Xanthone based Pb2+ selective turn on fluorescent probe for living cell staining†
Debasis
Karak
a,
Arnab
Banerjee
a,
Sisir
Lohar
a,
Animesh
Sahana
a,
Subhra Kanti
Mukhopadhyay
b,
Sushanta. S.
Adhikari
c and
Debasis
Das
*a
aDepartment of Chemistry, The University of Burdwan, Burdwan-713104, West Bengal, India. E-mail: ddas100in@yahoo.com; Fax: +91-342-2530452; Tel: +91-342-2533913
bDepartment of Microbiology, The University of Burdwan, Burdwan-713104, West Bengal, India
cDepartment of Chemistry, University College of Science and Technology, University of Calcutta, Kolkata-700009, India
First published on 30th October 2012
Abstract
A xanthone based Pb2+ selective turn-on fluorescent probe (L) was designed, synthesized, and characterized by different spectroscopic techniques. Binding of L to Pb2+ induced significant change in the absorption and/fluorescence properties. Other common cations, viz. Na+, K+, Ca2+, Mg2+, Ag+, Mn2+, Hg2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+ and Cr3+ did not interfere. The limit of detection of the method was 1.8 × 10−7 M. L can detect intra-cellular Pb2+ in living cells.
Pb2+, one of the most toxic heavy cations is believed to be responsible for memory loss, anemia, muscle paralysis, and mental retardation, particularly for children.1,2 Based on the toxicity of Pb2+ ion, the permissible limit set by the US Environmental Protection Agency (EPA) and Bureau of Indian Standards (BIS) is 0.015 mg L−1 and 0.1 mg L−1, respectively. As this metal has been used for a long time in batteries, gasoline, pigments, and drinking water distribution systems,3 it is widely distributed in the environment. Several methods are available for trace level determination of Pb2+, viz. atomic absorption spectrometry, ion-selective electrodes and inductively coupled plasma mass spectrometry.4 While these methods allow the low level determination of Pb2+ as required by the World Health Organization (15 ppb),5 they require expensive instrumentation and tedious sample pre-treatment. Nowadays, fluorescence spectroscopy is exhaustively used for the determination of Pb2+ because of its high sensitivity, simplicity, rapidity and low operational cost. An efficient fluorescent probe changes its emission intensity and/or spectral behaviour upon selective binding with the analyte.6,7 The xanthone nucleus which is present in numerous natural products, exhibits a wide range of interesting biological and pharmacological activities, e.g. antimicrobial,8,9 antioxidant,8,9 antimalarial,8,9 anticancer10 and anti-HIV activity.11 Presently, we have been engaged in the development of small fluorescent probes for the selective determination of toxic cations and anions.12 Moreover, the fluorescence property of the xanthone nucleus has encouraged us to design a highly selective, facile and inexpensive synthesis method for its derivative as a fluorescent indicator (L) for Pb2+. Recently, several chemosensors based on the fluorometric and/or colorimetric determination of Pb2+ have been reported.13–19 However, most of them have required tedious synthetic protocols involving multistep reactions. We have synthesized L in a single step from commercially available starting materials in a short time with high yield. L is less expensive than other reported fluorophores.
Absorption and emission spectra are recorded with a Shimadzu Multi Spec 1501 absorption spectrophotometer and Hitachi F-4500 fluorescence spectrophotometer, respectively. Stock solutions of L (100 μM) and tested metal ions (1000 μM) are prepared in DMSO and H2O respectively. All the experiments have been performed in DMSO–H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). Scheme 1 shows the synthesis of L. It is characterized by MALDI-TOF MS, FTIR and 1H NMR spectroscopic studies (Fig. S1, S2 and S3†).
1, v/v). Scheme 1 shows the synthesis of L. It is characterized by MALDI-TOF MS, FTIR and 1H NMR spectroscopic studies (Fig. S1, S2 and S3†).
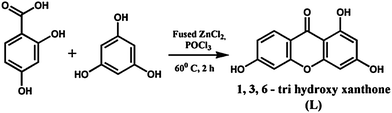 | ||
| Scheme 1 Synthesis of L. | ||
Fig. 1 illustrates the changes in the UV-Vis spectra of L (100 μM) in the presence of Pb2+. Upon gradual addition of Pb2+ to the solution of L, the intensity of a new broad shoulder at 405 nm gradually increased. The inset shows the linear range.
 | ||
| Fig. 1 Changes of the UV-Vis spectra of L (1 μM) with externally added Pb2+ (0, 10, 20, 30, 40, 50 μM). | ||
The effect of different metal ions (100 μM) viz. Na+, K+, Ca2+, Mg2+, Ag+, Mn2+, Hg2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Cr3+ and Pb2+ on the fluorescence properties of L (1 μM, λem = 510 nm, λex = 405 nm) is presented in Fig. 2. While Pb2+ enhances the emission intensity of L to a significant extent, two other cations viz. Cu2+ and Fe3+ quench the emission intensity to a very small extent and the remaining cations have no effect.
 | ||
| Fig. 2 Effect of different cations (100 μM) on the emission spectra of L (1 μM). Other metal ions: Na+, K+, Ca2+, Mg2+, Ag+, Mn2+, Hg2+, Cd2+ (λem = 510 nm, λex, 405 nm). | ||
The very weak emission intensity of L increases gradually up to a maximum of 9 fold upon the addition of Pb2+ (Fig. 3). The linear region of the plot of fluorescence intensity vs. externally added Pb2+ concentration (inset, Fig. 3) can be used for the determination of unknown Pb2+ concentration. The enhancement of fluorescence is ascribed as the chelation-enhanced fluorescence (CHEF) effect that reduces the intra-molecular charge transfer (ICT) process in L. The possibility of other types of binding of L with Pb2+ is ruled out on the basis of the 1H NMR of the [L–Pb2+] complex (discussed later) with the justification that the lone pair on carbonyl oxygen (sp2) is less available than the ether oxygen (sp3) for binding to Pb2+ and carbonyl oxygen is stabilized through intra-molecular hydrogen bonding as shown in Fig. 4.
![Emission spectra of L (1 μM) in the presence of 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 150 and 200 μM Pb2+. Inset: plot of emission intensity of Lvs. [Pb2+].](/image/article/2013/AY/c2ay25935d/c2ay25935d-f3.gif) | ||
| Fig. 3 Emission spectra of L (1 μM) in the presence of 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 150 and 200 μM Pb2+. Inset: plot of emission intensity of Lvs. [Pb2+]. | ||
 | ||
| Fig. 4 Proposed binding mode of L for Pb2+. | ||
The limit of detection (LOD) of Pb2+ was found to be 1.8 × 10−7 M (Fig. S4†). The binding constant of L with Pb2+ was estimated using the Benesi–Hildebrand equation,20 (F∞ − F0)/(Fx − F0) = 1 + 1/Ka[C]n, where F0, Fx and F∞ are the emission intensities of L in the absence of Pb2+, at an intermediate [Pb2+] and at a concentration of complete interaction, respectively. While, Ka is the binding constant, C is the concentration of Pb2+ and n is the number of Pb2+ bound per L (here, n = 1/2). The value of Ka was 0.125 × 104 M−1/2 with R2 = 0.96 (Fig. S5†). While Job's plot indicates 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (L
1 (L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pb2+, mole ratio) stoichiometry (Fig. S6†) of the [L–Pb2+] complex, the mass spectrum of the [L–Pb2+] complex (Fig. S7†) also supports the composition. FTIR of L shows three OH stretching frequencies, viz. 3200.36 cm−1 (hydrogen bonded, broad), 3494.3 and 3390.72 cm−1. Formation of the L–Pb2+ complex shifts the OH stretching frequency from 3494.3 to 3501.98 cm−1, keeping the other two OH stretching frequencies unaltered (Fig. S8†). Selectivity of L for Pb2+ has been tested and it was found that Cu2+ and Fe3+ interfere to a negligible extent (Fig. S9†), the interference was eliminated completely using thiocyanate as a masking agent. Thiocyanate has no adverse effect on the emission intensity of the [L–Pb2+] system.
Pb2+, mole ratio) stoichiometry (Fig. S6†) of the [L–Pb2+] complex, the mass spectrum of the [L–Pb2+] complex (Fig. S7†) also supports the composition. FTIR of L shows three OH stretching frequencies, viz. 3200.36 cm−1 (hydrogen bonded, broad), 3494.3 and 3390.72 cm−1. Formation of the L–Pb2+ complex shifts the OH stretching frequency from 3494.3 to 3501.98 cm−1, keeping the other two OH stretching frequencies unaltered (Fig. S8†). Selectivity of L for Pb2+ has been tested and it was found that Cu2+ and Fe3+ interfere to a negligible extent (Fig. S9†), the interference was eliminated completely using thiocyanate as a masking agent. Thiocyanate has no adverse effect on the emission intensity of the [L–Pb2+] system.
1H NMR titration of L with Pb2+ was performed in DMSO-d6 (Fig. 5). One –OH (Hb) is shifted downfield due to coordination of its lone pair to Pb2+ while no significant change to the chemical shift values for the other two –OH protons indicate their non-involvement in the coordination of Pb2+.
 | ||
| Fig. 5 Changes in the 1H NMR spectra of L with addition of Pb(NO3)2 in DMSO-d6: (A) free L; (B) L with 0.5 equivalent of Pb2+ (C) L with 1 equivalent of Pb2+. | ||
Fig. 6 clearly indicates that Pb2+ contaminated cells emit green fluorescence in presence of L under fluorescence microscope. Thus L will be useful for determination of Pb2+ toxicity in living cells.
 | ||
| Fig. 6 Fluorescence microscopic images of (A) Candida albicans cells; (B) Candida cells incubated with Pb2+; (C) Candida cells incubated with Pb2+ followed by addition of L; (D) Pollen cells; (E) Pollen cells incubated with Pb2+; (F) Pollen cells incubated with Pb2+ followed by addition of L. Bright field images of (G) Candida albicans cells incubated with Pb2+ followed by addition of L; (H) Candida cells incubated with Pb2+; (I) Pollen cells incubated with Pb2+ followed by addition of L; (J) Pollen cells incubated with Pb2+. | ||
We have successfully synthesized and characterized a biologically important xanthone based inexpensive turn on fluorescent probe for the selective determination of Pb2+. Fluorescence enhancement is attributed to the Pb2+ assisted CHEF process and inhibition of ICT. L has a fair binding ability to Pb2+ with an association constant (Ka) of 0.125 × 104 M−1/2. The LOD of the probe was 1.8 × 10−7 M. The probe is very efficient for the detection of intra-cellular Pb2+. Table S1†demonstrates that the present probe is very competitive and cheaper than the available Pb2+ selective sensors for living cell imaging.
Acknowledgements
Authors sincerely thank the UGC-DAE-KOLKATA center for financial support. A. Sahana and S. Lohar are thankful to CSIR, New Delhi for providing fellowships. Authors thank the Indian Institute of Chemical Biology (IICB), Kolkata for extending the mass spectrometer facilities. We sincerely acknowledge the University Science Instrumentation Center (USIC), Burdwan University for providing the fluorescence microscope facility.Notes and references
- J. S. Lin-Fu, Lead Poisoning, a Century of Discovery and Rediscovery, in Human Lead Exposure; H. L. Needleman, ed. Lewis Publishing, Boca Raton, FL, 1992 Search PubMed.
- A. Fetch, Crit. Rev. Anal. Chem., 1998, 28, 267 CrossRef.
- N. Castelino, P. Castelino and N. Sannolo, Inorganic Lead Exposure: Metabolism and Intoxication, The Agency for Toxic Substances and Disease Registry (ATSDR), U.S. Departement of Health and Human Services, accessed on 22 May 2008 Search PubMed.
- M. R. Cave, O. Butler, R. N. Chenery, J. M. Cook, M. S. Cresser and D. L. Miles, J. Anal. At. Spectrom., 2001, 16, 194 RSC.
- J. K. Fawell, Guidelines for Drinking Water Quality, World Health Organization, Geneva, 2nd edn, 1996, vol. 2, p. 940 Search PubMed.
- Chemosensors for Ion and Molecule Recognition, ed. J. P. Desvergne and A.W. Czarnik, Kluwer, Boston, 1997 Search PubMed.
- E. Bakker, P. Buhlmann and E. Pretsch, Chem. Rev., 1997, 97, 3083 CrossRef CAS.
- V. Peres and T. J. Nagem, Phytochemistry, 1997, 44, 191 CrossRef CAS.
- V. Peres, T. J. Nagem and F. F. De Olivera, Phytochemistry, 2000, 55, 683 CrossRef CAS.
- M. K. Schwaebe, T. J. Moran and J. P. Whitten, Tetrahedron Lett., 2005, 46, 827 CrossRef CAS.
- G. A. Cordell, A. D. Kinghorn, T. Pengsuparp, L. Cai, H. Constant, H. S. Fong, Z. L. Lin, J. M. Pezutto, K. Ingolfsdottir, H. Wagner and S. H. Hughes, J. Nat. Prod., 1995, 58, 1024 CrossRef.
- (a) A. Sahana, A. Banerjee, S. Das, S. Lohar, D. Karak, B. Sarkar, S. K. Mukhopadhyay, A. K. Mukherjee and D. Das, Org. Biomol. Chem., 2011, 9, 5523 RSC; (b) S. Das, S. Guha, A. Banerjee, S. Lohar, A. Sahana and D. Das, Org. Biomol. Chem., 2011, 9, 7097 RSC; (c) S. Guha, S. Lohar, A. Banerjee, A. Sahana, A. Chaterjee, S. K. Mukherjee, J. S. Matalobos and D. Das, Talanta, 2012, 91, 18 CrossRef CAS; (d) D. Karak, S. Lohar, A. Sahana, S. Guha, A. Banerjee and D. Das, Anal. Methods, 2012, 4, 1906 RSC; (e) A. Sahana, A. Banerjee, S. Guha, S. Lohar, A. Chattopadhyay, S. K.Mukhopadhyay and D. Das, Analyst, 2012, 137, 1544 RSC; (f) A. Banerjee, A. Sahana, S. Guha, S. Lohar, I. Hauli, S. K. Mukhopadhyay, J. S. Matalobos and D. Das, Inorg. Chem., 2012, 51, 5699 CrossRef CAS; (g) A. Banerjee, A. Sahana, S. Das, S. Lohar, S. Guha, B. Sarkar, S. K. Mukhopadhyay, A. K. Mukherjee and D. Das, Analyst, 2012, 137, 2166 RSC.
- E. Ranyuk, C. M. Douaihy, A. Bessmertnykh, F. Denat, A. Averin, I. Beletskaya and R. Guilard, Org. Lett., 2009, 11, 987 CrossRef CAS.
- J. Park and Y. Kim, Analyst, 2012, 137, 3246–3248 RSC.
- Y. Chen and J. Jiang, Org. Biomol. Chem., 2012, 10, 4782 CAS.
- A. K. Tyagi, J. Ramkumarb and O. D. Jayakumar, Analyst, 2012, 137, 760 RSC.
- X.-L. Ni, S. Wang, X. Zeng, Z. Tao and T. Yamato, Org. Lett., 2010, 13, 552 CrossRef.
- T. Lan, K. Furuyab and Y. Lu, Chem. Commun., 2010, 46, 3896 RSC.
- L.-J. Ma, Y.-F. Liu and Y. Wu, Chem. Commun., 2006, 2702 RSC.
- H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ay25935d |
| This journal is © The Royal Society of Chemistry 2013 |
