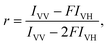Aqueous route for the synthesis of magnetite nanoparticles under atmospheric air: functionalization of surface with fluorescence marker†
Nagaprasad
Puvvada
,
Dhritabrata
Mandal
,
Pravas Kumar
Panigrahi
and
Amita
Pathak
*
Department of Chemistry, Indian Institute of Technology Kharagpur, Kharagpur-721302, India. E-mail: ami@chem.iitkgp.ernet.in
First published on 16th July 2012
Abstract
A simple chemical precipitation approach has been established for the synthesis of magnetite nanoparticles under atmospheric air conditions. The process involves precipitation of Fe3O4 nanoparticles from an aqueous solution containing ferric chloride, hydrochloric acid, sodium sulphite, ammonium ferrous sulphate and ammonia. Further, these nanoparticles were surface conjugated with aminated silica for the attachment of rhodamine 610. The crystallinity was assessed by X-ray diffraction and surface conjugation of aminated silica confirmed by Fourier transform infrared spectroscopy and Electron probe micro-analyzer. Fluorescent absorption measurements revealed a red shift with surface conjugated rhodamine 610 magnetite nanoparticles in comparison to free rhodamine 610 mixed with aminated magnetic nanoparticles. The magnetic measurements of prepared samples showed superparamagnetism, which led us to investigate its usage in internalization studies for biomedical applications. The internalization study of the conjugated magnetite nanoparticles in cell by fluorescent microscopy at different time intervals recommends its usage as an efficient drug delivery system.
Introduction
Magnetite (Fe3O4) nanoparticles are the most versatile magnetic materials, which have received considerable research attention due to their biological and chemical applications1 owing to their small size combined with superparamagnetism (SPM). In recent years, there has been growing interest in developing these materials for immobilization of biomolecules, cell separation systems, biosensors, nanoscale catalytic systems and in biomedical area akin to magnetic resonance imaging (MRI) scanning, therapeutic applications for cancer diagnosis etc.2–5 The biological issues in relation to SPM have increasingly addressed the preparation route for the magnetite nanoparticles (MNPs). In this view, various methods have been developed for the preparation of MNPs, which includes chemical precipitation,6 hydrothermal method,7 sol–gel,8 micro emulsion,9etc. However, the wet chemical process based on chemical precipitation is the most convenient and frequently used process for the synthesis of MNPs, which is suitable for biomedical applications. However, it still remains a great challenge to develop the MNPs under atmospheric air conditions with designed chemical components and controlled morphologies, which consequently may affect its properties.The difficulty of non-magnetic (nano and micro scale) therapeutic molecules (owing to their poor mobility) to localize within cells, virus, bacteria in current clinical trials led to the development of functionalized superparamagnetic nanoparticles and their introduction in nanobiomedicine. The magnetic nature of SPM nanoparticles assists them to deliver a drug at a target tumor along with spatial and temporal control to reach the intercellular spaces of cancer tissue.10 Furthermore, localization study of nanoparticles is required for drug delivery applications, which can be visualized through transmission electron microscopy (TEM) and MRI scanning, where in situ monitoring is not suitable. Confocal laser scanning microscopy (CLSM) is a highly sensitive technique for monitoring cellular uptake of the nanoparticles in internalization studies. On the other hand, functional nanomaterials have superior properties such as fluorescent labelling of biomolecules which will be useful in biomedical applications.11,12 Fluorescent tags that includes organic dyes and quantum dots is used frequently in biological and medical fields.13 The attachment of fluorescent probes on the surface of magnetite nanoparticles could be achieved by functional coating which is helpful in localization studies. The surface coating over the nanoparticles is essential to stabilize as well as to display various functional groups, which are more active in different cellular environments. For instance, Lu et al.14 coated the MNP surface with silica for facilitating fluorescent organic dye attachment resulting in increase in particle size around 50 nm. Another research team attempted dye incorporation15 on the surface of silanized MNPs. Unfortunately, they found that magnetism dropped from 65 in uncoated MNPs to 8.4 emu g−1 in coated MNPs. To date, though numerous research efforts have been focused on the design and preparation of organic functionalized magnetic hybrid nanomaterials for cellular uptake of cells, only a limited number of coatings (such as carboxylic acids,10 amines,4 and phosphonates5) are however conceivable.
Therefore, an effort has been made in this study to produce MNPs through a chemical precipitation method under atmospheric air conditions, and subsequent functionalization of the MNP surface. The chemistry employed to functionalize the MNP surface, which permits the facile conjugation of fluorescent labels, could work as nanocarriers in drug delivery applications. Our approach makes use of the simplicity of a drug delivery vehicle, which unloads its contents only at the internalization site under physiologically favourable conditions.
Materials and methods
Ferric chloride (≥96%), ammonium iron(II) sulfate (≥99%), hydrochloric acid (≥35.4%) and ammonia (25%) were purchased from Merck Ltd, India. Laser grade Rhodamine 610 (Rh 610) and amino propyl trimethoxy siliane (APTMS) was purchased from Exciton and Sigma Aldrich respectively. All the chemicals were of analytical grade and used without further purification.Synthesis of magnetite nanoparticles (MNPs)
In a typical synthetic procedure, 0.2 M FeCl3 solution was prepared and gently heated to about 80 °C. To this solution, appropriate amount of sodium sulphite solution (the amount depends on the estimated amount of dissolved O2 (Winkler method)) followed by a few drops of concentrated HCl were added until the solution turned pale yellow. Appropriate quantity of 0.1 M ammonium ferrous sulphate (with ferrous to ferric ion molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was then poured into the pale yellow solution. Finally, ammonia solution was added dropwise till the precipitation process was complete. The pH of the solution at this point was found to be 10. The so-obtained precipitate was then separated by magnet, and was washed four times with water and methanol, each time separating the precipitate by magnet. The precipitate was then dried in a vacuum desiccator and ground to obtain the MNPs in the powder form.
2) was then poured into the pale yellow solution. Finally, ammonia solution was added dropwise till the precipitation process was complete. The pH of the solution at this point was found to be 10. The so-obtained precipitate was then separated by magnet, and was washed four times with water and methanol, each time separating the precipitate by magnet. The precipitate was then dried in a vacuum desiccator and ground to obtain the MNPs in the powder form.
Characterization of Fe3O4 magnetic nanoparticles
Phase analysis of the synthesized powders was carried out by X-ray diffraction (XRD) using CuKα radiation over 2θ range of 20°–70° at a scan rate of 1.1° min−1 and with a sampling interval of 0.02 at 30 mA and 40 kV by using Philips PW 1710 diffractometer. The morphology and particle size of prepared MNPs were measured by high-resolution transmission electron microscope (HRTEM) using JEOL JEM-2100, with an acceleration voltage of 200 kV. Emission spectra were measured with Jobin Yvon-fluoromax-3. Magnetic measurements were carried out using SQUID VSM DC magnetometer (Quantam Design, USA). X-ray photoelectron spectroscopy (XPS) was performed using a omicron spectrometer with a MgKα X-ray source (1253.6 eV). Electron probe micro analysis (EPMA), was carried out for quantitative elemental detection using CAMECA SX-100.Cytotoxicity of MNPs was determined by conventional MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. MCF7 cells in exponential growth phase were seeded in a 96-well plate at a density of 4 × 103 cells per well in 0.1 ml DMEM complete medium. The cells were allowed to adhere and incubate at 37 °C for 24 h. After incubation, the medium was aspirated and replaced with 0.1 ml of fresh medium containing varying concentrations (0.1–1 mg ml−1) of magnetite nanoparticles. Control cells were treated with equivalent volumes of magnetite nanoparticle-free media. After 72 h of incubation, the medium was discarded and subsequently replaced with 0.1 ml of 1 mg ml−1 MTT in PBS. After 4–5 h incubation, the unreduced MTT solution was discarded. Further, DMSO (100 μl) was added to each well to dissolve the reduced formazan crystals formed by viable cells. The 96-well plate was shaken and absorbance of formazan dye was measured at 550 nm using a benchmark microplate reader. The assay was performed in triplicate. The cytotoxic effect of each treatment was expressed as percentage of cell viability relative to the untreated control cells (% control), which can be defined as: [[OD 550 nm treated cells]/[OD 550 nm control cells]] × 100.5
Cellular localization of magnetite nanoparticles was determined by seeding MCF 7 cells at a density of 2.5 × 104 cells on a sterile glass cover slip. The cover slips were pretreated with 0.01% poly-L-lysine before seeding and cells were treated with 250 μg ml−1 of Rh 610 labelled MNPs for different time periods. After incubation, the cover slips were removed and washed twice with PBS solution. Then cells were fixed for 20 min using 3.7% paraformaldehyde. The cells were washed with additional PBS, counter-stained with DAPI for 15 min. The MCF 7 cells were then analyzed using CLSM.
Results and discussion
The phase of the prepared samples was ascertained by XRD analysis and the observed diffractograms are shown in Fig. 1. The diffraction peaks of the resultant samples are well consistent with JCPDS card no 65-3107. Further, the absence of any noticeable unidentified XRD peaks indicates the purity of the synthesized magnetite nanoparticles (e.g., peaks due to Fe2O3) (Fig. 1a). The purity of the sample was further verified by XPS analysis, which shows Fe3+–Fe2+ molar ratio of around 1.998![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (inset of Fig. 1b). The average crystallite size of MNP and AMNP samples were found to be 11 and 12.2 nm, respectively, as calculated using XRD data in the well-known Scherrer's equation.
1 (inset of Fig. 1b). The average crystallite size of MNP and AMNP samples were found to be 11 and 12.2 nm, respectively, as calculated using XRD data in the well-known Scherrer's equation.
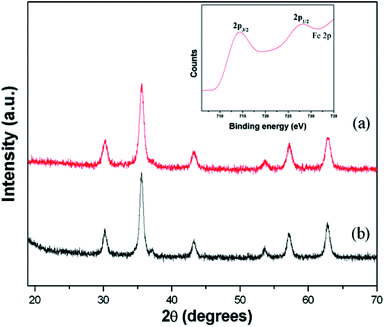 | ||
| Fig. 1 X-ray diffraction patterns of the samples; (a) MNPs and (b) AMNPs (XPS spectra of MNP as an inset). | ||
To decipher the functional groups present in the prepared nanoparticles, FTIR was employed as the functional group detecting tool and the resulted spectra taken in the range of 450–4000 cm−1 have been depicted in Fig. 2. The spectra show a broad band around 3414 cm−1 and a sharp band at 587 cm−1, indicating the presence of –OH and Fe–O groups related to the magnetic core. The amine functionalized magnetite nanoparticles depict FTIR peaks at 2878 and 2941 cm−1 corresponding to –CH2 stretching of the APTMS moieties present on the nanoparticles surface. Further, the weak peak observed at 1038 and a new peak at 1125 cm−1 may be assigned to Si–O and C–N stretching modes respectively. In addition to that a new peak is observed at 620 cm−1, which may be attributed to amine bending modes for the aminated MNP sample (Fig. S2†). This observation confirms the successful modification of the magnetite surface by primary amine groups. The functionalization of MNPs by amine was further ascertained by carrying out a series of chemical tests. First, we detected nitrogen using the Lassaign test and then carried out an azodye test. The non-appearance of red color in this test indicates the existence of a primary amine group. In addition, the primary amine is found to be aliphatic as is established by the Hinsberg reaction. In this test, amine-coated MNPs produced an insoluble precipitate in the presence of benzenesulfonyl chloride and hydrochloric acid. These results thus confirm the surface amine functionalization of nanoparticles. Further, silica coating was confirmed by Electron probe micro analysis (EPMA), which revealed that the AMNP sample contains Si–Fe in the weight ratio 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 89.
89.
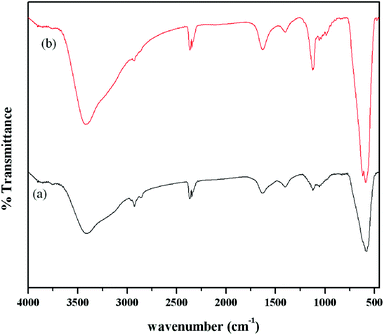 | ||
| Fig. 2 FTIR spectra of prepared magnetite samples; (a) MNPs and (b) AMNPs. | ||
The morphological characterization with transmission electron microscopy (TEM) reveals that the prepared samples are of nanosize (Fig. 3). The diameters of the prepared MNP and AMNP samples were found to be 12 ± 1 and 14 ± 2 nm respectively. A slight increase in the particle size of MNPs on treatment with APTMS can be correlated to the surface functionalization of MNPs with aminated silica (i.e., AMNPs). In order to get a clear view of the surface coating, TEM was obtained at higher magnification (Fig. S1†). The contrast variation in this HRTEM image (indicated by circle in Fig. S1†) suggests the formation of coating on MNPs. However, the demarcation of core-shell is not very clear, which may be attributed to very thin coating (∼2 nm). This observation is also supported by EPMA, FTIR and EDX (Fig. S1†) analysis.
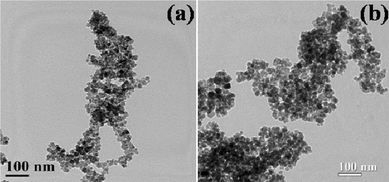 | ||
| Fig. 3 TEM images of the as-synthesized samples; (a) MNPs and (b) AMNPs. | ||
Magnetic measurements of the prepared MNPs were performed with superconducting quantum interference device (SQUID). Field dependent magnetization was performed as a function of temperature using standard zero field cooling (ZFC) and field cooling (FC) procedures. This analysis was carried out at temperatures in the range of 2 to 350 K with an applied magnetic field of 100 G. ZFC and FC magnetization curves of the MNPs and AMNPs are depicted in Fig. 4. The ZFC measurement involves cooling of the sample in the absence of an applied magnetic field. Under this condition, the magnetic moment of the particles are randomly orientated (due to Brownian motion). Hou et al.17 reported a similar observation in case of MNPs when all the spin orientations are blocked at a lower temperature far below the magnetic transition (Tc). Since there is no applied field to orient the spins along the field direction, the resultant magnetization is small. However, on application of an external magnetic field, the orientation of the dipole particles along the field direction generates a net magnetic moment, which increases with the temperature as a greater number of particles familiarize their magnetic moments parallel to the field. The ZFC curve in Fig. 4a illustrates a typical blocking process, reflecting the superparamagnetic behavior. From the curves, the blocking temperatures (TB) were found to be 140 and 242 K for MNP and AMNP samples respectively.17–19 The divergence in the ZFC and FC magnetization curves just above the TB may be indicative of some aggregation of the nanoparticles in the sample.20 In addition, the broadness of the magnetic transition peaks for both MNP and AMNP indicates that there may be strong magnetic dipole–dipole interaction among the particles.21
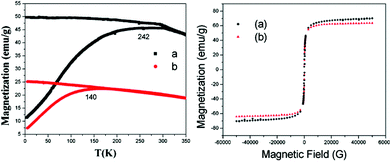 | ||
| Fig. 4 ZFC and FC magnetization curves with an applied field 100 G (a) of the MNPs and (b) AMNPS; the corresponding field-dependent magnetization studies of the sample showing the respective hysteresis loops measured at 300 K (same color lines). | ||
Fig. 4 shows the field-dependent magnetization M(H), performed for all the prepared samples at room temperature. Neither remanence nor coercivity in the hysteresis loops were observed at room temperature. Moreover, the prepared samples are superparamagnetic in nature as indicated from Langevin general equation fit measurement. The saturation magnetization for MNP and AMNP are 71.2 and 67.25 emu g−1 respectively.22 The slight difference in saturation magnetization of MNP and AMNP could be ascribed to the coating of the surface of MNP with aminated silica.23
For advanced studies, nanoparticles may require to act as selective carriers for those independent ingredients like molecular probes or drugs. This can be achieved through attachment of fluorescent probes on the MNPs surface via surface functionalization. However, the hydroxyl functional groups, which are generally located on the surface of MNPs, might not be useful for the attachment of fluorescent probes like Rh 610. Hence, the surfaces of MNPs were first functionalized with the help of APTMS and thereby providing surface amine groups for the attachment of florescent probes. On the other hand, it is well known that EDC can be used as a coupling agent for the formation of amide bond through the reaction between COOH and NH2 functional groups. The fluorescence emission, either from nanoparticles attached from molecular probes or molecular probe medium, was confirmed by fluorescence spectrophotometer and CLSM. The fluorescence spectrum recorded from 550–700 nm (shown in Fig. 5a) depicts a clear peak at 583 nm, which can be attributed to the AMNP covalently attached Rh 610 fluorescent dye. To verify this, we have carried out a fluorescence study on the Rh 610 bounded MNPs supernatant solution as well as AMNP mixed with Rh 610 in the absence of EDC. The dye bounded MNPs supernatant solution does not show any fluorescence as depicted in Fig. 5b, whereas AMNP mixed with Rh 610 in the absence of EDC exhibits fluorescence. In the latter case, the peak however appears at 576 nm, which may be ascribed to the availability of free Rh 610 in the AMNP suspension (Fig. 5c). The red shift (7 nm) of the emission maximum of the fluorescence band positions in Fig. 5a and 5c may be corroborated to the strong attachment of the Rh 610 dye to the AMNP particles surface.24
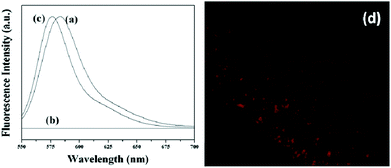 | ||
| Fig. 5 Emission spectra of (a) Rh-610 conjugated AMNPs, (b) the supernatant solution of Rh-610 conjugated AMNPs and (c) Magnetite nanoparticles dispersed in free Rh-610 and (d) typical CLSM image of the Rh-610 conjugated AMNPs. | ||
We have further estimated the anisotropy constant through the steady state anisotropy of dye attached to the surface of the nanoparticles or dye contained in the media. The anisotropy constant ‘r’ can be defined as:
To better understand the role of surface modification of Rh 610 labelled on the magnetite nanoparticles surface, the samples were examined under CLSM. The micrograph obtained from Rh 610 labelled AMNP shows red fluorescence (in Fig. 5d). This red fluorescence could be credited to the efficient binding of the dye molecules to the surfaces of the amino modified nano-colloid. Based on the analysis, it can thus be concluded that amino groups are available for selective modification through amide formation which is of covalent nature.
Further, we investigated the potential of the fluorescent nanoparticles for biomedical applications, in which cellular uptake was performed using MCF7 cells. The as-synthesized nanoparticles after surface modification were incubated with MCF7 cells at different time intervals, such as 30, 60, 180 and 360 min. The incubation process made these magnetic nanoparticles attached along the cell membrane and cytoplasm (Fig. 6). The cellular uptake of the magnetic nanoparticles might also be mediated by non-specific absorptive endocytosis mechanism. The strong binding ability of nanoparticles may be responsible for their high cellular uptake and this property of the synthesized MNPs can be utilized for drug delivery applications. We performed cytotoxicity of MNPs and AMNPs on MCF 7 cells through an MTT assay. MNPs displayed slight toxicity compared to AMNPs at higher concentrations. MNPs exhibited 84% of cell surveillance, while AMNPs displayed 95% of cell surveillance at 1 mg ml−1 concentration with respect to control cells (Fig. 6f). Further, since the nanoparticles were integrated in non-phagocytosing animal cells without using additional agents and exclusive of exerting cytotoxic effects, the synthesized nanoparticles are considered as superior to most of the organic fluorophores and may pave their way in biomedical applications. The uptake mechanism of the nanoparticle and the detailed intracellular localization are currently in progress.
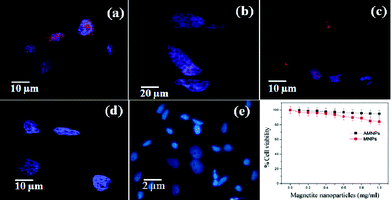 | ||
| Fig. 6 CLSM images after (a) 30, (b) 60 (c) 180 and (d) 360 min for the localization of R-610 tagged AMNPs treated on MCF 7 cell line (e) control. (f) Cell viability assay of the prepared samples on MCF 7 cells. | ||
Conclusions
In this paper, we successfully prepared magnetite nanoparticles under atmospheric air conditions and surface functionalized with silica to attach Rh-610 for biomedical applications. The XRD of prepared samples revealed its purity and crystallite size of around 11 nm. MNP and AMNP both exhibit typical SPM behavior with well-defined blocking temperatures (at 140 and 242 K respectively). The amined silica coated MNP shows lower blocking temperature and saturation magnetization, which indicates that the magnetization strength reduces due to surface coating. The localization study of nanoparticles recommends its usage as an efficient delivery system in biological applications.Acknowledgements
The authors would like to acknowledge MHRD, Government of India, for financial support. The authors are grateful to Professor M. Mahitosh for supporting the cellular uptake measurement, School of Medical Science and Technology, IIT Kharagpur, India. The authors are also grateful to Professor B. Viswanathan for helping with the XPS measurement, NCCR, Indian Institute of Technology Madras.Notes and references
- C. S. Yeh, C. C. Huang, K. Y. Chuang, C. P. Chou, M. T. Wu, H. S. Sheu, D. B. Shieh, C. Y. Tsai, C. H. Su and H. Y. Lei, J. Mater. Chem., 2011, 21, 7472–7479 RSC.
- Y. Zhang, N. Kohler and M. Zhang, Biomaterials, 2002, 23, 1553–1561 CrossRef CAS.
- R. N. Muller, S. Laurent, D. Forge, M. Port, A. Roch, C. Robic and L. V. Elst, Chem. Rev., 2008, 108, 2064–2110 CrossRef.
- Y. Ge, Y. Zhang, S. He, F. Nie, G. Teng and N. Gu, Nanoscale Res. Lett., 2009, 4, 287–295 CrossRef CAS.
- M. Das, D. Mishra, T. K. Maiti, A. Basak and P. Pramanik, Nanotechnology, 2008, 19, 415101 CrossRef.
- Q. Zhang, M. S. Thompson, A. Y. Carmichael-Baranauskas, B. L. Caba, M. A. Zalich, Y. N. Lin, O. T. Mefford, R. M. Davis and J. S. Riffle, Langmuir, 2007, 23, 6927–6936 CrossRef CAS.
- C. Han, W. Cai, W. Tang, G. Wang and C. Liang, J. Mater. Chem., 2011, 21, 11188–11196 RSC.
- J. Xu, H. Yang, W. Fu, K. Du, Y. Sui, J. Chen, Y. Zeng, M. Li and G. Zou, J. Magn. Magn. Mater., 2007, 309, 307–311 CrossRef CAS.
- Z. L. Liu, X. Wang, K. L. Yao, G. H. Du, Q. H. Lu, Z. H. Ding, J. Tao, Q. Ning, X. P. Luo, D. Y. Tian and D. Xi, J. Mater. Sci., 2004, 39, 2633–2636 CrossRef CAS.
- M. Das, P. Dhak, S. Gupta, A. Basak and P. Pramanik, Nanotechnology, 2010, 21, 125103 CrossRef.
- A. Quarta, R. Di Corato, L. Manna, S. Argentiere, R. Cingolani, G. Barbarella and T. Pellegrino, J. Am. Chem. Soc., 2008, 130, 10545–10555 CrossRef CAS.
- J. H. Gao, H. W. Gu and B. Xu, Acc. Chem. Res., 2009, 42, 1097–1107 CrossRef CAS.
- L. Y. Wang, Z. H. Yang, Y. Zhang and L. Wang, J. Phys. Chem. C, 2009, 113, 3955–3959 CAS.
- Y. Lu, Y. Yin, B. T. Mayers and Y. Xia, Nano Lett., 2002, 2, 183–186 CrossRef CAS.
- H. Lu, G. Yi, S. Zhao, D. Chen, L.-H. Guo and J. Cheng, J. Mater. Chem., 2004, 14, 1336–1341 RSC.
- G. K. Kouassi and J. Irudayaraj, Anal. Chem., 2006, 78, 3234–3241 CrossRef CAS.
- Y. L. Hou, J. F. Yu and S. Gao, J. Mater. Chem., 2003, 13, 1983–1987 RSC.
- A. G. Roca, M. P. Morales, K. O'Grady and C. J. Serna, Nanotechnology, 2006, 17, 2783–2788 CrossRef CAS.
- R. L. Rebodos and P. J. Vikesland, Langmuir, 2010, 26, 16745–16753 CrossRef CAS.
- P. Guardia, B. Batlle-Brugal, A. G. Roca, O. Iglesias, M. P. Morales, C. J. Serna, A. Labarta and X. Batlle, J. Magn. Magn. Mater., 2007, 316, E756–E759 CrossRef CAS.
- J. H. Gao, G. L. Liang, J. S. Cheung, Y. Pan, Y. Kuang, F. Zhao, B. Zhang, X. X. Zhang, E. X. Wu and B. Xu, J. Am. Chem. Soc., 2008, 130, 11828–11833 CrossRef CAS.
- N. Puvvada, P. K. Panigrahi, D. Mandal and A. Pathak, RSC Adv., 2012, 2, 3270–3273 RSC.
- N. Zhao, W. Ma, Z. M. Cui, W. G. Song, C. L. Xu and M. Y. Gao, ACS Nano, 2009, 3, 1775–1780 CrossRef CAS.
- Y. Q. Ge, Y. Zhang, S. Y. He, F. Nie, G. J. Teng and N. Gu, Nanoscale Res. Lett., 2009, 4, 287–295 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2tx20004j |
| This journal is © The Royal Society of Chemistry 2012 |