Electrospun PLGA fibre sheets incorporating fluorescent nanosensors: self-reporting scaffolds for application in tissue engineering†
H. C.
Harrington
a,
F. R. A. J.
Rose
b,
Y.
Reinwald
b,
L. D. K.
Buttery
b,
A. M.
Ghaemmaghami
c and
J. W.
Aylott
*a
aLaboratory of Biophysics and Surface Analysis, School of Pharmacy, University of Nottingham, UK. E-mail: jon.aylott@nottingham.ac.uk
bDivision of Drug Delivery and Tissue Engineering, Centre for Biomolecular Sciences, University of Nottingham, UK
cAllergy Research Group, School of Molecular Medical Sciences, University of Nottingham, UK
First published on 27th November 2012
Abstract
Ratiometric analyte responsive nanosensors have been incorporated into electrospun poly(lactic-co-glycolic) acid (PLGA) fibres to create self-reporting scaffolds. It has been demonstrated that the self-reporting scaffolds could be utilised to monitor microenvironment conditions without damaging the fabricated scaffold or the cells being cultured upon the construct. This presents opportunities to fully understand, monitor and optimise the growth of 3D model tissue constructs in vitro.
To address the issue of a current lack of process monitoring and control of in vitro tissue regeneration processes we have developed a self-reporting scaffold that can report the analyte concentration within the scaffold microenvironment. The developed biocompatible self-reporting scaffolds would allow microenvironment analyte concentration to be reported in situ and in real-time, permitting long-term experiments to be monitored without disruption. Monitoring in situ would offer the potential to control factors such as nutrient exchange and metabolic waste removal during all stages of tissue production.1 The use of the developed self-reporting scaffolds for tissue engineering applications could lead to a greater understanding of the conditions required to optimise growth in 3D culture systems.2,3
Scaffolds that act as sensing devices by monitoring fluorescence activity have been prepared previously, where the sensing device was either the actual polymeric scaffold constructed by electrospinning or through the use of an analyte responsive dye which is incorporated into the polymer prior to scaffold formation.4,5 However, these sensing devices are prone to interference from other analytes and therefore may give erroneous optical outputs. The use of a ratiometric sensing device holds the potential to eliminate these possible adverse effects and provide a response specific to the analyte in question. We report the incorporation of fluorescent, analyte responsive ratiometric nanosensors into the PLGA fibres to develop a novel self-reporting scaffold.
Ratiometric optical nanosensors incorporate both a reference and an analyte responsive indicator dye within a biocompatible matrix. The matrix is permeable to the analyte in question yet protects cells from the potentially toxic effects of the incorporated dyes.6,7 Analytes can diffuse through the pores of the matrix and interact with the entrapped dyes resulting in a change in fluorescence intensity from the analyte responsive dye, while the emission from the reference dye remains constant. The change in fluorescence will be proportional to the target analyte concentration thus enabling the response of cells to various stimuli to be measured. Taking a ratio of the responsive to reference fluorescent intensities at known analyte concentrations calibrates the system to which unknown concentrations can be compared. Ratiometric measurements are beneficial as the result is not affected by conditions such as fluctuations in temperature, light source intensity or by parameters such as heterogeneous dye concentrations.
Nanosensors have been prepared to monitor various analytes including pH, oxygen, glucose and calcium.8 In this work we prepared pH and oxygen nanosensors using a modified Stöber process9 (methods are provided in the accompanying ESI†). The nanosensors produced were determined by scanning electron microscopy (SEM) to have an average diameter of 300–400 nm dimensions (Fig. 1).
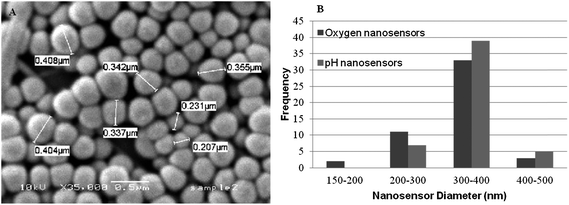 | ||
| Fig. 1 (A) SEM micrograph of oxygen responsive sol–gel nanosensors produced using the Stöber process. (B) Histogram showing particle diameters of oxygen and pH nanosensors. | ||
The fluorescence emission spectra of oxygen responsive nanosensors was measured at different dissolved oxygen concentrations over a biologically relevant range (0–21% (v/v)). It was demonstrated that the fluorophores retained their solution based properties when incorporated into the sol–gel matrix, with tris(4,7-diphenyl-1,10-phenanthroline)ruthenium(II) chloride (Ru(dpp)32+) (λexcitation 488 nm, λemission 630 nm) being dynamically quenched by oxygen, and Oregon Green Dextran (λexcitation 488 nm, λemission510 nm) being the reference dye (Fig. 2A). Calibration of the nanosensor response was determined from the ratio of the maximum fluorescence intensity of sensing dye to that of the reference dye using the Stern–Volmer equation. The linear Stern–Volmer plot (Fig. 2B) suggests that there are negligible matrix heterogeneity effects and minimal dye leaching from the sol–gel matrix. The dyes used for pH sensing remained optically responsive when incorporated into the sol–gel matrix where the fluorescence emission from 5-(and-6)-carboxyfluorescein (FAM) (λexcitation 488 nm, λemission 524 nm) changed at different pH while the emission response from the reference dye 6-carboxytetramethylrhodamine (TAMRA) (λexcitation 550 nm, λemission 580 nm) remained constant (Fig. 2C). Calibrations of pH response were performed over the pH range 5.5–8.0 and resulted in sigmoidal curves typical to pH measurements (Fig. 2D).10
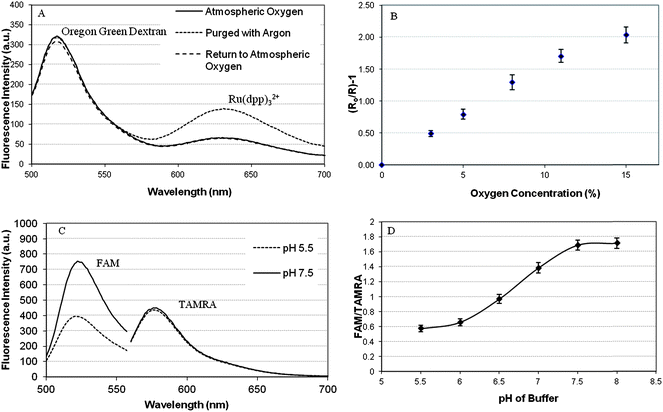 | ||
| Fig. 2 (A) Fluorescence emission spectra of oxygen responsive sol–gel nanosensors; showing the response of the incorporated dyes to oxygen. (B) Stern–Volmer plot for oxygen responsive sol–gel nanosensors over a biologically relevant oxygen concentration range. (C) Fluorescence emission spectra for pH responsive sol–gel performed at pH 5.5 and pH 7.5. (D) Calibration curve for pH responsive sol–gel nanosensors; calculated using the ratio of the fluorescence intensities FAM and TAMRA over a biologically relevant pH range. Error bars represent SEM N = 3. | ||
Analyte responsive nanosensors were incorporated into PLGA fibres by electrospinning (see accompanying ESI† for methodology). Fibre morphology was similar to polymer fibres electrospun under the same conditions but not containing nanosensors (Fig. 3A). The ability to produce scaffold fibres containing nanosensors of a regular morphology comparable to control samples suggests that the incorporation of the nanosensors does not compromise the electrospinning process. In addition the nanosensors were observed to be associated with the fibres as shown in Fig. 3B–D. The capability of the nanosensors to retain their fluorescence properties following incorporation into the PLGA fibres was verified using confocal laser scanning microscopy (CLSM). The CLSM images of the PLGA containing nanosensors responsive to pH are shown in Fig. 3C and D, where the dyes FAM and TAMRA can be observed to emit fluorescence in the green and red regions respectively when excited using an argon laser.
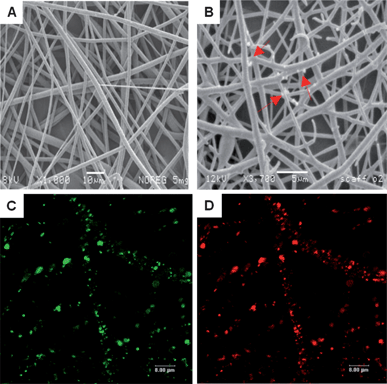 | ||
| Fig. 3 Representative SEM micrographs showing (A) control, PLGA fibres (not incorporating nanosensors). Scale bar 10 μm (B) PLGA fibres electrospun with 5 mg mL−1 nanosensors where the arrows indicate nanosensors associated with the fibres. Scale bar 5 μm. Representative CLSM images of nanosensors incorporated into PLGA scaffolds to create self-reporting scaffolds. (C) FAM. (D) TAMRA. Scale bars 8 μm. | ||
The self-reporting scaffolds are intended to not only serve as sensing devices, but also to provide mechanical support for tissue growth by allowing cell attachment and proliferation, whilst allowing cell migration through the interstices of the 3D electrospun structure. To determine if the prepared PLGA self-reporting scaffolds could support cellular growth, the cell line 3T3 fibroblast, was cultured upon UV sterilised and pre-wetted PLGA scaffolds. Following 3 days in culture upon the scaffolds, cell attachment and viability were confirmed using CLSM and SEM.
The SEM micrographs taken on day 3 following seeding showed the suitability of the self-reporting scaffold to support cell growth, where it was seen that the immortalised primary mouse embryonic fibroblast cell line (3T3) fibroblasts attached and spread along the PLGA scaffold fibres (Fig. 4A).
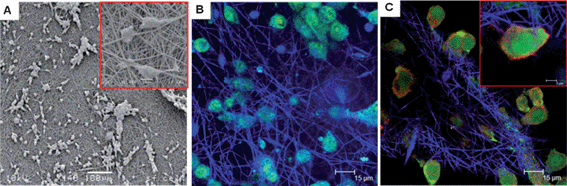 | ||
| Fig. 4 Cell viability studies. (A) SEM micrographs of 3T3 fibroblasts cultured upon self-reporting PLGA scaffolds for 3 days. Scale bars 100 μm and 20 μm for magnified inset. (B) Live/Dead® staining of 3T3 fibroblasts cultured upon PLGA scaffold for 3 days. Reflectance of PLGA scaffold pseudo coloured blue, green fluorescence of live cells. Dead cells depicted red fluorescence. Scale bar 15 μm. (C) Representative images evaluating the production of the ECM proteins fibronectin (green) and elastin (red) in 3T3 fibroblasts cultured upon PLGA scaffold. Scale bar 15 μm and 5 μm for magnified inset. | ||
The structural role of the scaffold is evident where 3T3 fibroblasts were observed to have attached and spread, their cell growth being guided by the fibre architecture with some cells aligning with the scaffold fibres and displaying typical filopodia extensions. Cell morphology was thought to be slightly spherical (rather than the characteristic spindle shape) and literature review reflected that this could be resolved following extended growth periods or by modifying the surface chemistry of the scaffold.11,12
Assessment of 3T3 fibroblast cell viability whilst being cultured upon control PLGA scaffolds (not incorporating nanosensors) was performed using a Live/Dead® assay. Cells incubated with the Live/Dead® stain were examined using CLSM (Fig. 4B), where viable cells are stained green and dead cells stained red. The confocal images show an abundance of green fluorescence and very little red fluorescence in the overlaid image, demonstrating that the majority of cells have remained alive following culture upon control PLGA scaffolds for 3 days. Scaffold fibres were visualised by observing the reflected light of the 488 nm argon laser, for image clarity this has been pseudo coloured blue.
A known limitation of synthetic scaffolds such as PLGA is that they lack the native biological cues that natural materials possess to promote the production of desirable cellular responses such as the production of matrix proteins.13 In designing scaffold systems the intention is that the supporting scaffold not only mimics the geometric capabilities of the natural extracellular matrix (ECM) but also the biochemical microenvironment. As the 3T3 fibroblast cells proliferate and migrate through the scaffold the cells will fill the inter-fibre spaces and secrete ECM proteins supportive to their growth and function. Fibroblasts are the main cellular component of the dermis; the main ECM proteins that they produce are collagen, fibronectin and elastin, to form connective tissues that provide the skin with its tensile strength.14 Immunofluorescence techniques coupled with CLSM imaging confirmed the presence of the ECM proteins, fibronectin and elastin, produced by 3T3 fibroblasts whilst being cultured upon PLGA scaffolds (Fig. 4C). Positive staining for the presence of ECM proteins in this study suggests that electrospun PLGA scaffolds are a suitable matrix to support the growth and function of fibroblast cells. Experiments performed for cell viability and ECM identification utilised PLGA scaffolds that did not incorporate nanosensors due to the same fluorescent channels being required for visualising the immunofluorescent dyes as the nanosensor emission. The 3T3 fibroblasts in this study have maintained viability following transfer from a monolayer culture plate to culture upon a PLGA scaffold as evidenced by their ability to secrete ECM proteins. The ability of the fibroblasts cultured upon PLGA scaffolds to produce ECM proteins suggests that the fibroblasts are able to function whilst being cultured in vitro within the scaffold environment.
The self-reporting scaffolds were examined further by CLSM to produce calibration curves. Self-reporting scaffolds incorporating oxygen responsive nanosensors were assessed for their ability to respond to changes in dissolved oxygen concentrations. Stern–Volmer plot was produced (Fig. 5A), demonstrating that self-reporting scaffolds are responsive to changes in dissolved oxygen concentration in the biologically relevant range. Self-reporting scaffolds responsive to pH were immersed into phosphate buffered saline solutions ranging from pH 5.5 to pH 8.0. Calibration plots displaying sigmoidal curves typical of pH measurements were produced indicating that incorporating the nanosensors into PLGA scaffold by the electrospinning process has not affected the ability of the nanosensors to respond to changes in environmental pH (Fig. 5B).
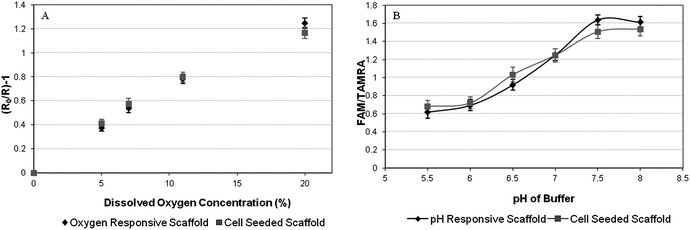 | ||
| Fig. 5 (A) The response of oxygen sensitive sol–gel nanosensors incorporated into PLGA scaffold with cells cultured upon the scaffold is compared to an oxygen sensing scaffold without cells upon it. Error bars represent SEM N = 3. (B)The response of pH sensitive sol–gel nanosensors incorporated into a PLGA scaffold with cells cultured upon the scaffold is compared to a pH sensing scaffold without cells being cultured upon it. | ||
Calibrations were performed upon pH and oxygen sensing scaffolds whilst supporting 3T3 fibroblasts. The calibration curves produced were comparable to that of the scaffolds without fibroblasts. The ability to produce calibration curves for both oxygen and pH whilst cells were cultured upon the self reporting scaffolds demonstrates that the sterilisation process of the self-reporting scaffolds by application of UV light and also preparation of the self-reporting scaffold for cell culture does not cause any detrimental effects to the nanosensor capabilities of the self-reporting scaffold.
Self-reporting scaffolds offer the potential of in situ, real-time assessment of the microenvironment in 3D constructs during the tissue growth process. Reporting the microenvironment analyte concentration could allow assessment of the transport of cell nutrients within a 3D construct and also determine if there is accumulation of metabolic waste.15 Monitoring the pH within the engineered construct could indicate whether acidic by-products produced as the scaffold degrades are being effectively removed, an area that remains understudied.16 Self-reporting scaffolds for oxygen could be used to monitor the oxygen levels within 3D constructs so that it can be determined whether the cells throughout the constructs are receiving adequate oxygen to support growth or, for instance, there are local hypoxic regions. Until now this has been limited to measuring oxygen concentration at an isolated point within the scaffold using an oxygen probe.17
Acknowledgements
This research was supported by the Biotechnology and Biological Sciences Research Council (BBSRC) through grant BB/H011293/1.Notes and references
- N. B. E. Sawyer, L. K. Worrall, J. A. Crowe, S. L. Waters, K. M. Shakesheff, F. Rose and S. P. Morgan, Biotechnol. Bioeng., 2008, 100, 159–167 Search PubMed.
- D. Wendt, S. A. Riboldi, M. Cioffi and I. Martin, Adv. Mater., 2009, 21, 3352–3367 CrossRef CAS.
- Y. Martin and P. Vermette, Biomaterials, 2005, 26, 7481–7503 CrossRef CAS.
- X. Y. Wang, C. Drew, S. H. Lee, K. J. Senecal, J. Kumar and L. A. Sarnuelson, Nano Lett., 2002, 2, 1273–1275 CrossRef CAS.
- Y. Yang, H. H. P. Yiu and A. J. El Haj, Analyst, 2005, 130, 1502–1506 RSC.
- H. Xu, J. W. Aylott and R. Kopelman, Analyst, 2002, 127, 1471–1477 RSC.
- J. W. Aylott, Analyst, 2003, 128, 309–312 RSC.
- Y.-E. K. Lee and R. Kopelman, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2009, 1, 98–110 Search PubMed.
- H. Xu, J. W. Aylott, R. Kopelman, T. J. Miller and M. A. Philbert, Anal. Chem., 2001, 73, 4124–4133 CrossRef CAS.
- E. Bakker and W. Simon, Anal. Chem., 1992, 64, 1805–1812 CrossRef CAS.
- X. J. Xin, M. Hussain and J. J. Mao, Biomaterials, 2007, 28, 316–325 CrossRef CAS.
- G. Khang, S. J. Lee, J. H. Jeon, J. H. Lee and H. B. Lee, Polymer, 2000, 24, 869–876 Search PubMed.
- E. S. Place, J. H. George, C. K. Williams and M. M. Stevens, Chem. Soc. Rev., 2009, 38, 1139–1151 RSC.
- K. A. Blackwood, R. McKean, I. Canton, C. O. Freeman, K. L. Franklin, D. Cole, I. Brooks, P. Farthing, S. Rimmer, J. W. Haycock, A. J. Ryan and S. MacNeil, Biomaterials, 2008, 21, 3091–3104 Search PubMed.
- S. J. Hollister, Nat. Mater., 2005, 4, 518–524 CrossRef CAS.
- H. J. Sung, C. Meredith, C. Johnson and Z. S. Galis, Biomaterials, 2004, 25, 5735–5742 CrossRef CAS.
- U. Cheema, T. Alekseeva, E. A. Abou-Neel and R. A. Brown, Eur. Cells Mater., 2010, 20, 274–281 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ay25771h |
| This journal is © The Royal Society of Chemistry 2013 |
