotube arrays: Fabrication, material properties, and solar energy applications, Sol. Energy Mater. Sol. Cells, 2006, 90(14), 2011–2075 CrossRef CAS.
- J. J. Norman and T. A. Desai, Methods for fabrication of nanoscale topography for tissue engineering scaffolds, Ann. Biomed. Eng., 2006, 34(1), 89–101 CrossRef.
- M. J. Biggs, et al., Regulation of implant surface cell adhesion: characterization and quantification of S-phase primary osteoblast adhesions on biomimetic nanoscale substrates, J. Orthop. Res., 2007, 25(2), 273–282 CrossRef CAS.
- A. S. Curtis, et al., Substratum nanotopography and the adhesion of biological cells. Are symmetry or regularity of nanotopography important?, Biophys. Chem., 2001, 94(3), 275–283 CrossRef CAS.
- M. J. Dalby, et al., Investigating the limits of filopodial sensing: a brief report using SEM to image the interaction between 10 nm high nano-topography and fibroblast filopodia, Cell Biol. Int., 2004, 28(3), 229–236 CrossRef CAS.
- M. J. Biggs, et al., Interactions with nanoscale topography: adhesion quantification and signal transduction in cells of osteogenic and multipotent lineage, J. Biomed. Mater. Res. A., 2009, 91(1), 195–208 CrossRef.
- M. J. Biggs, et al., The effects of nanoscale pits on primary human osteoblast adhesion formation and cellular spreading, J. Mater. Sci.: Mater. Med., 2007, 18(2), 399–404 CrossRef CAS.
- M. J. Dalby, N. Gadegaard and C. D. Wilkinson, The response of fibroblasts to hexagonal nanotopography fabricated by electron beam lithography, J. Biomed. Mater. Res. A., 2008, 84(4), 973–979 CrossRef.
- M. J. Dalby, et al., Osteoprogenitor response to defined topographies with nanoscale depths, Biomaterials, 2006, 27(8), 1306–1315 CrossRef CAS.
- C. Vieu, et al., Electron beam lithography: Resolution limits and applications, Appl. Surf. Sci., 2000, 164(1–4), 111–117 CrossRef CAS.
- C. Tan, et al., Ordered nanostructures written directly by laser interference, Nanotechnology, 2009, 20(12), 125303 CrossRef CAS.
- D. W. Hamilton, et al., Migration of periodontal ligament fibroblasts on nanometric topographical patterns: influence of filopodia and focal adhesions on contact guidance, PLoS One, 2010, 5(12), e15129 CAS.
- J. de Boor, et al., Three-beam interference lithography: upgrading a Lloyd's interferometer for single-exposure hexagonal patterning, Opt. Lett., 2009, 34(12), 1783–1785 CrossRef.
- K. V. Sreekanth, J. K. Chua and V. M. Murukeshan, Interferometric lithography for nanoscale feature patterning: a comparative analysis between laser interference, evanescent wave interference, and surface plasmon interference, Appl. Opt., 2010, 49(35), 6710–6717 CrossRef.
- M. A. Wood, Colloidal lithography and current fabrication techniques producing in-plane nanotopography for biological applications, J. R. Soc. Interface, 2007, 4(12), 1–17 CrossRef CAS.
- M. A. Wood, M. Riehle and C. D. W. Wilkinson, Patterning colloidal nanotopographies, Nanotechnology, 2002, 13(5), 605–609 CrossRef CAS.
- P. Hanarp, et al., Control of nanoparticle film structure for colloidal lithography, Colloids Surf., A, 2003, 214(1–3), 23–36 CrossRef CAS.
- M. J. Dalby, et al., Changes in fibroblast morphology in response to nano-columns produced by colloidal lithography, Biomaterials, 2004, 25(23), 5415–5422 CrossRef CAS.
- R. G. Harrison, On the stereotropism of embryonic cells, Science, 1911, 34(870), 279–281 CAS.
- P. Weiss, Experiments on cell and axon orientation in vitro; the role of colloidal exudates in tissue organization, J. Exp. Zool., 1945, 100, 353–386 CrossRef CAS.
- A. S. Curtis and M. Varde, Control of cell behavior: topological factors, J. Natl. Cancer Inst., 1964, 33, 15–26 CAS.
- Y. A. Rovensky, I. L. Slavnaja and J. M. Vasiliev, Behaviour of fibroblast-like cells on grooved surfaces, Exp. Cell Res., 1971, 65(1), 193–201 CrossRef CAS.
- G. A. Dunn and J. P. Heath, A new hypothesis of contact guidance in tissue cells, Exp. Cell Res., 1976, 101(1), 1–14 CrossRef CAS.
- P. T. Ohara and R. C. Buck, Contact guidance in vitro. A light, transmission, and scanning electron microscopic study, Exp. Cell Res., 1979, 121(2), 235–249 CrossRef CAS.
- A. F. von Recum and T. G. van Kooten, The influence of micro-topography on cellular response and the implications for silicone implants, J. Biomater. Sci., Polym. Ed., 1995, 7(2), 181–198 CrossRef CAS.
- A. Curtis and C. Wilkinson, Topographical control of cells, Biomaterials, 1997, 18(24), 1573–1583 CrossRef CAS.
- X. F. Walboomers and J. A. Jansen, Cell and tissue behavior on micro-grooved surfaces, Odontology, 2001, 89(1), 2–11 CrossRef CAS.
- R. G. Flemming, et al., Effects of synthetic micro- and nano-structured surfaces on cell behavior, Biomaterials, 1999, 20(6), 573–588 CrossRef CAS.
- C. C. Berry, et al., Human fibroblast and human bone marrow cell response to lithographically nanopatterned adhesive domains on protein rejecting substrates, IEEE Trans. NanoBiosci., 2007, 6(3), 201–209 CrossRef CAS.
- K. Takeuchi, et al., Enhanced intrinsic biomechanical properties of osteoblastic mineralized tissue on roughened titanium surface, J. Biomed. Mater. Res. A., 2005, 72(3), 296–305 CrossRef.
- P. T. de Oliveira and A. Nanci, Nanotexturing of titanium-based surfaces upregulates expression of bone sialoprotein and osteopontin by cultured osteogenic cells, Biomaterials, 2004, 25(3), 403–413 CrossRef.
- P. T. de Oliveira, et al., Enhancement of in vitro osteogenesis on titanium by chemically produced nanotopography, J. Biomed. Mater. Res. A., 2007, 80(3), 554–564 CrossRef.
- K. Kubo, et al., Microtopography of titanium suppresses osteoblastic differentiation but enhances chondroblastic differentiation of rat femoral periosteum-derived cells, J. Biomed. Mater. Res. A, 2008, 87(2), 380–391 CrossRef.
- M. Ball, et al., The effect of different surface morphology and roughness on osteoblast-like cells, J. Biomed. Mater. Res. A, 2008, 86(3), 637–647 CrossRef.
- T. Ogawa, et al., Ti nano-nodular structuring for bone integration and regeneration, J. Dent. Res., 2008, 87(8), 751–756 CrossRef CAS.
- K. Kubo, et al., Cellular behavior on TiO2 nanonodular structures in a micro-to-nanoscale hierarchy model, Biomaterials, 2009, 30(29), 5319–5329 CrossRef CAS.
- I. H. Bae, et al., Anodic oxidized nanotubular titanium implants enhance bone morphogenetic protein-2 delivery, J. Biomed. Mater. Res. B: Appl. Biomater., 2010, 93(2), 484–491 CrossRef.
- K. C. Popat, et al., Influence of engineered titania nanotubular surfaces on bone cells, Biomaterials, 2007, 28(21), 3188–3197 CrossRef CAS.
- C. Yao, E. B. Slamovich and T. J. Webster, Enhanced osteoblast functions on anodized titanium with nanotube-like structures, J. Biomed. Mater. Res. A., 2008, 85(1), 157–166 CrossRef.
- W. Q. Yu, et al., In vitro behavior of MC3T3-E1 preosteoblast with different annealing temperature titania nanotubes, Oral Dis., 2010, 16(7), 624–630 CrossRef CAS.
- K. Burns, C. Yao and T. J. Webster, Increased chondrocyte adhesion on nanotubular anodized titanium, J. Biomed. Mater. Res. A., 2009, 88(3), 561–568 CrossRef.
- B. Ercan and T. J. Webster, The effect of biphasic electrical stimulation on osteoblast function at anodized nanotubular titanium surfaces, Biomaterials, 2010, 31(13), 3684–3693 CrossRef CAS.
- J. Y. Lim, et al., Osteoblast adhesion on poly(L-lactic acid)/polystyrene demixed thin film blends: effect of nanotopography, surface chemistry, and wettability, Biomacromolecules, 2005, 6(6), 3319–3327 CrossRef CAS.
- J. Y. Lim, et al., Human foetal osteoblastic cell response to polymer-demixed nanotopographic interfaces, J. R. Soc. Interface, 2005, 2(2), 97–108 CrossRef CAS.
- M. J. Dalby, et al., Fibroblast reaction to island topography: changes in cytoskeleton and morphology with time, Biomaterials, 2003, 24(6), 927–935 CrossRef CAS.
- M. J. Dalby, et al., Interactions of human blood and tissue cell types with 95-nm-high nanotopography, IEEE Trans. NanoBiosci., 2002, 1(1), 18–23 CrossRef.
- M. J. Dalby, et al., Polymer-demixed nanotopography: control of fibroblast spreading and proliferation, Tissue Eng., 2002, 8(6), 1099–1108 CrossRef CAS.
- M. J. Dalby, et al., Rapid fibroblast adhesion to 27 nm high polymer demixed nano-topography, Biomaterials, 2004, 25(1), 77–83 CrossRef CAS.
- J. M. Rice, et al., Quantitative assessment of the response of primary derived human osteoblasts and macrophages to a range of nanotopography surfaces in a single culture model in vitro, Biomaterials, 2003, 24(26), 4799–4818 CrossRef CAS.
- J. C. Hansen, et al., Effect of surface nanoscale topography on elastic modulus of individual osteoblastic cells as determined by atomic force microscopy, J. Biomech., 2007, 40(13), 2865–2871 CrossRef.
- J. D. Salvi, J. Y. Lim and H. J. Donahue, Increased mechanosensitivity of cells cultured on nanotopographies, J. Biomech., 2010, 43(15), 3058–3062 CrossRef.
- M. J. Dalby, et al., Investigating filopodia sensing using arrays of defined nano-pits down to 35 nm diameter in size, Int. J. Biochem. Cell Biol., 2004, 36(10), 2005–2015 CrossRef CAS.
- M. J. Dalby, et al., Fibroblast response to a controlled nanoenvironment produced by colloidal lithography, J. Biomed. Mater. Res., 2004, 69(2), 314–322 CrossRef.
- E. T. den Braber, et al., Quantitative analysis of fibroblast morphology on microgrooved surfaces with various groove and ridge dimensions, Biomaterials, 1996, 17(21), 2037–2044 CrossRef CAS.
- X. F. Walboomers, et al., Attachment of fibroblasts on smooth and microgrooved polystyrene, J. Biomed. Mater. Res., 1999, 46(2), 212–220 CrossRef CAS.
- W. A. Loesberg, et al., The threshold at which substrate nanogroove dimensions may influence fibroblast alignment and adhesion, Biomaterials, 2007, 28(27), 3944–3951 CrossRef CAS.
- M. R. Lee, et al., Direct differentiation of human embryonic stem cells into selective neurons on nanoscale ridge/groove pattern arrays, Biomaterials, 2010, 31(15), 4360–4366 CrossRef CAS.
- W. Hu, et al., Effects of nanoimprinted patterns in tissue-culture polystyrene on cell behavior, J. Vac. Sci. Technol., B, 2005, 23(6), 2984–2989 CAS.
- A. I. Teixeira, et al., Epithelial contact guidance on well-defined micro- and nanostructured substrates, J. Cell Sci., 2003, 116(Pt 10), 1881–1892 CrossRef CAS.
- C. J. Bettinger, et al., Enhancement of in vitro capillary tube formation by substrate nanotopography, Adv. Mater., 2008, 20(1), 99–103 CrossRef CAS.
- E. Rebollar, et al., Proliferation of aligned mammalian cells on laser-nanostructured polystyrene, Biomaterials, 2008, 29(12), 1796–1806 CrossRef CAS.
- C. H. Choi, et al., Cell interaction with three-dimensional sharp-tip nanotopography, Biomaterials, 2007, 28(9), 1672–1679 CrossRef CAS.
- E. Lamers, et al., The influence of nanoscale topographical cues on initial osteoblast morphology and migration, Eur. Cell Mater., 2010, 20, 329–343 CAS.
- J. Y. Yang, et al., Quantitative analysis of osteoblast-like cells (MG63) morphology on nanogrooved substrata with various groove and ridge dimensions, J. Biomed. Mater. Res., Part A, 2009, 90A(3), 629–640 CrossRef CAS.
- E. K. F. Yim, S. W. Pang and K. W. Leong, Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage, Exp. Cell Res., 2007, 313(9), 1820–1829 CrossRef CAS.
- M. J. Dalby, Topographically induced direct cell mechanotransduction, Med. Eng. Phys., 2005, 27(9), 730–742 CrossRef.
- M. J. Dalby, Cellular response to low adhesion nanotopographies, Int. J. Nanomedicine, 2007, 2(3), 373–381 CAS.
- M. H. You, et al., Synergistically enhanced osteogenic differentiation of human mesenchymal stem cells by culture on nanostructured surfaces with induction media, Biomacromolecules, 2010, 11(7), 1856–1862 CrossRef CAS.
- L. Prodanov, et al., The interaction between nanoscale surface features and mechanical loading and its effect on osteoblast-like cells behavior, Biomaterials, 2010, 31(30), 7758–7765 CrossRef CAS.
- T. J. Webster, et al., Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics, J. Biomed. Mater. Res., 2000, 51(3), 475–483 CrossRef CAS.
- T. Brevig, et al., The recognition of adsorbed and denatured proteins of different topographies by beta2 integrins and effects on leukocyte adhesion and activation, Biomaterials, 2005, 26(16), 3039–3053 CrossRef CAS.
- Y. Z. Yang, R. Cavin and J. L. Ong, Protein adsorption on titanium surfaces and their effect on osteoblast attachment, J. Biomed. Mater. Res., 2003, 67A(1), 344–349 CrossRef CAS.
- D. E. MacDonald, et al., Thermal and chemical modification of titanium-aluminum-vanadium implant materials: effects on surface properties, glycoprotein adsorption, and MG63 cell attachment, Biomaterials, 2004, 25(16), 3135–3146 CrossRef CAS.
- Y. Arima and H. Iwata, Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers, Biomaterials, 2007, 28(20), 3074–3082 CrossRef CAS.
- L. Ponsonnet, et al., Relationship between surface properties (roughness, wettability) of titanium and titanium alloys and cell behaviour, Mater. Sci. Eng., C, 2003, 23(4), 551–560 CrossRef.
- F. Rupp, et al., Roughness induced dynamic changes of wettability of acid etched titanium implant modifications, Biomaterials, 2004, 25(7–8), 1429–1438 CrossRef CAS.
- K. Cai, J. Bossert and K. D. Jandt, Does the nanometre scale topography of titanium influence protein adsorption and cell proliferation?, Colloids Surf., B, 2006, 49(2), 136–144 CrossRef CAS.
- M. N. Sela, et al., Adsorption of human plasma proteins to modified titanium surfaces, Clin. Oral Implants Res., 2007, 18(5), 630–638 CrossRef.
- K. Rechendorff, et al., Enhancement of protein adsorption induced by surface roughness, Langmuir, 2006, 22(26), 10885–8 CrossRef CAS.
- M. B. Hovgaard, et al., Fibronectin adsorption on tantalum: the influence of nanoroughness, J. Phys. Chem. B, 2008, 112(28), 8241–8249 CrossRef CAS.
- T. J. Webster, et al., Mechanisms of enhanced osteoblast adhesion on nanophase alumina involve vitronectin, Tissue Eng., 2001, 7(3), 291–301 CrossRef CAS.
- A. Dolatshahi-Pirouz, et al., Bovine serum albumin adsorption on nano-rough platinum surfaces studied by QCM-D, Colloids Surf., B, 2008, 66(1), 53–59 CrossRef CAS.
- X. Wang, et al., Influence of physicochemical properties of laser-modified polystyrene on bovine serum albumin adsorption and rat C6 glioma cell behavior, J. Biomed. Mater. Res. A, 2006, 78(4), 746–754 CrossRef.
- D. V. Nicolau, et al., Protein immobilisation on micro/nanostructures fabricated by laser microablation, Biosens. Bioelectron., 2010, 26(4), 1337–1345 CrossRef CAS.
- J. M. Anderson, A. Rodriguez and D. T. Chang, Foreign body reaction to biomaterials, Semin. Immunol., 2008, 20(2), 86–100 CrossRef CAS.
- J. M. Anderson, Biological responses to materials, Annu. Rev. Mater. Res., 2001, 31, 81–110 CrossRef CAS.
- B. D. Ratner, Replacing and renewing: synthetic materials, biomimetics, and tissue engineering in implant dentistry, J. Dent. Educ., 2001, 65(12), 1340–1347 CAS.
- T. Albrektsson and L. Sennerby, Direct bone anchorage of oral implants: clinical and experimental considerations of the concept of osseointegration, Int. J. Prosthodont., 1990, 3(1), 30–41 CAS.
- L. Le Guehennec, et al., Surface treatments of titanium dental implants for rapid osseointegration, Dent. Mater., 2007, 23(7), 844–854 CrossRef CAS.
- D. F. Williams, The Williams Dictionary of Biomaterials, Liverpool University Press, Liverpool, 1999, xvii, 343 p Search PubMed.
- G. Giavaresi, et al., In vitro and in vivo response to nanotopographically-modified surfaces of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and polycaprolactone, J. Biomater. Sci., Polym. Ed., 2006, 17(12), 1405–1423 CrossRef CAS.
- X. Wu, et al., Nano-TiO2/PEEK bioactive composite as a bone substitute material: in vitro and in vivo studies, Int. J. Nanomedicine, 2012, 7, 1215–1225 CAS.
- H. Liu and T. J. Webster, Ceramic/polymer nanocomposites with tunable drug delivery capability at specific disease sites, J. Biomed. Mater. Res. A, 2010, 93(3), 1180–1192 Search PubMed.
- L. M. Bjursten, et al., Titanium dioxide nanotubes enhance bone bonding in vivo, J. Biomed. Mater. Res. A, 2010, 92(3), 1218–1224 Search PubMed.
- N. Wang, et al., Effects of TiO2 nanotubes with different diameters on gene expression and osseointegration of implants in minipigs, Biomaterials, 2011, 32(29), 6900–6911 CrossRef CAS.
- G. C. Smith, et al., Soft tissue response to titanium dioxide nanotube modified implants, Acta Biomater., 2011, 7(8), 3209–3215 CrossRef CAS.
- L. Meirelles, et al., Effect of hydroxyapatite and titania nanostructures on early in vivo bone response, Clin. Implant Dent. Relat. Res., 2008, 10(4), 245–254 Search PubMed.
- S. S. Liao, et al., Hierarchically biomimetic bone scaffold materials: nano-HA/collagen/PLA composite, J. Biomed. Mater. Res., 2004, 69(2), 158–165 CrossRef CAS.
- V. C. Mendes, R. Moineddin and J. E. Davies, Discrete calcium phosphate nanocrystalline deposition enhances osteoconduction on titanium-based implant surfaces, J. Biomed. Mater. Res. A, 2009, 90(2), 577–585 CrossRef.
- V. C. Mendes, R. Moineddin and J. E. Davies, The effect of discrete calcium phosphate nanocrystals on bone-bonding to titanium surfaces, Biomaterials, 2007, 28(32), 4748–4755 CrossRef CAS.
- R. Jimbo, et al., Genetic responses to nanostructured calcium-phosphate-coated implants, J. Dent. Res., 2011, 90(12), 1422–1427 CrossRef CAS.
- A. Palmquist, et al., Biomechanical, histological, and ultrastructural analyses of laser micro- and nano-structured titanium alloy implants: a study in rabbit, J. Biomed. Mater. Res. A, 2010, 92(4), 1476–1486 Search PubMed.
- A. Palmquist, et al., Biomechanical, histological and ultrastructural analyses of laser micro- and nano-structured titanium implant after 6 months in rabbit, J. Biomed. Mater. Res. B: Appl. Biomater., 2011, 97(2), 289–298 CrossRef.
- A. Palmquist, et al., Acute inflammatory response to laser-induced micro- and nano-sized titanium surface features, Clin. Implant Dent. Relat. Res., 2011 DOI:10.1111/j.1708-8208.2011.00361.x.
- A. Palmquist, et al., Bone-titanium oxide interface in humans revealed by transmission electron microscopy and electron tomography, J. R. Soc. Interface, 2012, 9(67), 396–400 CrossRef CAS.
- L. M. Svanborg, M. Andersson and A. Wennerberg, Surface characterization of commercial oral implants on the nanometer level, J. Biomed. Mater. Res. B: Appl. Biomater., 2010, 92(2), 462–469 Search PubMed.
| This journal is © The Royal Society of Chemistry 2013 |
