Simultaneous determination of pentachlorophenol, niclosamide and fenpropathrin in fishpond water using an LC-MS/MS method for forensic investigation
Haipeng Jiang*a, Yinhua Zhangb, Xiangguo Chenb, Jizhong Lvb and Jing Zou*a
aKey Laboratory for Green Chemical Process of Education Ministry and School of Chemical Engineering and Pharmacy, Wuhan Institute of Technology, Wuhan 430073, Hubei, PR China. E-mail: jianghp@mail.wit.edu.cn; Fax: +86 027-87194591; Tel: +86 027-87194622
bPhysical and Chemical Investigation Laboratory of Hubei Province Public Security Department, Wuhan 430073, Hubei, PR China
First published on 5th October 2012
Abstract
Pentachlorophenol, niclosamide and fenpropathrin are agrochemicals commonly used as molluscicides or pesticides; however, criminal poisoning aquaculture products using the above three agrochemicals, by motive of illegal economical competition or ill-willing reprisal, occur in high frequency in some fishery regions. Identifying the poison is important to the forensic investigation of such cases of criminal poisoning. In this work, an LC-MS method was developed for the simultaneous determination of the three toxicants. Selective ion monitoring (SIM) was applied for the determination of pentachlorophenol, and multiple reaction monitoring (MRM) was applied for the determination of niclosamide and fenpropathrin. These methods showed excellent reliability and assay performance, and can serve as a reference method for forensic investigations of fish poisoning, So far, this method has been successfully applied in some cases of criminal poisoning.
1. Introduction
Fisheries have an important role in the regional and national economy of some countries, and aquaculture income plays a critical role in providing a livelihood for families in some regions.1 However, due to criminal poisoning, the large scale death of pond-reared fish occasionally happens. The determination of which poisons are used in these cases is important to forensic investigations.2–5 According to previous criminal cases, pentachlorophenol, niclosamide and fenpropathrin (see Fig. 1) are the most widely used fishery poisons.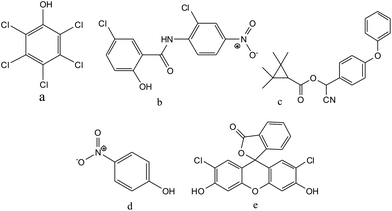 | ||
| Fig. 1 Chemical structures of pentachlorophenol (a), niclosamide (b), fenpropathrin (c), p-nitrophenol (IS) (d), and 2,7-dichlorofluorescein (IS) (e). | ||
Pentachlorophenol is very toxic to fish6,7 (see also Sigma-Aldrich's MSDS data, Index-no: 604-002-00-8, pentachlorophenol has an LC50 for rainbow trout of 0.075 mg L−1, 96.0 h). It was initially used in aquaculture as a pond-cleaning reagent to control grubs, which often plague pond-reared fishes.8 Although the use of pentachlorophenol as a molluscicide in China has been banned since 1997,9 fish poisoning with pentachlorophenol still occurs.
Niclosamide is another severe toxicant to fish and other aquaculture products10,11 (see Sigma-Aldrich's MSDS data, niclosamide has an LC50 for Danio rerio of 0.11 mg L−1, 96 h). Similar to pentachlorophenol, niclosamide was used as a molluscicide to control grubs.11,12 Since niclosamide spontaneously and rapidly degrades under aqueous photolytic conditions, it can be used as an ideal pond-cleaning reagent to kill residual fish which may prey upon fish fry.12–14 Niclosamide is unavoidably used for criminal poisoning due to its wide usage in aquaculture.
Fenpropathrin is a highly potent pyrethroid insecticide used widely for crop farming, and shows significant toxicity to fish15,16 (see also Sigma-Aldrich's MSDS data, Index-no: 607-239-00-5, fenpropathrin has an LC50 for rainbow trout of 0.002 μg L−1, 96.0 h). Since fenpropathrin is easily obtained from commercial sources, the incidence of fishery poisonings using this chemical has remained high.
Fishery poisonings are troublesome cases because of the difficulties in identifying the poisons. Therefore, the development of an analytical method for determining the toxicants is necessary. However, so far there has been no analytical method for the simultaneous determination of the above mentioned toxicants. As for identifying the poison, GC–MS analysis has been the main method, for it can provide valuable identification data for unknown substances.5 However, due to the nature of these chemicals being more polar, in our experience, they are most likely undetected when analyzed by direct GC–MS or a GC–MS with derivatization method.17 In this study, we describe a direct high-performance liquid chromatography tandem mass spectrometry (LC-MS/MS) method for the simultaneous determination of pentachlorophenol, niclosamide and fenpropathrin in fishpond water. The method has been proved to have excellent reliability and assay performance, thus can be used as a reference method for the forensic investigation of fish poisoning.
2. Experimental
2.1. Reagents and chemicals
Pentachlorophenol, niclosamide and fenpropathrin were purchased from J & K Chemical Ltd. p-Nitrophenol and 2,7-dichlorofluorescein were purchased from Sigma-Aldrich Chemical Co. Inc. All organic solvents were HPLC grade and were purchased from Fisher Scientific. Ultrapure water was obtained from Milli-Q Ultrapure Water Purification System. Ammonium acetate (NH4Ac, 98%) and acetic acid (HAc, 99%) were from CNW Technologies GmbH.2.2. Instrument
The HPLC system was made up of the Dionex Ultimate 2000 HPLC system, a 96-vial autosampler, a temperature control compartment and a binary solvent delivery pump. An ESI interface, Ion MAX™, coupled the LC system to a novel linear ion trap mass spectrometer, LTQ-MS (Thermo Finnigan, San Jose, CA, USA). Data were acquired and processed using Xcalibur 2.1 software.2.3. HPLC conditions
Chromatographic separations were achieved using an Acclaim™ 120-C18 column (3 μm, 150 mm × 3 mm i.d.), maintained at a constant temperature of 30 °C. The mobile phase consisted of 20 mmol L−1 ammonium acetate of pH 4.5 (eluent A) and methanol (eluent B), delivered at a flow rate of 0.3 mL min−1. The chromatographic gradient used was: from 0 to 4 min, the percentage of eluent B maintained at 85%; from 4 to 13 min, percentage of eluent B linearly increases from 85% to 90%, and is maintained at 90% for 1 min, then returned to 85% in 1 min and is maintained at 85% for 4 min.2.4. Mass spectrometer conditions
The mass spectrometer was operated in the positive or negative mode, the spray voltage was 4.5 kV for the positive or negative mode, and the temperature of the heated capillary was set at 250 °C. Nitrogen was used as the nebulizer gas and auxiliary gas. The flow rates of the sheath gas, and sweep gas were set (in arbitrary units min−1) at 20 and 2, respectively. Selective ion monitoring at 263, 265, 267 experiments were conducted for pentachlorophenol in the negative mode, multiple reaction monitoring (MRM) experiments were conducted for niclosamide (325/289,327/291) in the negative mode, fenpropathrin (350/125, 350/199) in the positive mode. Other parameters were optimized automatically by infusion of the standard solution into the LC effluent via a T-shape connector using a syringe pump. With an ultra-fast cycle time and short polarity switch time, the LTQ XL can fulfill the task of simultaneous quantitation in both positive and negative ionization modes. In the present method, the data acquisition was divided into two segments: it is conducted to monitor pentachlorophenol and the internal standards, p-nitrophenol and 2,7-dichlorofluorescein, from 0 to 6 min; then the data acquisition is switched into monitoring niclosamide and fenpropathrin.2.5. Preparation of standard solutions
Individual stock standard solutions (200 μg mL−1) were prepared by dissolving 2.0 mg of compound in 10 mL of methanol and stored at −20 °C. Working solutions at serial concentrations were obtained by diluting aliquots of stock solutions with methanol, 10 μL of each internal standard stock solution were spiked to 1 mL solution which corresponds to a final concentration of 10 ng mL−1. In recovery tests, three different concentration levels of individual solutions were added to the blank fish-pond water sample prior to extraction. Stock standard solutions were stored at −20 °C and were stable for 6 months. Working standard solutions were stored at 4 °C and were stable for at least 1 month.2.6. Sample preparation and extraction
A 50 mL water sample was first fortified with mixed internal standard (10 μL) which corresponds to a concentration of 0.2 ng mL−1 of internal standard in the fishpond water sample. 500 μL of acetic acid was then added to the 50 mL water sample. Then each sample was extracted three times with 10 mL organic solvent (dichloromethane and acetone with volume ratio 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The combined organic layers were collected and evaporated to dryness under a stream of nitrogen at 50 °C, then redissolved in 1 mL of methanol for analysis.
1). The combined organic layers were collected and evaporated to dryness under a stream of nitrogen at 50 °C, then redissolved in 1 mL of methanol for analysis.3. Results and discussion
3.1. Optimization of LC-MS/MS analytic method
The analysis of pentachlorophenol by LC-MS/MS has its challenges. During efforts to optimize the MRM method for pentachlorophenol, it was found that the molecular ion was very difficult to fragment, even at increasing normalized collision energies up to 70. The even higher collision energy results in an acute signal decrease for both the molecular ion and fragment ions. It is obvious that direct analysis of pentachlorophenol by LC-MS/MS without derivatization would yield low sensitivity. So, selective ion monitoring (SIM) at 263, 265, 267 experiments were conducted for the analysis of pentachlorophenol, and the detection parameters were optimized to make the base peak of 263, 265, 267 have a good signal value.Niclosamide and fenpropathrin were analyzed by the multiple reaction monitoring method, their detection parameters were optimized automatically by infusion of the standard solution into the LC effluent. The optimized parameters for each compound, are summarized in Table 1.
| Analyte | SIM or MRM trace (m/z) | Optimal normalized collision energy | Capillary voltage (V) | Tube lens (V) | Gate lens (V) |
|---|---|---|---|---|---|
| a Selective ion or fragmentation transition monitored for quantitative purposes.b As internal standard to calculate the response factor of pentachlorophenol and niclosamide.c As internal standard to calculate the response factor of fenpropathrin. | |||||
| Pentachlorophenol | 263 | — | −1.3 | −68.42 | 46.79 |
| 265a | — | ||||
| 268 | — | ||||
| Niclosamide | 325/289a | 35 | −1.3 | −68.42 | 46.79 |
| 327/291 | 35 | ||||
| Fenpropathrin | 350/125a | 19 | 23 | 130 | −69.52 |
| 350/199 | 19 | ||||
| p-Nitrophenol | 138/108b | 55 | −1.3 | −68.42 | 46.79 |
| 2,7-Dichlorofluorescein | 401/383c | 55 | 23 | 130 | −69.52 |
The HPLC elution program was optimized to obtain the best peak shape and resolution. During the method development, it was found that the pH of the mobile phase has an obvious influence on the retention time and peak shape of pentachlorophenol and niclosamide; when the elution strength was increased too rapidly to 90% methanol, niclosamide and fenpropathrin had similar retention times and this resulted in lower resolution. Considering overall resolution and peak shape, the pH of the mobile phase was adjusted to 4.5, and the gradient profile was optimized to the program mentioned under the section “HPLC conditions”.
Different buffers (ammonium formate/formic acid and ammonium acetate/acetic acid) were tested, in positive and negative ion modes. The results showed that the MS signals were increased when using ammonium acetate/acetic acid in positive ion mode. Thus, 20 mM ammonium acetate/acetic acid at pH 4.5 was selected in order to obtain optimal signals.
Organic components used in the mobile phase, including CH3CN or MeOH, have influence on the ion suppression in LC-MS. Different gradient programs using aqueous binary mixtures of pH 4.5 with MeOH or CH3CN as the organic component were also checked, it was found that fenpropathrin has low sensitivity when using CH3CN as the organic component, especially fenpropathrin eluted by 100% CH3CN. So, MeOH was selected as the organic component.
3.2. Assay performance
To check the specificity of the method, interference from the matrix was investigated by the analysis of five blank water samples from different fishponds. Results shows that no interference was observed (see Fig. 2). As to the specificity of pentachlorophenol, as it contains five chlorine atoms, the ratio of the isotope peaks of 263![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 265
265![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 267 = 9
267 = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 can be used to help with chemical identification. The same can be said of niclosamide, which contains two chlorine atoms, and the ratio of the isotope peaks of 325
10 can be used to help with chemical identification. The same can be said of niclosamide, which contains two chlorine atoms, and the ratio of the isotope peaks of 325![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 327 = 3
327 = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in the mass spectrum.
2 in the mass spectrum.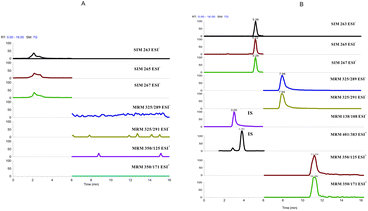 | ||
| Fig. 2 Representative chromatograms of (A) blank processed fishpond water sample. (B) Blank water sample spiked with a standard solution of pentachlorophenol, niclosamide, fenpropathrin and 10 ng mL−1 of the internal standards, p-nitrophenol and 2,7-dichlorofluorescein. | ||
The linearity of the chromatographic response was tested with matrix extracted calibration curves using five calibration points. Blank fishpond water samples, which were proved by LC-MS to have none of the above mentioned toxicant and internal standards, were used as the matrix for the preparation of calibration curves. Blank water samples fortified with working standards in a sequence of concentrations and 10 ng mL−1 of the corresponding internal standard, were subjected to the full extraction procedure. The calibration curves were prepared by plotting the response factor (the ratio of the peak area of analyte over the peak area of the internal standard) against analyte concentration. For pentachlorophenol, the response factor was calculated by dividing the peak area of SIM at 265 by the peak area of MRM at 138/108 for p-nitrophenol (see Table 1). For niclosamide, the response factor was calculated by dividing the peak area of MRM at 325/289 by the peak area of MRM at 138/108 for p-nitrophenol. For fenpropathrin, the response factor was calculated by dividing the peak area of MRM at 350/125 by the peak area of MRM at 401/383 for 2,7-dichlorofluorescein. Each calibration curve is constructed by linear curve fitting using the least-squares linear regression calculation. The linearity was good for all analytes in the whole range of concentrations tested, as proved by the correlation coefficients (r2) ranging from between 0.9912 and 0.9986 for all curves (Table 2).
| Analyte | Linear regression equation | Correlation coefficient, r2 | Linear range (ng mL−1) | LOD (ng mL−1) | Lower limit of linearity (ng mL−1) |
|---|---|---|---|---|---|
| Pentachlorophenol | y = 11.955x − 11.717 | 0.9986 | 1–20 | 0.8 | 1 |
| Niclosamide | y = 6.5807x − 0.2158 | 0.9912 | 0.04–2 | 0.02 | 0.04 |
| Fenpropathrin | y = 0.1168x + 0.0838 | 0.9958 | 1.4–20 | 1.2 | 1.4 |
The sensitivity was evaluated by determining the limits of detection (LOD) and lower limit of linearity. The limit of detection (LOD) was determined by successive analyses of spiked matrices with increasing amounts of every standard until a signal-to-noise ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was reached for the least intense transition; the lower limit of linearity was adopted as the lowest concentration of linear curve to give r2 > 0.99 for the most intense transition.
1 was reached for the least intense transition; the lower limit of linearity was adopted as the lowest concentration of linear curve to give r2 > 0.99 for the most intense transition.
The precision and accuracy of the method were investigated using working standard solutions. Intra- and inter-day precision for each toxicant at high, intermediate and low concentration levels were investigated. The precision of the method was expressed by RSD (relative standard deviation); the RSD ranges of the three toxicants analyzed by the present method were 1.5–5.8% for intra-day precision (N = 3) and 1.2–7.2% for inter-day precision (N = 3). The recoveries of each toxicant at high, intermediate and low concentration levels were also investigated. They ranged from 90.6–100.6% (see Table 3).
| Analyte | Concentration (ng mL−1) | Recovery (%) | RSD (%) | ||
|---|---|---|---|---|---|
| Added | Found | Intra-day | Inter-day | ||
| Pentachlorophenol | 1 | 0.906 | 90.6 | 5.8 | 6.4 |
| 4 | 4.024 | 100.6 | 1.2 | 1.9 | |
| 20 | 19.66 | 98.3 | 1.7 | 5.8 | |
| Niclosamide | 0.04 | 0.038 | 95.0 | 2.5 | 1.2 |
| 0.4 | 0.386 | 96.5 | 1.5 | 3.2 | |
| 2 | 1.936 | 96.8 | 4.3 | 5.4 | |
| Fenpropathrin | 1.4 | 1.31 | 93.5 | 3.8 | 7.2 |
| 6 | 6.03 | 100.5 | 1.2 | 1.5 | |
| 20 | 19.68 | 98.4 | 4.2 | 4.6 | |
3.3. Real sample analysis
Hubei province was the largest cultured-pearls base in China. A case of large scale death of pond-reared pearls occurred in the summer of 2010. Due to the premature pearls have little market value, the economic loss was evaluated to be about $750![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. The pond water was analyzed by the developed method and 0.086 ng mL−1 niclosamide was found (see Fig. 3). Because the victim had never used niclosamide for fishpond aquaculture purposes, it initiated a forensic investigation into the case. Subsequently, a large amount of yellow powder, secretly covered by a boat on one corner of the pond, was found to be the source of the poison. The yellow powder was identified as niclosamide and the case was characterized as ill-will poisoning.
000. The pond water was analyzed by the developed method and 0.086 ng mL−1 niclosamide was found (see Fig. 3). Because the victim had never used niclosamide for fishpond aquaculture purposes, it initiated a forensic investigation into the case. Subsequently, a large amount of yellow powder, secretly covered by a boat on one corner of the pond, was found to be the source of the poison. The yellow powder was identified as niclosamide and the case was characterized as ill-will poisoning.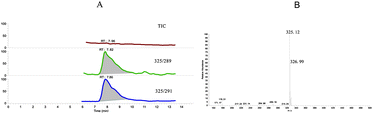 | ||
Fig. 3 Application of the LC-MS/MS method to the water sample from the poisoned fishpond where niclosamide was found. (A) Chromatograms of TIC (total ion current) and two transitions of niclosamide; (B) the isotope peak height of 325![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 327 = 3 327 = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 extracted from the TIC spectrum. 2 extracted from the TIC spectrum. | ||
3.4. Discussion
GC-MS analysis has been the main method used to identify poisons,5 however, the most used toxicants to fish are pentachlorophenol, niclosamide and fenpropathrin, which are medium to high polarity chemicals, and they are likely to be undetected when analysed just by GC-MS. The present method employs LC-MS for the simultaneous determination the three most used fish poisons in one run.The limits of detection (LOD) for the three toxicants are all below the LC50 for each one. Although niclosamide degrades rapidly in aqueous photolytic conditions, the highest LC50/LOD ratio can compensate for such a degradation. However, it is still strongly recommended that the fishpond water samples be kept out of light and sent for detection as soon as possible. Fenpropathrin has the lowest LC50/LOD ratio among the three poisons, but the poisoning criminal often administers large amounts of fenpropathrin, and not just concentrations around the LC50 of the chemical. Even so, a more sensitive GC-MS method should be used.18–20
4. Conclusions
A LC-MS method for the simultaneous determination of the three most used fishery poisons was developed. The method showed good specificity, linearity, sensitivity, precision and accuracy, and can be served as a reference method for forensic investigations into fish poisonings.Acknowledgements
The author thanks Hubei Province Public Security Department and district Public Security Bureau for sample collection.Notes and references
- Y. Guo, H. Y. Yu, B. Z. Zhang and E. Y. Zeng, J. Agric. Food Chem., 2009, 57, 3674–3680 CrossRef CAS.
- W. Vycudilik and G. Gmeiner, Drug Test. Anal., 2009, 1, 177–183 CrossRef CAS.
- R. A. Musah, M. A. Domin, M. A. Walling and J. R. E. Shepard, Rapid Commun. Mass Spectrom., 2012, 26, 1109–1114 CrossRef CAS.
- Q. Liu, L. Zhou, N. Zheng, L. Zhuo, Y. Liu and L. Liu, Forensic Sci. Int., 2009, 193, 88–94 CrossRef.
- J. F. Van Bocxlaer, K. M. Clauwaert, W. E. Lambert, D. L. Deforce, E. G. Van den Eeckhout and A. P. De Leenheer, Mass Spectrom. Rev., 2000, 19, 165–214 CrossRef CAS.
- C. Goodnight, Ind. Eng. Chem., 1942, 34, 868–872 CrossRef CAS.
- J. M. McKim, P. K. Schmieder, R. W. Carlson, E. P. Hunt and G. J. Niemi, Environ. Toxicol. Chem., 1987, 6, 295–312 CAS.
- R. L. Lane and J. E. Morris, Tech. Bull. Series, Iowa State University, 2000, vol. 115 Search PubMed.
- X. Jin, J. Zha, Y. Xu, J. P. Giesy and Z. Wang, Environ. Sci. Pollut. Res., 2011, 1–10 Search PubMed.
- M. A. Boogaard, T. D. Bills and D. A. Johnson, J. Great Lakes Res., 2003, 29, 529–541 CrossRef CAS.
- J. Dai, W. Wang, Y. Liang, H. Li, X. Guan and Y. Zhu, Parasitol. Res., 2008, 103, 405–412 CrossRef.
- P. W. Graebing, J. Chib, T. D. Hubert and W. H. Gingerich, J. Agric. Food Chem., 2004, 52, 870–878 CrossRef CAS.
- P. W. Graebing, J. Chib, T. D. Hubert and W. H. Gingerich, J. Agric. Food Chem., 2004, 52, 5924–5932 CrossRef CAS.
- M. P. Frank, P. Graebing and J. Chib, J. Agric. Food Chem., 2002, 50, 2607–2614 CrossRef CAS.
- I. Wengatz, D. W. Stoutamire, S. J. Gee and B. D. Hammock, J. Agric. Food Chem., 1998, 46, 2211–2221 CrossRef CAS.
- Z. Yuqin, L. Lina and L. Jianhua, Environ. Pollut. Control J., 2008, 11, 53–57 Search PubMed.
- D. F. Rendle, Chem. Soc. Rev., 2005, 34, 1021–1030 RSC.
- P. Serôdio and J. M. F. Nogueira, Anal. Bioanal. Chem., 2005, 382, 1141–1151 CrossRef.
- E. Van Hoeck, F. David and P. Sandra, J. Chromatogr., A, 2007, 1157, 1–9 CrossRef CAS.
- J. L. Martínez Vidal, P. Espada, A. Garrido Frenich and F. J. Arrebola, J. Chromatogr., A, 2000, 867, 235–245 CrossRef.
| This journal is © The Royal Society of Chemistry 2013 |
