Estimation of human equivalent exposure from rat inhalation toxicity study of silver nanoparticles using multi-path particle dosimetry model
Jun Ho
Ji
a and
Il Je
Yu
*b
aSamsung Electronics Co. Ltd., Suwon, Korea
bInstitute of Nanoproduct Safety Research, Hoseo University, Asan, Korea. E-mail: u1670916@chollian.net; Fax: +82-41-540-9846; Tel: +82-41-540-9630
First published on 19th September 2012
Abstract
Respiratory tract dosimetry is a useful tool to estimate the exposure concentrations of an inhaled substance that will produce the same result at a target site of the respiratory tract in both rats and humans. Thus, to enable the results of animal inhalation studies to be extrapolated to human equivalent exposure levels, the MPPD (multi-path particle dosimetry) model was used to estimate the differences in the respiratory dosimetry of rats and humans. In our previous study, when animals were subchronically exposed to silver nanoparticles over a period of 90 days, a no observable adverse effect level (NOAEL) of 100 μg m−3 was suggested. Therefore, this study used results from a previous animal study, including the test aerosol information and estimated clearance rate of silver nanoparticles after a 90 day inhalation toxicity test. As a result, the human equivalent workplace exposure concentration of silver nanoparticles was estimated as 59 μg m−3 compared to the rat NOAEL of 100 μg m−3.
1. Introduction
While nanotechnology is anticipated to be the spring-board for many technological innovations in the 21st century, environmental, health, and safety problems related to the rapid commercialization of nanotechnology represent a serious obstacle for the further development of nanotechnology. In particular, the antimicrobial properties of silver nanoparticles have been widely applied in many consumer products, such as disinfectants, deodorants, antimicrobial sprays and powders, bedding, washers, water purification, toothpaste, shampoo and rinse, nipples and nursing bottles, fabrics, filters, kitchen utensils, toys, and humidifiers. Among 580 consumer nanotechnology-based products, the most commonly mentioned material in the product descriptions was silver-based nanoparticles.1 Thus, the risk of human exposure to silver nanoparticles is steadily rising for both workers at nanosilver-manufacturing workplaces and consumers using nanosilver-containing products.As the deposition, clearance, and physical or chemical effects of inhaled particles produce diverse responses in humans, animal models have been used in inhalation toxicity studies. Yet, while animal models are important for understanding the fate and effects of inhaled nanomaterials, the inhaled dose and exposure concentrations in rat studies are not representative of the actual dose in humans. Nonetheless, if the effects in humans and rats occur at the same target sites and show similar accumulated doses, animal studies can be used to estimate the human equivalent exposure from an accumulated dose, deposited dose, or inhaled dose. The same retained dose per unit surface area in the respiratory tract of rats and humans is assumed to yield similar health effects, provided that the underlying mechanisms and sensitivity between the two species are the same. Thus, based on this assumption, a human equivalent concentration can be estimated for any particle size distribution considering the deposited and inhaled human doses.2,3
Previously, the current authors conducted several inhalation toxicity studies of silver nanoparticles.4–7 In a 90-day subchronic inhalation toxicity study using Sprague–Dawley rats, the target organs for silver nanoparticles were considered to be the lungs and liver in both male and female rats.7 Lung function changes were observed when the animals were subchronically exposed to silver nanoparticles over a period of 90 days.6 Plus, a no observable adverse effect level of 100 μg m−3 was suggested from this experiment.7 In a subsequent study, the recovery from such lung function changes in rats was investigated during 12 weeks after the cessation of nanoparticle exposure,8 which allowed the clearance rate of silver nanoparticles to be calculated.
Accordingly, this study describes an approach to calculate the human equivalent exposure, as suggested by Oberdörster2 and Oller and Oberdörster3 and uses results from a previous animal study, including the test aerosol information and estimated clearance rate of silver nanoparticles. To enable the animal inhalation study results to be extrapolated to human exposure levels, the MPPD model is used to investigate the dosimetric differences between rats and humans. As the most significant differences are found in the alveolar region, the current calculations are focused on this region. Finally, the human equivalent exposure is estimated for the rat NOAEL of 100 μg m−3 and rat clearance rates resulting from a 90-day inhalation toxicity study.
2. Human equivalent exposure from rat data
Certain dosimetric principles need to be considered when extrapolating the results of rat inhalation experiments to humans, as exposure to the same concentration of an airborne substance does not lead to the same uptake in the organs of each species. Respiratory track dosimetry is a useful tool for estimating the exposure concentration of an inhaled substance that results in the same dose at a target site in the respiratory track for both rats and humans. Fig. 1 shows a schematic of the concept of extrapolating the human equivalent exposure from a rat inhalation study when taking account of the differences between the two species using the dosimetry of the inhaled substance in the respiratory track. This dose only depends on the inhaled volume or mass and exposure concentration, and is independent of the particle size of the inhaled particles.3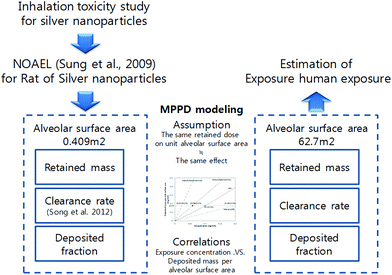 | ||
| Fig. 1 Dosimetric extrapolation of inhaled particles for rats and humans.2 | ||
The deposited dose can be expressed as micrograms per square centimeter of epithelial surface area or micrograms per gram of lung tissue. The physical and biochemical processes in the lungs, including mechanical clearance, uptake in the epithelial cells, and interstitium or systemic circulation, can result in a retained or, in the case of repeated exposure, accumulated dose at a local or systemic target site, which can eventually have an effect (Fig. 1).
The retained (accumulated) dose is normally expressed as micrograms per gram of the target organ weight. However, since the lungs are the target organ in this case, it can also be expressed as micrograms per square centimeter of the alveolar surface area. Furthermore, if it is assumed that the effects in humans and rats occur at the same target site and at the same accumulated doses, the human equivalent exposure can be estimated using the accumulated dose to calculate the human deposited dose and human inhaled dose (Fig. 1).
3. Model for human and rat airway particle dosimetry: multiple-path particle dosimetry model
Various mathematical models have already been applied to describe the respiratory deposition, clearance, and retention of aerosols. As such, the numerical modeling used in this study was based on the multiple-path particle dosimetry (MPPD) model that was jointly developed by the Chemical Industry Institute of Toxicology (CIIT, currently The Hamner Institutes for Health Sciences) and the Dutch National Institute for Public Health and the Environment (RIVM). MPPD is widely used for particle dosimetry calculations as it is easy to apply and available online. Thus, the MPPD model can calculate the deposition and clearance of monodisperse and polydisperse aerosols in the respiratory tracts of rats and human adults and children (deposition only) for particles ranging in size from ultrafine to coarse. Such models are based on single-path and multiple-path methods for tracking the air flow and calculating the aerosol deposition in the lungs. The single-path method calculates the deposition in a typical path per airway generation, while the multiple-path method calculates the particle deposition in all airways of the lungs and provides lobar-specific and airway-specific information.9 Within each airway, the deposition is calculated using theoretically derived efficiencies for deposition by diffusion, sedimentation, and impaction within the airway or airway bifurcation. Plus, the filtration of aerosols by the nose and mouth is determined using empirical efficiency functions. Fig. 2 shows some MPPD model comparisons of rat and human exposure to silver nanoparticles, where Fig. 2(a) shows the deposition fractions of the total and alveolar region according to the particle diameter, while Fig. 2(b) shows the rat to human deposition fraction ratios according to the unit density and a silver density of 10.49. For silver nanoparticles measuring less than 100 nm, the density difference was not effective and the alveolar deposition fractions for rats and humans were very similar.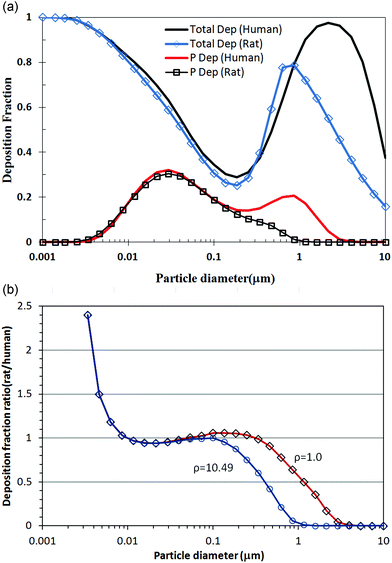 | ||
| Fig. 2 Comparison of predicted total and alveolar deposition fraction between rats and humans for silver nanoparticles. (a) Total and alveolar deposition fractions between rats and humans for silver nanoparticles according to particle diameter and (b) deposition fraction ratios (rats/humans) according to unit density and silver density of 10.49. | ||
4. Results
This approach provides a comparison of animal exposure levels and human exposure at the deposited dose level, regardless of whether the animals and humans are exposed to the same or different particle size aerosols. The deposited dose fraction in the alveolar region per unit surface area differed between rats and humans, as shown in Fig. 2. For the MPPD modeling, the data set was obtained from a previous study.7,8 The lung morphometry, particle properties, exposure conditions, and clearance settings are all listed in Table 1. As shown, the geometric mean diameter, geometric standard deviation, and NOAEL concentration were 18 nm, 1.5, and 100 μg m−3, respectively. Plus, from our previous work,8 the average clearance rate of silver nanoparticles was 0.00515. The other parameters were based on the program default values. The MPPD modeling then estimated the retained mass in the alveolar region after 13 weeks of exposure.| Model parameters | Rat | Human (with light exercise) |
|---|---|---|
| 1. Lung morphometry | ||
| Model | Asymmetric multiple path | Yeh/Schum 5 lobe |
| Functional residual capacity (FRC) | 4.0 ml (default) | 3300 ml (default) |
| Upper respiratory tract (URT) volume | 0.42 ml (default) | 50 ml (default) |
| Surface area of alveolar region | 0.409 m2 (ref. 3) | 62.7 m2 (ref. 3) |
| 2. Particle properties (silver) | ||
| Density | 10.49 g cm−3 | 10.49 g cm−3 |
| Geometric mean diameter | 18 nm7 | 18 nm7 |
| Geometric standard deviation | 1.57 | 1.57 |
| 3. Exposure conditions | ||
| Aerosol concentration (NOAEL of silver nanoparticles) | 0.1 mg m−3 | — |
| Tidal volume | 2.1 ml | 1024 ml (light exercise) |
| Air breathing frequency | 102 per minute | 20 per minute (light exercise) |
| Exposure hours per day | 6 h/day | 8 h/day |
| Exposure days per week | 5 days/week | 5 days/week |
| Exposure weeks | 13 weeks | 13 weeks |
| 4. Clearance setting | ||
| Fast human clearance rate | 0.02 per day (default) | |
| Medium human clearance rate | 0.001 per day (default) | |
| Slow human clearance rate | 0.0001 per day (default) | |
| Rat clearance rate | 0.00105652 per day (default) | |
| (Average for experimental data) | 0.00515 per day8 | |
| (Male experimental data) | 0.0059 per day8 | |
| (Female experimental data) | 0.0044 per day8 | |
Fig. 3 shows the total deposited mass in the alveolar region for rats and humans, respectively, when exposed to a particle concentration of 100 μg m−3. Based on the conditions in Table 1 for rats and humans, the silver nanoparticle exposure was six or eight hours per day and five days per week. As shown in Fig. 3, for rats, the deposited mass in the alveolar region increased for six hours and then decreased over the subsequent eighteen hours. Meanwhile, for humans, the deposited mass in the alveolar region increased for eight hours and then decreased over the subsequent sixteen hours for humans. These deposition patterns were repeated for five days, and then the deposited mass decreased during the weekend, as no silver nanoparticles were supplied and only the clearance process worked on the alveolar region.
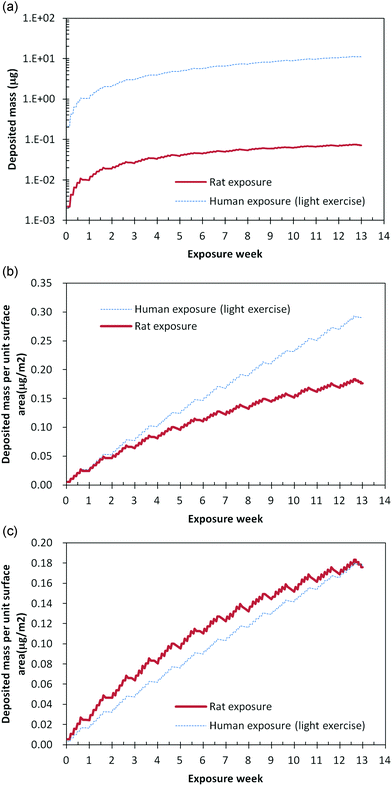 | ||
| Fig. 3 Deposition pattern according to exposure time, (a) deposited mass in alveolar region at same exposure concentration of 100 μg m−3, (b) deposited mass per unit surface area in alveolar region at same exposure concentration of 100 μg m−3, and (c) deposited mass per unit surface area in alveolar region at exposure concentration of 100 μg m−3 for rats and human equivalent exposure concentration of 59 μg m−3. | ||
As the tidal volume of a rat is much smaller than that of a human, the total deposited mass in the rat alveolar region was also very small, as shown in Fig. 3(a). Notwithstanding, as shown in Fig. 3(b), the deposited mass per surface area of the alveolar region was similar at 0.29 for humans under the condition of light exercise and 0.176 for rats, even though the human deposition mass per surface area of the alveolar region under the condition of light exercise was more than that of the rat deposition mass. In addition, Fig. 3(c) shows the deposition pattern during thirteen weeks. At the end of thirteen weeks, the deposited mass per surface area in the alveolar region was the same for rats and humans, although the deposition patterns according to time differed slightly.
Fig. 4 shows the predicted deposited mass per unit surface area in the alveolar region according to the exposure concentration after 13 weeks of silver nanoparticle inhalation exposure. Under the conditions of light exercise, meaning human activity in the workplace, a human equivalent exposure concentration of 59 μg m−3 corresponded to a rat exposure concentration of 100 μg m−3. Meanwhile, under resting conditions, a rat exposure concentration of 100 μg m−3 corresponded to a human equivalent exposure concentration of 196 μg m−3. Plus, the human equivalent exposure concentration for heavy exercise was 23 μg m−3, as the conditions of heavy exercise requires more breathing.
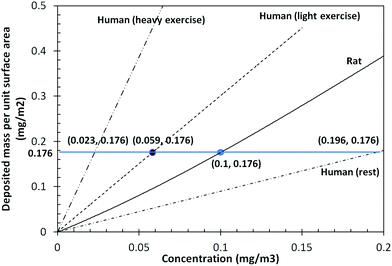 | ||
| Fig. 4 Predicted deposited mass per unit surface area in alveolar regions after 13 weeks of silver nanoparticle exposure. | ||
5. Conclusions
This study calculated the human equivalent exposure to silver nanoparticles based on NOAEL data from a previous inhalation study of rats. The animal study results included the test aerosol conditions and estimated clearance rate of silver nanoparticles after a 90 day inhalation toxicity test. To extrapolate the animal inhalation study results into human exposure results, the MPPD model was used, which enables easy derivation of a human equivalent concentration for particular areas of the respiratory tract when using relevant parameters (as shown in Table 2). The dosimetric differences between rats and humans were investigated using the MPPD model, and based on the rat NOAEL exposure condition of 100 μg m−3, the human equivalent exposure concentration of silver nanoparticles was estimated as 59 μg m−3.| dpg = 18 nm (σg = 1.5) | Rat | Human |
|---|---|---|
| Exposure time | 6 h/day | 8 h/day |
| 5 days/week | 5 days/week | |
| 13 weeks | 13 weeks | |
| Lung surface area (m2) | 0.409 | 62.7 |
| Activity | Rest | Rest | Light exercise | Heavy exercise |
|---|---|---|---|---|
| Breathing frequency (#/min) | 102 | 12 | 20 | 26 |
| Tidal volume (ml) | 2.1 | 625 | 1024 | 1920 |
| Equivalent exposure concentration (μg m−3) | 100 | 196 | 59 | 23 |
When comparing animal and human exposure levels associated with a particular health endpoint, the application of dosimetric adjustment is very important in order to examine the sensitivity of an animal model to predict human risks.3 However, some reservations still remain as regards certain parameter assumptions of the MPPD model.
Acknowledgements
This research was supported by the Nano R&D program through the National Research Foundation of Korea funded by the Korean Ministry of Education, Science and Technology (2011-0019171).References
- Woodrow Wilson International Center for Scholars, http://www.nanotechproject.org/inventories/consumer/ . Accessed 11 Jun 2012.
- G. Oberdörster, Dosimetric principles for extrapolating results of rat inhalation studies to humans, using an inhaled Ni compound as an example, Health Phys., 1989, 57(Suppl 1), 213–220 CrossRef.
- A. R. Oller and G. Oberdörster, Incorporation of particle size differences between animal studies and human workplace aerosols for deriving exposure limit values, Regul. Toxicol. Pharmacol., 2010, 57, 181–194 CrossRef CAS.
- J. H. Ji, J. H. Jung, I. J. Yu and S. S. Kim, Long-term stability characteristics of nanoparticle generator using small ceramic heater for inhalation toxicity study, Inhalation Toxicol., 2007, 19, 745–751 CrossRef CAS.
- J. H. Ji, J. H. Jung, S. S. Kim, J. U. Yoon, J. D. Park and B. S. Choi, et al., Twenty-eight-day inhalation toxicity study of silver nanoparticles in Sprague Dawley rats, Inhalation Toxicol., 2007, 19, 857–871 CrossRef CAS.
- J. H. Sung, J. H. Ji, J. U. Yun, D. S. Kim, M. Y. Song and J. Jeong, et al., Lung function changes in Sprague Dawley rats after prolonged inhalation exposure to silver nanoparticles, Inhalation Toxicol., 2008, 20(6), 567–574 CrossRef CAS.
- J. H. Sung, J. H. Ji, J. D. Park, J. U. Yoon, D. S. Kim and K. S. Jeon, et al., Subchronic inhalation toxicity of silver nanoparticles, Toxicol. Sci., 2009, 108(2), 452–461 CrossRef CAS.
- K. S. Song, J. H. Sung, J. H. Ji, J. H. Lee, J. S. Lee, H. R. Ryu, J. K. Lee, Y. H. Chung, H. M. Park, B. S. Shin, H. K. Chang, B. Kelman and I. J. Yu, Recovery from silver-nanoparticle-exposure-induced lung inflammation and lung function changes in Sprague Dawley rats, Nanotoxicology, 2012 DOI:10.3109/17435390.2011.648223.
- ARA (Applied Research Associates), multiple-path particle dosimetry model (MPPD V 2.11), http://www.ara.com/products/mppd.htm. Accessed 8 Jan 2011.
| This journal is © The Royal Society of Chemistry 2012 |
