Development of an automatic photometric titration procedure to determine olive oil acidity employing a miniaturized multicommuted flow-batch setup
Carla C.
Crispino
and
Boaventura F.
Reis
*
Centro de Energia Nuclear na Agricultura, Universidade de São Paulo, Av. Centenário, 303, CP 96, 13400-970 Piracicaba, SP, Brazil. E-mail: reis@cena.usp.br
First published on 29th October 2013
Abstract
The present work proposes an automatic procedure for photometric determination of olive oil acidity, employing a miniaturized multicommuted flow-batch analysis setup without the use of calibration curves. Under microcomputer control, the proposed setup was able to mimic the manual procedure suggested by the European Commission Regulation. A homemade LED-based photometer was designed by coupling a radiation source and a photodetector to the titration chamber, creating a compact and downsized unit. The photometric titration of olive oil was carried out using potassium hydroxide solution in an n-propanol medium, which was done without any previous pretreatment, thereby allowing that a true photometric titration procedure in a non-aqueous medium was carried out for the first time. After establishing better operational conditions, the proposed procedure was applied to determine acidity in olive oil samples. The accuracy was assessed by applying the paired t-test to results obtained using a reference method, and there was no significant difference at a 95% confidence level. Other useful features were achieved, including a relative standard deviation of 1.8% (n = 6), a sample consumption of 125 μL per determination, an effluent generation of 1.4 mL per titration, and a sampling throughput ranging from 40 to 50 determinations per hour, whereby indicating that it could be an effective alternative for routine analysis of olive oil.
1. Introduction
In Mediterranean countries, such as Italy, France, Portugal, Spain, and Greece, the incidence of heart disease is lower than in the countries of northern Europe.1 In general, the inhabitants of these countries have a diet rich in olive oil, which contains substances that prevent coronary arteriosclerosis.2 Olive oil is considered a complex compound containing fatty acids, vitamins, pigments, antioxidant chemical species, and volatile substances.3 Among the fatty acids, attention has been paid to oleic acid, a carboxylic acid with a long chain and whose structure has 18 carbon atoms and 1 double bond. This unsaturation feature makes it an essential fatty acid (omega-9). This compound comprises a class of lipids vital in the construction of the cell membrane, participating in the metabolism and in the synthesis of hormones.4The hydrolysis of olive oil forms free fatty acids (FFA) and glycerol residue. The FFA content expressed as oleic acid is used to classify the olive oil in different categories. For virgin olive oil, the maximum acidity is 1%,3 while for extra virgin, the maximum acidity is 0.8%.5 The formation of FFA is the main cause of olive oil flavor deterioration, thereby decreasing its useful life.3 Acidity is the main parameter to define the quality of the olive oil, thus if acidity is greater than 3%, the olive oil is considered unsuitable for human consumption.3,5 Virgin olive oil is obtained by the extraction of the fruit, using exclusively mechanical processes such as pressure, centrifugation, or/and filtration, thus preserving its sensory characteristics and nutritional value.1 But processing and storage conditions may affect the product quality.6
The importance of olive oil acidity becomes evident when we note that products available on the market display the acidity value as a quality parameter. The labeled acidity expressed as a percentage indicates the amount of free oleic acid per 100 g.7 The FFA concentration in olive oil is one of the main parameters used to characterize its quality, which has been achieved by using analytical techniques such as nuclear magnetic resonance,8 infrared spectroscopy with Fourier transform,9 and a robotized analytical procedure.10
Because of the importance of olive oil acidity, this parameter should be evaluated during the production process, and as well as during storage and marketing.6 European Commission Regulation no. 2568/91 (ECC 1991) classifies olive oil according to the degree of acidity11 and suggested a titration procedure for FFA determination.5 The method is simple, nevertheless it is very slow and consumes a large amount of samples and organic solvent, requiring also an experienced operator to handle the titrant solution and to visualize the end of the titration. The availability of a feasible analytical procedure that consumes less organic solvent is essential to attain these requirements, which have been accomplished by resorting of any automatic process, such as flow injection analysis.11–13
Olive oil viscosity is a parameter that could depreciate the effectiveness of the analytical procedure, because it can affect the rate of pumping and dispersion, compromising the quality of results. This drawback was overcome by using n-propanol or ethanol–diethylether as conditioning fluids for the sample.11–14 In the first three cases, the procedures were based on the photometric titration using phenolphthalein as an indicting dye, while in the fourth a Cu2+ pyridine solution was employed as a chromogenic reagent.14 The referred procedures were implemented using flow injection analysis approaches, presenting facilities such as high throughput and simplicity of operation.
Photometric titration based on flow injection analysis was proposed by Ruzicka and Hansen in 1977 and implemented in an aqueous medium.15 Titration in a non-aqueous medium was proposed ten years later for pharmaceutical formulations,16 which was also employed to develop procedures for the determination of olive oil acidity.11,12 In these studies, photometric titration procedures required the processing of a set of reference solutions to obtain calibration curves, which were used to allow oil acidity determination. Titration strategies to allow acidity determination without using calibration curves were accomplished later employing the multicommuted flow analysis (MCFA) and monosegmented approaches (MSFA).17–20
Nowadays, attention has been given to the analytical procedures that provide results of high quality and that consume less reagent while generating a low volume of waste (as suggested by the Green Analytical Chemical (GAC) guidelines).21,22 These requirements have been achieved by developing analytical procedures employing a miniaturized flow setup.23–28
In this work, we intend to develop a miniaturized flow setup and an analytical procedure for the determination of olive oil acidity, which will be accomplished by employing a photometric titration strategy using a multicommuted flow-batch approach,29–31 which affords facilities to mimic the manual titration procedure, thus eliminating the use of calibration curves to find the acid concentration. Aiming to comply with the GAC requirements,21,22 the flow system will be designed with downsized dimensions, which will be done by attaching a radiation source (LED) and a photodetector to the titration chamber in order to form a compact unit.
2. Experimental
2.1. Apparatus
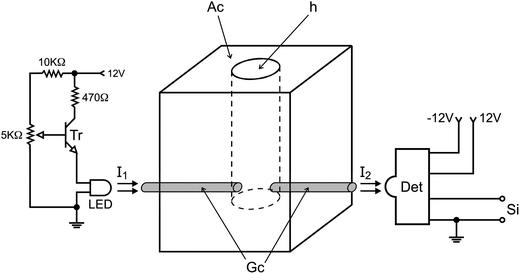
Equipment and accessories include a microcomputer furnished with an electronic interface card PCL-711S (Advantech Corp, OH, USA), running software written in Quick BASIC 4.5; a Ismatec IPC-8 peristaltic, furnished with pumping tubes (Tygon MHLL, Ismatec); 4 three-way solenoid valves P/N:HPBPP031 and a solenoid pinch valve P/N:225P091-21 (NResearch, New Jersey, NJ, USA); a 12 V stabilized power supply with a current intensity of 2 A to feed solenoid valves; a digital interface to control valves as described elsewhere;32 a homemade titration chamber as shown below; a dc motor (12 V) of 2000 RPM; a high brightness LED (intensity 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mcd) with maximum emission at λ = 530 nm and a narrow viewing angle (<20°); a photodiode IPL 10530 DAL; a transistor BC547; resistors of 270, 1 k, and 5 kΩ; and a polyethylene tube (200 cm, 0.8 mm i.d.) to make the flow lines. A metallic box was used to accommodate the titration chamber and the photometer with the following dimensions: 15 cm width, 20 cm height and 12 cm in depth.
000 mcd) with maximum emission at λ = 530 nm and a narrow viewing angle (<20°); a photodiode IPL 10530 DAL; a transistor BC547; resistors of 270, 1 k, and 5 kΩ; and a polyethylene tube (200 cm, 0.8 mm i.d.) to make the flow lines. A metallic box was used to accommodate the titration chamber and the photometer with the following dimensions: 15 cm width, 20 cm height and 12 cm in depth.
2.2. Reagent solutions and samples
All solutions were prepared with analytical grade chemicals and stored in glass or polyethylene bottles when necessary.n-Propanol from Sigma Aldrich (USA) was used as a carrier fluid and to prepare titrant and indicator solutions. A 0.1 mol L−1 potassium hydroxide solution (Merck, Germany) was prepared by dissolving 1.12 g of solid in 200 mL of n-propanol, which was stored in an Amber vessel and when not in use it was maintained in a freezer at 4 °C. Before starting the titration run, an aliquot of 25 mL of this solution was standardized using the phthalate methodology. A 0.1% (w/v) phenolphthalein solution (Carlo Erba, Italy) was prepared by dissolving 0.1 g of solid in 100 mL of n-propanol.
Oil samples were purchased from a local market and processed without any previous handling. For comparison of results, samples were processed employing the method suggested by the European Commission Regulation no. 2568/91 (ECC 1991).5
2.3. Flow analysis setup and procedure
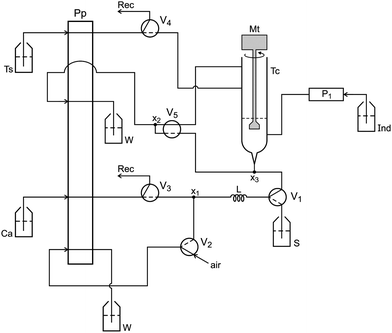
The flow analysis setup designed to implement the photometric titration is shown in Fig. 1 on standby. Under these conditions, only the peristaltic pump is run. Because all valves are switched OFF, the carrier fluid (Cs) and titrant solution are circulating through their storing vessels.The setup was controlled using a microcomputer furnished with digital interface and running software in Quick BASIC 4.5. When the software initiated, the solenoid valves V3 and V4 were switched ON for 15 s to fill their coupling flow lines up to the titration chamber (Tc). At the same time, the solenoid mini-pump was switched ON/OFF 10 times to fill the flow line with dye solution. Solenoid valve V5 remained ON for 15 s to empty the titration chamber. Solenoid valves V3 and V5 were then switched ON sequentially for 15 s each. This washing step was repeated twice to ensure a complete washing of the titration chamber. The pumping flow rate of the carrier fluid was increased to 130 μL s−1 to save time.
The photometer and the titration chamber were assembled as a compact unit, which was accommodated into a small metallic box as described above, and the assembling diagram is shown in Fig. 2.
When the photometer is powered, the calibration step can be carried out prior to beginning the analytical run by carrying out the following steps. 1000 μL of carrier fluid is delivered into the titration chamber by maintaining valve V3 switched ON for 10 s. The intensity of the radiation beam (I1) emitted by the LED is adjusted by the variable resistor (5 kΩ) wired to the base of the transistor (Fig. 2). The radiation beam (I2) coming from the titration chamber (Tc) is collected by the photodiode, generating a difference of electric potential (mV), which presents a direct relationship with the radiation beam intensity. This signal was converted from analog to digital by the PCL711 interface and read by the microcomputer. The brightening of the LED was varied until the microcomputer displayed a reading value of 2000 mV on the screen. This measured value (Vf) was saved as a reference to identify the end point of the titration.
The titration chamber washing step described above was performed prior to beginning a new titration run, which was accomplished as follows. At the first step, V1 and V2 (Fig. 1) were switched ON for 15 s to fill the sampling loop (L) with olive oil (S). Afterwards, valve V3 was switched ON to direct the carrier fluid (n-propanol) towards the sampling loop (L). While this valve state was maintained, the sample aliquot was displaced by the carrier fluid (Cs) towards the titration chamber (Tc). After displacing the sample aliquot, valve V3 was switched OFF. The mini-pump P1 was switched ON/OFF three times to insert an aliquot (30 μL) of the dye indicator (phenolphthalein) into the titration chamber. The titration run began by switching ON both the DC motor (Mt) and valve V4, in order to allow that mixing proceeded while the titrant solution was added to the titration chamber. Simultaneously, the microcomputer read the signal (Si) generated by the photometer. When a signal variation (ΔV = Vf − Si) exceeded a previously settled threshold value, valve V4 was switched OFF to interrupt the addition of the titrant solution. After 0.5 s, the DC motor was turned OFF, and the analytical signal was read again. If this signal was lower than the threshold value, valve V4 was switched ON for 0.2 s while the DC motor was left ON for 2.0 s and the signal was read again. This sequence of events was repeated to achieve a signal higher than the threshold value. Assays carried out using a standardized sample of olive oil indicated that 200 mV was a convenient value for the threshold, which was selected as a parameter to be used to find the end point of the titration. Prior to beginning the next titration run, the titration chamber was emptied and washed as described previously.
To ensure the precision of the measurements, switching valve V4 was synchronized to insert the titrant solution with the pumping pulsation pattern. Prior to switching ON valve V4, the microcomputer awaited the signal from the peristaltic pump, which was read through the analog input of the PCL711 interface card that was coupled to the pump roller count interface.
The flow rate of the titrant solution varied from 2.7 up to 5.5 μL s−1, and the amount of titrant solution used was controlled by time. To find the end of the titration, valve V4 was switched ON several times. These time intervals were summed to calculate the volume of titrant solution used. After completing the titration, valve V5 was turned ON to empty the titration chamber.
Once the operational conditions were established, to prove the effectiveness of the proposed procedure, a set of olive oil samples was processed. For accuracy, samples were analyzed using a reference method.5
3. Results and discussion
3.1. General comments
According to the European Commission Regulation guidelines, the acid concentration of olive oil must be expressed as a percentage (w/w) of oleic acid,5 which was done using the following equation:| Ac = [(V × C × M)/m] × 100 |
This equation was included in the control software, so that when the software initiated, the microcomputer requested actualization of the values of C and m parameters, which could be changed prior to beginning the titration run. The molar mass of oleic acid was previously included in the software as a permanent parameter.
Preliminary assays were carried out using chloroform and n-propanol as diluents to select the best one to minimize oil viscosity, which could impair the effectiveness of the proposed procedure. We observed that oil dissolved more easily in an n-propanol medium, which was also efficient for cleaning the titration chamber. This organic solvent was less aggressive than chloroform, thus allowing the use of acrylic pieces and polyethylene tube to construct the flow system manifold. These assays were carried out at a laboratory temperature of 25 °C that was maintained for further experiments.
The previous studies related to the determination of olive oil acidity11–13 employed titration procedures that did not satisfy the equivalency criterion established by the IUPAC,33 hence requiring the use of calibration curves to determine the acidity concentration, which was done by processing a set of acid standard solutions.11–13 In the current work, this drawback was overcome by developing an automatic titration procedure for acidity determination without using calibration curves (true titration). The procedure was accomplished by employing a multicommuted flow-batch setup designed for this purpose, allowing that, for the first time, a true titration procedure in a non-aqueous medium was carried out.
3.2. Effect of the titrant solution flow rate and concentration
A high flow rate of titrant solution could make it difficult to select the smaller volume required to find the end point, while a low flow rate would extend the time interval to finish the titration run. Aiming to find the better compromise to save time without impairing the quality of results, assays were done by setting flow rates of 2.7, 4.0, and 5.5 μL s−1, yielding the results shown in Table 1.Employing a flow rate of 2.7 μL s−1, the time intervals elapsed to complete the titration were 35 s and 140 s, when the labeled samples' acidities were 0.2 and 1.0%, respectively. While employing a flow rate of 4.0 μL s−1, the time intervals were 3.0 and 12.0 s, respectively. While performing the assays, we observed that while employing a flow rate of 5.5 μL s−1, the color change occurred quickly, generating a very intense pink color, indicating that a greater excess of titrant solution was delivered to the titration chamber. Under these conditions, the fine adjustment described in the Experimental section could not be applied. Considering the precision of the measurements, both flow rates 2.7 and 4.0 μL s−1 yielded acceptable results, but to save time, the flow rate of 4.0 μL s−1 was selected for the titrant solution. These assays were carried out using a 0.1 mol L−1 potassium hydroxide solution. Additional assays were performed using titrant solutions with a concentration of 0.05 and 0.15 mol L−1. In the first case, the time interval at the end of the titration was two-fold, while in the second one the precision of the measurements was worsened, thereby the titrant solution concentration of 0.1 mol L−1 was selected.
3.3. Effect of sample volume
To ascertain the effect of the sample volume, assays employing oil samples and titrant solution described above and sampling loops with lengths of 12.5, 25, and 50 cm (volumes of 62.5, 125 and 250 μL) yielded the results shown in Table 2. By analyzing the results, we observe that data related to the sampling loop with a volume of 62.5 μL yielded errors higher than 8%, which is not acceptable. Data for sampling loops with volumes of 125 and 250 μL presented relative errors lower than 2.0%. Although the sample aliquot was collected using a sampling loop, these results show that the sample volume can affect the precision of the results. An intrinsic sampling error minimized by increasing the volume of sample aliquot and by increasing the titration solution volume could improve the results. As both loops gave results with similar precision, and to save time and titrant solution, the loop with a volume of 125.0 μL was selected.To assess the correct amount of olive oil collected by the sample loop (125.0 μL), the loop was filled and weighed five times, yielding a mass value of 0.135 ± 0.002 g, which was included in the control software to facilitate acidity determination.
3.4. Performance comparison
The main features of the proposed procedure and other studies published before are summarized in Table 3 to allow a comparison of performance.| Parameters | Proposed procedure | Ref. 11 | Ref. 12 | Ref. 13 | Ref. 14 |
|---|---|---|---|---|---|
| a Sample determination per hour. b Waste generated per determination. c Relative standard deviation. | |||||
| Sample (μL) | 125 | 280 | 175 | 130 | >170 |
| Throughputa | 40–50 | 60 | 30–100 | 60 | 12 |
| Wasteb (mL) | 1.2–1.6 | 2–10 | 3–7 | 3.6 | 21 |
| Concentration range (%) | 0.10–1.5 | 0.1–1.3 | 0.15–8.0 | 0.15–0.81 | 0.06–9.0 |
| RSDc (%) | 1.8 | <5 | 2.1 | 1.5–2.50 | 4.9–7.8 |
By analyzing the results shown in Table 3, we can observe that the overall performance of the proposed procedure is very favorable, presenting the advantages of better relative standard deviation and low volume of waste generated per determination. The concentration range is narrower than those presented in ref. 12 and 14 but it is enough to analyze the olive oils usually found in the market.
3.5. Comparison of results
To prove the effectiveness and accuracy of the proposed procedure, a set of olive oil samples were analyzed by employing both the proposed procedure and a reference method,5 yielding the results shown in Table 3.Plotting the data obtained by a reference method and the proposed procedure on the ordinate and abscise axis, respectively, presented the following linear equation: Y = 1.0115X − 0.0077 (R2 = 0.9937). Since slope and intersect values are near unit and zero, respectively, we can consider that the results were similar. Applying the paired t-test to the results for a 95% confidence level (considering the degree of freedom of fifteen), the calculated value is tcal = 0.12015 while the theoretical value is t = 1.75305, indicating no statistically significant difference between the results.
The sampling throughput and waste volume can vary as a function of the acid concentration, owing to the throughput decrease when processing samples with higher acid concentration. Processing the samples shown in Table 4, the consumption of titrant solution per titration run varied within the range of 0.056 to 0.084 μg.
| Samples | Maximum acidity labeled value | Proposed method | Reference method5 |
|---|---|---|---|
| Acidity (%) | |||
| a Results are the average of 6 consecutive measurements that were carried out using a 0.085 mol L−1 titrant solution. b Aged olive oil. | |||
| A1 | 0.2 | 0.191 ± 0.003 | 0.18 |
| A2 | 0.3 | 0.342 ± 0.001 | 0.33 |
| A3 | 0.4 | 0.373 ± 0.003 | 0.37 |
| A4 | 0.5 | 0.371 ± 0.001 | 0.37 |
| A5 | 0.6 | 0.470 ± 0.005 | 0.48 |
| A6 | 0.7 | 0.564 ± 0.004 | 0.56 |
| A7 | 0.8 | 0.595 ± 0.003 | 0.60 |
| A8 | 1.0 | 0.892 ± 0.003 | 0.92 |
| A9 | 0.5 | 0.442 ± 0.002 | 0.43 |
| A10 | 0.5 | 0.377 ± 0.003 | 0.38 |
| A11 | 0.4 | 0.373 ± 0.001 | 0.36 |
| A12b | 0.5 | 0.932 ± 0.003 | 0.97 |
| A13 | 1.0 | 0.771 ± 0.003 | 0.78 |
| A14 | 1.0 | 0.865 ± 0.003 | 0.88 |
| A15 | 1.0 | 0.896 ± 0.002 | 0.86 |
| A16 | 1.0 | 0.937 ± 0.003 | 0.89 |
4. Conclusions
The overall performance of the flow system module, the photometer, and the analytical procedure yielded good results. The equipment setup can work for several days without any maintenance. The robustness of the proposed system was ascertained by running them for 3 hours per day for two weeks. The results obtained by processing a sample previously standardized showed a variation around 2%. The setup is simple to operate and does not require experienced personnel. The lower volume of waste generated allow us to confirm that the miniaturized setup is an excellent tool to develop an analytical procedure in accordance with the GAC guidelines.21,22The accuracy of the results is an unequivocal indication of the system's effectiveness. The results achieved without using a calibration curve would also be considered as an advantage compared with previous studies.11–13 The high throughput associated with low volume of waste generation and good quality of results allow us to conclude that the proposed procedure is a viable alternative to the European Community's official method.5 Considering the effectiveness of the titration setup we can conclude that it would be used to accomplish titration of other kinds of organic samples.
Acknowledgements
The authors acknowledge the financial support from CNPq, CAPES, FAPESP and INCTAA.References
- M. Ruiz-Canela and M. A. Martínez-González, Maturitas, 2011, 68, 245 CrossRef CAS PubMed.
- M. Kratz, P. Cullen, A. Kassner, M. Fobker, P. M. Abuja, G. Assmann and U. Wahrburg, Eur. J. Clin. Nutr., 2002, 56, 72 CrossRef CAS PubMed.
- R. M. El-Abassy, P. Donfack and M. Arnulf, J. Am. Oil Chem. Soc., 2009, 86, 507 CrossRef CAS.
- A. C. Rustan and C. A. Drevon, Fatty Acids: Structures and Properties, eLS, Wiley Online Library, 2005 DOI:10.1038/npg.els.0003894.
- International Olive Council, COI/T.15/NC.no. 3. Rev.2, 24, 2006, http://www.internationaloliveoil.org, accessed 15 November 2012.
- J. A. Pereira, S. Casal, A. Bento and M. B. P. P. Oliveira, J. Agric. Food Chem., 2002, 50, 6335 CrossRef CAS PubMed.
- http://www.gallo.pt/#/tudo-sobre-azeite/classificacao-do-azeite, accessed 19 November 2012.
- P. Dais, A. Spyros, S. Christophoridou, E. Hatzakis, G. Fragaki, A. Agiomyrgianaki, E. Salivaras, G. Siragakis, D. Daskalaki, M. Tasioula-Margari and M. Brenes, J. Agric. Food Chem., 2007, 55, 577 CrossRef CAS PubMed.
- A. Al-Alawi, F. R. van de Voort and J. Sedman, Spectrosc. Lett., 2005, 38, 389 CrossRef CAS.
- A. Velasco-Arjona and M. D. L. De Castro, J. Am. Oil Chem. Soc., 1998, 75, 1849 CrossRef CAS.
- E. Mariotti and M. Mascini, Food Chem., 2001, 73, 235 CrossRef CAS.
- P. G. Nouros, C. A. Georgiou and M. G. Polissiou, Anal. Chim. Acta, 1997, 351, 291 CrossRef CAS.
- P. Linares, M. D. L. de Castro and M. Valcárcel, Anal. Chim. Acta, 1989, 225, 431 CrossRef CAS.
- Z. L. Zhi, A. Rios and M. Valcárcel, Anal. Chim. Acta, 1996, 318, 187 CrossRef CAS.
- J. Ruzicka, E. H. Hansen and H. Mosbaek, Anal. Chim. Acta, 1977, 92, 235 CrossRef CAS.
- C. A. Georgiou and M. A. Koupparis, Analyst, 1988, 113, 755 RSC.
- M. Korn, L. F. B. P. Gouveia, E. de Oliveira and B. F. Reis, Anal. Chim. Acta, 1995, 313, 177 CrossRef CAS.
- A. P. S. Paim and B. F. Reis, Anal. Sci., 2000, 16, 487 CrossRef CAS.
- E. P. Borges, P. B. Martelli and B. F. Reis, Microchim. Acta, 2000, 135, 179 CrossRef CAS.
- E. V. Aquino, J. J. R. Rohwedder and C. Pasquini, Anal. Chim. Acta, 2001, 438, 67 CrossRef.
- S. Armenta, S. Garrigues and M. de la Guardia, TrAC, Trends Anal. Chem., 2008, 27, 497 CrossRef CAS.
- W. R. Melchert, B. F. Reis and F. R. P. Rocha, Anal. Chim. Acta, 2012, 714, 8 CrossRef CAS PubMed.
- M. A. Feres and B. F. Reis, Talanta, 2005, 68, 422 CrossRef CAS PubMed.
- S. S. Borges, J. D. Peixoto, M. A. Feres and B. F. Reis, Anal. Chim. Acta, 2010, 668, 3 CrossRef CAS PubMed.
- Y. L. Yu, Y. Jiang and R. H. He, Talanta, 2012, 88, 352 CrossRef CAS PubMed.
- D. J. Cocovi-Solberg, M. Miró, V. Cerdà, M. Pokrzywnicka, L. Tymecki and R. Koncki, Talanta, 2012, 96, 113 CrossRef CAS PubMed.
- G. P. Vieira, S. R. W. Perdigão, M. F. Fiore and B. F. Reis, Sens. Actuators, B, 2012, 161, 422 CrossRef CAS.
- M. B. Lima, I. S. Barreto, S. I. E. Andrade, M. S. S. Neta, L. F. Almeida and M. C. U. Araújo, Talanta, 2012, 98, 118 CrossRef CAS PubMed.
- R. S. Honorato, M. C. U. Araújo, R. A. C. Lima, E. A. G. Zagatto, R. A. S. Lapa and J. L. F. C. Lima, Anal. Chim. Acta, 1999, 396, 91 CrossRef CAS.
- E. P. Medeiros, E. C. L. Nascimento, A. C. D. Medeiros, J. G. V. Neto, E. C. da Silva and M. C. U. Araujo, Anal. Chim. Acta, 2004, 511, 113 CrossRef CAS.
- M. B. da Silva, C. C. Crispino and B. F. Reis, J. Braz. Chem. Soc., 2010, 21, 1854 CrossRef CAS.
- E. Rodenas-Torralba, F. R. P. Rocha, B. F. Reis, A. Morales-Rubio and M. de la Guardia, J. Autom. Methods Manage. Chem., 2006, 2006, 20384 CrossRef PubMed.
- H. M. N. H. Irving, H. Freiser and T. S. West, IUPAC Compendium of Analytical Nomenclature, Definitive Rules 1977, Pergamon, Oxford, 41, 1978 Search PubMed.
| This journal is © The Royal Society of Chemistry 2014 |