Responsive imaging probes for metabotropic glutamate receptors†
Anurag
Mishra
*a,
Sven
Gottschalk
b,
Jörn
Engelmann
b and
David
Parker
*a
aDepartment of Chemistry Durham University South Road, Durham, DH1 3LE, England. E-mail: anurag.mishra@durham.ac.uk; david.parker@durham.ac.uk; Fax: (+44-191-3342051)
bHigh Field MR Center Max Planck Institute for Biological Cybernetics, Spemannstrasse 41, Tuebingen, 72076, Germany
First published on 27th September 2011
Abstract
The design, synthesis and evaluation of eight contrast agents for metabotropic glutamate receptors is reported. Each of the contrast agents contains a selective mGluR5 binding moiety linked to a ‘DOTA’-derived gadolinium complex. The potential of these systems was evaluated in vitro for application as responsive MR imaging probes. The targeting moieties mGluR5antagonists based on aromatic alkyne and dipyridyl/heterobiaryl amide derivatives integrated in a modular fashion, involving linkage to the macrocyclic DOTA ligand to allow specific binding to the mGluR5 receptors. Signal intensity enhancements of up to 27% were observed by MRI in primary astrocyte suspensions and the reversibility of probe binding to the receptor sites, induced by added glutamate, was demonstrated using optical emission and the antagonistic activity of complexes was defined by calcium binding assays.
Introduction
A range of techniques is being used by neuroscientists to address the details of brain function in vivo. Methods range from electrophysiological measurements with single or multiple electrodes, pharmacological testing and non-invasive computational methods to Blood-Oxygen-Level-Dependent (BOLD) functional Magnetic Resonance Imaging (fMRI). Investigations over the last few decades strongly suggest that stimulus- or task-related neural activity is distributed over large parts of the brain, covering different cortical and sub-cortical areas.1 Nonetheless, large gaps in the nature and quality of information exist between the different techniques available for studies of the nervous system. The emergence of fMRI—a technique for measuring haemodynamic changes after enhanced neural activity—in the early 1990s has had a major impact on basic cognitive neuroscience research.1 However, BOLD fMRI has its limitations: it measures a surrogate signal, the spatial specificity and temporal response of which is subject to both physical and biological constraints. The existing fMRI methods alone are not sufficient and there is a need for the development of fresh approaches to provide new information for cognitive research.Glutamate is several times more abundant than any other neurotransmitter; indeed, brain tissue contains 5–15 mM Glu per kg. It plays a critical role in mediating excitatory signals in the mammalian central nervous system and is involved in most characteristics of normal brain function, including cognition, memory and learning.2,3 On the arrival of a nerve impulse (or action potential) at the terminal button, voltage gated calcium channels open and calcium ions enter the presynaptic neurons, triggering vesicles to discharge Glu into the narrow synaptic cleft. Glu diffuses to the postsynaptic neurons and binds to glutamate receptors (GluRs), activating the postsynaptic neuronal cell. The released Glu is then cleared away by excitatory amino acid transporters (present on astroglial support cells) and, later, by transporters on postsynaptic neurons.4
Glutamate mediates its effect through both G-protein-coupled metabotropic receptors (mGluRs) and ligand-gated ionotropic receptors (iGluRs). Only the mGluR subtype-5 (mGluR5) are found to be actively involved in transducing excitatory signals between neurons through Glu.3 These receptors are widely localized on the postsynaptic membrane and are distributed in various brain regions, including the spinal cord, thalamic nuclei and the hippocampus.3 Next to neurons, mGluR5 is also expressed on astrocytes, where it is thought to be part of Glu transmission by astrocytes. In addition, a role in the Glu-mediated astrocyte-to-neuron signalling has also been discussed.5
Magnetic resonance imaging is a powerful tool to study brain function. The sensitivity and specificity of MR imaging can be further amplified by the use of responsive contrast agents (RCAs). Reports on pH, enzyme and metal-ion-sensitive CAs have been published.6 In principle, the relaxivity of these responsive agents can be tailored to be dependent on certain variables involved in neuronal signalling, such as receptor systems, calcium levels and ion channel functions, participating in the up-regulation of signals following synaptic stimulation.
Of particular interest for brain imaging are responsive contrast agents that can report changes in neural activity. Contrast agents that selectively bind to glutamate receptors (GluRCA) offer one way forward in this context. Such probes may bind both to neuronal postsynaptic receptors as well as to those expressed in astrocytes. They can therefore act both as “markers” of receptor density and as indicators of neuronal activation. The application to image mGluR5-density is of particular interest under pathophysiological conditions. This receptor has been shown to play important roles in several disease states, such as Parkinson's and Alzheimer's diseases, neuropathic pain, anxiety, depression and also in drug addiction or withdrawal.7a
In a responsive system, Glu is released from presynaptic neurons into the synaptic cleft following stimulation. We hypothesise that the GluRCA will be displaced from the mGluR5 receptors and replaced by Glu. This sequence of processes leads to changes in relaxivity reflecting the amount of free and bound GluRCA, respectively. The Glu burst is believed to occur over a period of milliseconds following stimulation and re-equilibration is believed to occur over a period of about one second,7b allowing time for data acquisition using fast MRI pulse techniques.1 Assuming that the local concentration of the CA does not change significantly over this timescale, the concentration changes in Glu that occur on neural activation can be detected and quantified by modulation of the water proton relaxation rate, created by the presence of the glutamate-responsive CA.
In this work, the glutamate-responsive imaging probes (GluRCAs) were designed to exhibit a change in relaxivity due to alteration of the rotational correlation time, τr. It is well-known that, in the medium field range, τr depends on the molecular volume of the complex. A change in relaxivity ensues provided that there is some degree of motional coupling between the Gd-bound water (and associated second-sphere waters) and the overall tumbling motion of the conjugate.8 When the GluRCA interacts with its designated binding site at the receptor, τr is increased (and the second-sphere and prototropic exchange contributions to overall relaxivity may also vary), resulting in a large relaxivity change, revealed by R1 modulation (R1 = 1/T1).6
We report the design, synthesis and evaluation of eight GluRCAs containing various selective mGluR5 binding moieties linked to ‘DOTA’ derived gadolinium complexes, exploring their potential application as targeted and responsive MR imaging probes. These binding moieties are established specific mGluR5antagonists2,10 (alkynes and dipyridyl/heterobiaryl amides derivative) and have been integrated into these structures in a modular fashion, involving linkage to the macrocyclic ligand core (i.e. ‘DOTA’) to allow the targeting of mGluR5 receptors (Scheme 1).
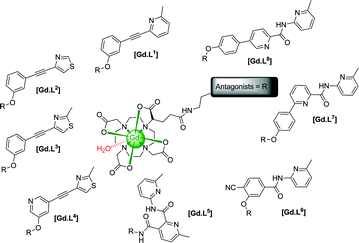 | ||
| Scheme 1 | ||
Results and discussion
Ligand and complex synthesis
Several synthetic steps are involved in preparing the GluRCAs. The amine precursors 10/14/19/24/31/38/43/48 were synthesised using a range of synthetic transformations including Suzuki/Sonogashira coupling, alkylation, amide formation, protection and de-protection steps (ESI, Schemes S1 and S2†). For example, tBu ester 7 was obtained by stepwise alkylation of (1,4,7-tris-carboxymethyl-1,4,7,10-tetraaza-cyclododec-1-yl)-acetic acid t-butyl ester, with (S)-5-benzyl 1-tert-butyl 2-bromopentanedioate in acetonitrile followed by debenzylation.The intermediate tetra-esters were synthesized by coupling the amine 10/14/19/24/31/38/43/48 and the appropriate mono-acid, e.g.7, [EDC/HOBt/NMM] in anhydrous DMF, from which ligands L1–81–8 were obtained by hydrolysis with TFA:CH2Cl2 (ESI, Scheme S3†). Ligands, L1–81–8 were loaded with gadolinium using GdCl3.6H2O in water at pH 5.5 (Scheme 2). The final concentration of Gd3+ was determined by inductively coupled plasma mass spectrometry (ICP-MS). The proton longitudinal relaxivities, r1p, of [Gd.L1–81–8] were measured to be 4.80–5.90 mM−1s−1 in buffered aqueous solution [310 K, 1.4 T, pH 7.4, 0.1M PBS)] (ESI, Table 1†).
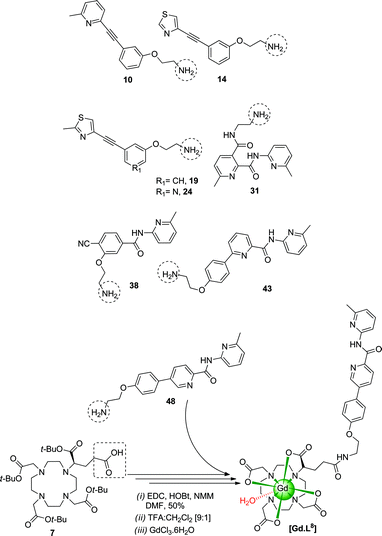 | ||
| Scheme 2 | ||
Probe-receptor and probe-protein binding studies
Primary rat astrocytes were chosen as the cellular model, as these cells are known to express mGluR5 efficiently. However, we did not use additional differentiation of the cells with a G5-supplement, as sufficient expression of the receptor in our model was revealed by immunofluorescence staining studies (ESI, Fig. S1†). Higher expression levels occur with primary cell cultures compared to the secondary cell cultures that have been used in previous receptor studies.11The cytotoxicity of the gadolinium complexes [Gd.L1–81–8] was examined with a proliferation assay (XTT: mitochondrial redox perturbation) in combination with a cell number assay (Hoechst 33342: stains DNA in cells). Apart from [Gd.L33], none of the complexes exhibited significant effects in both assays over the range 50–200μM after 24 h. However, [Gd.L33] did give rise to an increase in metabolic activity in primary astrocytes, although the number of cells was not significantly influenced, which is consistent with a minor cytotoxic effect under these conditions (ESI, Fig. S2†).
Cellular labeling of mGluR5-expressing primary astrocytes with [Gd.L1–81–8] was assessed by measuring T1-weighted MR images on a 3T Siemens human whole body MR scanner and calculating the cellular longitudinal relaxation rates R1,cell (Fig. 1). In the first set of experiments, primary astrocytes were treated for 45 min with 100 μM of each of the gadolinium complexes, then washed to remove any unbound complex, re-suspended in fresh buffer and T1-weighted MR images were acquired (see ESI for details†). From the structurally similar [Gd.L1–41–4], [Gd.L33] showed statistically significant increases in R1,cell (126% of control), comparable to that of [Gd.L22] (125% of control), while [Gd.L11] and [Gd.L44] also exhibited significant increases, but to a lesser extent (Fig. 1A). Amongst the dipyridyl/heterobiaryl amide analogues [Gd.L5–85–8], only [Gd.L55] and [Gd.L88] had cellular relaxation rates R1,cell (112% and 116% of control, respectively) that were significantly different from untreated control cells. The increased R1,cell is consistent with receptor binding or uptake into the cells, as each process has been shown to increase the measured proton relaxation rate.12 To further elucidate which of these two is responsible for the observed increase in R1,cell, the following experiment was conducted. The antagonist-arm of [Gd.L11] is structurally identical to the highly efficient, established mGluR5-antagonist MPEP (2-methyl-6-(phenylethynyl)pyridine).10 Hence, in one series of experiments, primary astrocytes were pre-treated with 200μM MPEP for 15 min and then incubated with 100μM [Gd.L11] for another 45 min with MPEP still present in the incubation solution. With this pre-treatment, no increase in R1,cell was observed after treatment with [Gd.L11] (Fig. 1A). This result indicates that the increases observed in R1,cell are very likely to be associated with receptor binding and not simply a non-specific cellular internalisation process.
![Cellular 1H MR relaxation rates R1,cell in cell suspensions (3T, 298 K) after treatment of primary astrocytes under various conditions. Control: cells incubated with HEPES buffered saline solution without CA. [Gd.DOTA] served as a negative control. (A) Treatments with 100μM of [Gd.DOTA] or [Gd.L1–81–8] for 45 min. Data are the mean (±SEM) of 2–4 experiments with 2 replicates each. ***P < 0.001, ns: not significant vs. Control; ###P < 0.001 vs. [Gd.DOTA]. ANOVA with Bonferroni's multiple comparison post test. (B) Incubations for 45 min with increasing concentrations of [Gd.DOTA] or [Gd.L22]. Data are means of n = 1–6 (±SEM). ***P < 0.001 vs. control. #P < 0.05, ###P < 0.001 vs. [Gd.DOTA] 100μM. ANOVA with Bonferroni's multiple comparison post test. A relaxivity value r1,cell for [Gd.L22] of 22.8 mM−1s−1 was determined by examining the measured relaxation rate over four concentrations (see ESI). (C) Representative T1-weighted MR-images of 1 × 107cells treated for 45 min with 100 μM [Gd.DOTA] or [Gd.L22]. Images were obtained using a turbo spin echo technique with a matrix of 256 × 256 voxels over a field-of-view of 110 × 110 mm2, slice thickness 1 mm (voxel size 0.4 × 0.4 × 1.0 mm), TR 1000 ms, TE 13 ms, Ti 23 ms and 20 averages.](/image/article/2012/SC/c1sc00418b/c1sc00418b-f1.gif) | ||
| Fig. 1 Cellular 1H MR relaxation rates R1,cell in cell suspensions (3T, 298 K) after treatment of primary astrocytes under various conditions. Control: cells incubated with HEPES buffered saline solution without CA. [Gd.DOTA] served as a negative control. (A) Treatments with 100μM of [Gd.DOTA] or [Gd.L1–81–8] for 45 min. Data are the mean (±SEM) of 2–4 experiments with 2 replicates each. ***P < 0.001, ns: not significant vs. Control; ###P < 0.001 vs. [Gd.DOTA]. ANOVA with Bonferroni's multiple comparison post test. (B) Incubations for 45 min with increasing concentrations of [Gd.DOTA] or [Gd.L22]. Data are means of n = 1–6 (±SEM). ***P < 0.001 vs. control. #P < 0.05, ###P < 0.001 vs. [Gd.DOTA] 100μM. ANOVA with Bonferroni's multiple comparison post test. A relaxivity value r1,cell for [Gd.L22] of 22.8 mM−1s−1 was determined by examining the measured relaxation rate over four concentrations (see ESI†). (C) Representative T1-weighted MR-images of 1 × 107cells treated for 45 min with 100 μM [Gd.DOTA] or [Gd.L22]. Images were obtained using a turbo spin echo technique with a matrix of 256 × 256 voxels over a field-of-view of 110 × 110 mm2, slice thickness 1 mm (voxel size 0.4 × 0.4 × 1.0 mm), TR 1000 ms, TE 13 ms, Ti 23 ms and 20 averages. | ||
To investigate the possible concentration-effects, primary rat astrocytes were treated with increasing concentrations of [Gd.L22] or [Gd.DOTA] (as a negative control) for 45 min. The results from these dose-response experiments for [Gd.L22] (Figs 1B and C) revealed a significant concentration-dependent increase in R1,cell compared to untreated cells (control) and to [Gd.DOTA]-treated cells (negative control). The measured relaxivity, r1,cell, for [Gd.L22] in the cell suspensions was 22.8 mM−1s−1 (3T, 298 K), determining the Gd concentration over four concentrations of added agent by ICP-MS (ESI†). The enhancement of the relaxivity must be associated with the higher relaxivity of the receptor-bound complex. Such effects are strongly field-dependent, so a preliminary study was undertaken to examine the effect of (non-specific) protein binding on measured relaxivity at 1.4 T. At this field, relaxivity increases associated with longer rotational correlation times should lead to increased relaxivity values, notwithstanding the lower affinity of the probe for the protein compared to the mGluR5receptor.
Human serum albumin (HSA) is the major protein constituent in the circulatory system in mammalians and its effect on the measured relaxivity of [Gd.L33] and [Gd.L88] (both 0.1 mM) was assessed at 1.4 T in the presence of up to 0.6 mM protein. This led to an increase in r1p of 115% (5.85 mM−1 s−1 to 12.6 mM−1 s−1, at 0.1 mM added protein) and 49% (6.77 mM−1 s−1 to 10.1 mM−1 s−1, at 0.1 mM added protein) for [Gd.L33] and [Gd.L88] respectively (ESI, Fig. S3†). The apparent association constant for protein binding, assuming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of interaction, was estimated to be log K = 3.45(±0.03) and log K = 3.38(±0.02) for [Gd.L33] and [Gd.L88], respectively (50 mM TRIS pH 7.4, 2.5 mM Ca2+, 10 mM Mg2+, 10% sucrose, 310 K).
1 stoichiometry of interaction, was estimated to be log K = 3.45(±0.03) and log K = 3.38(±0.02) for [Gd.L33] and [Gd.L88], respectively (50 mM TRIS pH 7.4, 2.5 mM Ca2+, 10 mM Mg2+, 10% sucrose, 310 K).
Antagonistic activity and the reversibility of probe-receptor binding in the presence of glutamate
The antagonistic activity of [Gd.L33] and [Gd.L88] was assessed using a functional cellular calcium fluorescence assay. Activation of mGluR5 by Glu leads to transient changes in the intracellular calcium concentration7,13 (Fig. 2A). These calcium-transients can be measured in cells that are labelled intracellularly with the calcium-sensitive fluorescence dye fluo-4.14 Pre-treatment of the cells with antagonists prior to exposure to Glu results in a diminished calcium-transient and an increase in the EC50‡ value for Glu.13 Cross-reactivity with other GluRs is not expected, as it has been reported that astrocytes solely express active mGluR513 and, therefore, the use of inhibitors for other GluRs was not considered necessary. Both gadolinium complexes, [Gd.L33] and [Gd.L88] had a pronounced antagonistic activity (Fig. 2B), each significantly increasing the EC50 of Glu by a factor of 3.9(±0.9) and 3.1(±0.3), respectively (both ***P<0.001 vs. cells pre-treated with buffer only).![Assays for glutamate-receptor function for [Gd.L33] and [Gd.L88]. The presence of [Gd.L33] or [Gd.L88] decreases glutamate-induced calcium transients. Primary astrocytes were grown in poly-d-lysine-coated 96 well plates for 10–15 days in DMEM, which was changed to glutamine-free DMEM the day before the calcium assay. (A) Representative fluorescence recordings (normalized to the average of 20 s baseline) of a control well vs. a well pre-treated with 100 μM [Gd.L33] for at least 15 min showing the antagonistic effect of [Gd.L33] on the glutamate induced calcium transient. (B) The shift in the concentration of glutamate that gives a response half-way between the bottom and top of the fitting curve (i.e.EC50) to a higher concentration after pre-treatment of cortical primary astrocytes with [Gd.L33] or [Gd.L88]. Curves were generated from at least three independent experiments and were calculated for each condition using data normalized to the maximum obtainable change in calcium and nonlinear regression analysis. Data points represent means ± SEM. [HBSS: Hanks' Balanced Salt Solution].](/image/article/2012/SC/c1sc00418b/c1sc00418b-f2.gif) | ||
| Fig. 2 Assays for glutamate-receptor function for [Gd.L33] and [Gd.L88]. The presence of [Gd.L33] or [Gd.L88] decreases glutamate-induced calcium transients. Primary astrocytes were grown in poly-D-lysine-coated 96 well plates for 10–15 days in DMEM, which was changed to glutamine-free DMEM the day before the calcium assay. (A) Representative fluorescence recordings (normalized to the average of 20 s baseline) of a control well vs. a well pre-treated with 100 μM [Gd.L33] for at least 15 min showing the antagonistic effect of [Gd.L33] on the glutamate induced calcium transient. (B) The shift in the concentration of glutamate that gives a response half-way between the bottom and top of the fitting curve (i.e.EC50)‡ to a higher concentration after pre-treatment of cortical primary astrocytes with [Gd.L33] or [Gd.L88]. Curves were generated from at least three independent experiments and were calculated for each condition using data normalized to the maximum obtainable change in calcium and nonlinear regression analysis. Data points represent means ± SEM. [HBSS: Hanks' Balanced Salt Solution]. | ||
For practical reasons, it is not feasible to monitor using MRI probe–receptor binding reversibility induced by Glu in this primary astrocyte cellular model. The time required to measure the relaxation rates in our current cellular model far exceeds the time over which the cells take up Glu. Hence, no measurable change in R1,cell was observed when the cells were washed with Glu-containing buffer subsequent to probe-treatment, as described above (data not shown). One of the greatest disadvantages of MR imaging techniques compared with other imaging modalities is their intrinsic insensitivity. The presence of a gadolinium complex creates an increase in water proton signal intensity at concentrations of the order of 0.01 mM in vivo. Monitoring by optical imaging allows the observation of effects at concentrations in the nanomolar to micromolar range. Fortunately, out of the eight complexes, [Gd.L88] exhibited a strong absorbance and fluorescence (ϕem = 0.08). The favourable absorption (λmax 312 nm, ε = 20 mM−1 cm−1) and emission characteristics (λexc/λem 340/450 nm) of [Gd.L88] allowed binding studies to be made at micromolar complex concentrations.
No changes in the emission spectra (50 mM TRIS pH = 7.4, 2.5 mM Ca2+, 10 mM Mg2+, 10% sucrose, 298 K) were observed in the presence of up to two added equivalents of HSA, suggesting that the interaction of this control protein does not perturb the chromophoric properties of the antagonist moiety (data not shown). The addition of human mGluR5membrane preparations to [Gd.L88], (λexc 340/λem 450, 50 mM TRIS pH = 7.4, 2.5 mM Ca2+, 10 mM Mg2+, 10% sucrose, 298 K) gave rise to increases in the emission intensity. Up to a four-fold intensity change was observed in the emission maximum (Fig. 3), suggesting that the probe is binding to the receptors, thereby enhancing the local rigidity of the fluorophore and suppressing deactivation processes associated with vibrational relaxation. Subsequent additions of Glu, in the 5–100 nM range, restored the emission intensity to the value of the unbound complex, which is consistent with the reversibility of probe binding to receptors induced by Glu addition.
![Emission spectral changes (λexc 340 nm) for [Gd.L88] (L) with [[Gd.L88]:human mGluR5 complex = LR], in the absence and presence of increasing amounts of glutamate. Fitting the decrease in emission intensity with [Glu] gives an apparent binding constant, log K = 8.0 (±0.1) (50 mM TRIS pH = 7.4, 2.5 mM Ca2+, 10 mM Mg2+, 10% sucrose, 298 K).](/image/article/2012/SC/c1sc00418b/c1sc00418b-f3.gif) | ||
| Fig. 3 Emission spectral changes (λexc 340 nm) for [Gd.L88] (L) with [[Gd.L88]:human mGluR5 complex = LR], in the absence and presence of increasing amounts of glutamate. Fitting the decrease in emission intensity with [Glu] gives an apparent binding constant, log K = 8.0 (±0.1) (50 mM TRIS pH = 7.4, 2.5 mM Ca2+, 10 mM Mg2+, 10% sucrose, 298 K). | ||
Summary and conclusions
In summary, the responsive contrast agents [Gd.L33] and [Gd.L88] are promising candidates as imaging probes that report on mGluR5receptor density. They exhibit a significant relaxation rate increase in the presence of the mGluR5 receptors on primary astrocytes, and function as antagonists, as revealed by functional calcium binding assays. The reversibility of binding to the receptors is further exemplified in fluorescence studies with [Gd.L88], demonstrating that addition of glutamate leads to release of the complex from the receptor binding site. Thus, they may also offer scope to allow the monitoring of events induced by fluctuations in local Glu concentrations. Future work will seek to extend these encouraging preliminary results by following changes in MR signal intensity in an animal model. We aim to follow changes in the binding of the probe to the mGluR5 receptors in real time, induced by an external stimulus, while the contrast agent is directly infused to create a steady-state local concentration.Acknowledgements
We thank the EC for a Marie Curie Fellowship, PIEF-GA-2009-237253 (AM), the German Ministry for Education and Research, BMBF, FKZ:01EZ0813 (SG), ESF COST D38 and Max Planck Society for their support. The authors would like to thank Marie-Luise Schwesinger for excellent technical assistance.References
- (a) N. K. Logothetis, Nature, 2008, 453, 869 CrossRef CAS; (b) N. K. Logothetis, H. Guggenberger, S. Peled and J. Pauls, Nat. Neurosci., 1999, 2(6), 555 CrossRef CAS.
- F. G. Siméon, A. K. Brown, S. S. Zoghbi, V. M. Patterson, R. B. Innis and V. W. Pike, J. Med. Chem., 2007, 50, 3256 CrossRef.
- D. Featherstone, ACS Chem. Neurosci., 2010, 1, 4 CrossRef CAS; C. G. Rousseaux, J. Toxicol. Pathol., 2008, 21, 25 CrossRef.
- J. D. Rothstein, Nature, 2000, 407, 141 CrossRef CAS.
- S. Goursaud, E. N. Kozlova, J.-M. Maloteaux and E. Hermans, J. Neurochem., 2009, 108, 1442 CrossRef CAS.
- (a) C. S. Bonnet and E. Toth, Future Med. Chem., 2010, 2, 367–384 CrossRef CAS; (b) A. Mishra, N. K. Logothetis and D. Parker, Chem.–Eur. J., 2011, 17, 1529 CrossRef CAS; (c) E. L. Que, E. Gianolio, S. L. Baker, S. Aime and C. J. Chang, Dalton Trans., 2010, 39, 469 RSC; (d) Y. You, E. Tomat, K. Hwang, T. Atanassijevic, W. Nam, A. P. Jasannof and S. J. Lippard, Chem. Commun., 2010, 46, 4139 RSC; (e) J. L. Major, R. M. Boiteau and T. J. Meade, Inorg. Chem., 2008, 47, 10788 CrossRef CAS; (f) E. L. Que and C. J. Chang, Chem. Soc. Rev., 2010, 39, 51 RSC; (g) M. P. Lowe, D. Parker, O. Reany, S. Aime, M. Botta, G. Castellano and E. Gianolio, J. Am. Chem. Soc., 2001, 123, 7601 CrossRef CAS; (h) M. Andrews, A. J. Amoroso, C. P. Harding and S. J. A. Pope, Dalton Trans., 2010, 39, 3407 RSC.
- (a) C. G. Rousseaux, J. Toxicol. Pathol., 2008, 21, 133 CrossRef CAS; (b) S. A. Hires, Y. Zhu and R. Y. Tsien, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 4411 CrossRef CAS.
- D. A. Fulton, M. O'Halloran, D. Parker, P. K. Senanayake, M. Botta and S. Aime, Chem. Commun., 2005, 474 RSC; D. Parker, E. Elemento, D. A. Fulton, S. Aime and M. Botta, Chem. Commun., 2006, 1064 Search PubMed.
- The chemistry of contrast agents in medical resonance imaging, ed. A. E. Merbach and E. Toth, John Wiley, New York, 2001 Search PubMed.
- (a) F. I. Carroll, Ann. N. Y. Acad. Sci., 2008, 1141, 221 CrossRef CAS; (b) N. D. P. Cosford, J. Roppe, L. Tehrani, E. J. Schweiger, T. J. Seiders, A. Chaudary, S. Rao and M. A. Varney, Bioorg. Med. Chem. Lett., 2003, 13, 351 CrossRef CAS; (c) C. Bonnefous, J.-M. Vernier, J. H. Hutchinson, J. Chung, G. Reyes-Manalo and T. Kamenecka, Bioorg. Med. Chem. Lett., 2005, 15, 1197 CrossRef CAS; (d) S. Kulkarni and A. H. Newman, Bioorg. Med. Chem. Lett., 2007, 17, 2074 CrossRef CAS; (e) S. Kulkarni, M.-F. Zou, J. Cao, J. R. Deschamps, A. L. Rodriguez, P. J. Conn and A. H. Newman, J. Med. Chem., 2009, 52, 3563 CrossRef CAS.
- R. D. Peavy, M. S. S. Chang, E. Sanders-Bush and P. J. Conn, J. Neurosci., 2001, 21, 9619 CAS.
- (a) A. Mishra, J. Pfeuffer, R. Mishra, J. Engelmann, A. K. Mishra, K. Ugurbil and N. K. Logothetis, Bioconjugate Chem., 2006, 17, 773 CrossRef CAS; (b) R. Mishra, W. Su, R. Pohmann, J. Pfeuffer, M. G. Sauer, K. Ugurbil and J. Engelmann, Bioconjugate Chem., 2009, 20, 1860 CrossRef CAS.
- Y. Zhang, A. L. Rodriguez and P. J. Conn, J. Pharmacol. Exp. Ther., 2005, 315, 1212 CrossRef CAS.
- K. R. Gee, K. A. Brown, W. N. Chen, J. Bishop-Stewart, D. Gray and I. Johnson, Cell Calcium, 2000, 27, 97 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental section. See DOI: 10.1039/c1sc00418b |
| ‡ Here, the EC50 is the concentration of Glu that gives a response half-way between the bottom and top of the fitting curve, as reported by the fluo-4 indicator for calcium. |
| This journal is © The Royal Society of Chemistry 2012 |
