Ultrasensitive and selective detection of alkaline-earth metal ions using ion-imprinted Au NPs composites and surface plasmon resonance spectroscopy†
Yaniv
Ben-Amram
,
Ran
Tel-Vered
,
Michael
Riskin
,
Zhen-Gang
Wang
and
Itamar
Willner
*
Institute of Chemistry, Center for Nanoscience and Nanotechnology, The Hebrew University of Jerusalem, Jerusalem, 91904, Israel. E-mail: willnea@vms.huji.ac.il; Fax: +972-2-6527715; Tel: +972-2-6585272
First published on 13th October 2011
Abstract
Au nanoparticles (NPs) functionalized with electropolymerizable thioaniline units and with dithiothreitol ligands were synthesized. The NPs were electropolymerized onto Au-coated glass surfaces in the presence of the alkaline-earth metal ions Mg2+, Ca2+, Sr2+ or Ba2+, to yield the respective ion-imprinted bis-aniline-bridged Au NPs composites on the Au surfaces. After elimination of the ions from the crosslinked matrices, specific imprinted ion recognition sites were generated in the composites. Selective association of the respective ions to the imprinted sites consisting of the dithiothreitol ligands is demonstrated. The high affinities of the metal ions to the respective imprinted sites lead to impressive sensitivities (fM range). The association of the ions to the imprinted sites is monitored by surface plasmon resonance spectroscopy, and the coupling between the localized plasmon of the NPs and the surface plasmon wave is used as an amplification mechanism.
Introduction
In a series of recent studies we reported on the imprinting of molecular recognition sites in Au nanoparticles (NPs) composites assembled on Au-coated surfaces, and on the application of the imprinted matrices for selective and ultrasensitive sensing using surface plasmon resonance (SPR) as a readout signal.1–5 The NPs composites were generated by the electropolymerization of Au NPs functionalized with a mixed monolayer consisting of thioaniline and mercaptoethane sulfonic acid on Au-coated glass supports, which were modified with thioaniline. The electropolymerization led to three-dimensional Au NPs composites crosslinked by bis-aniline units. The generation of imprinted matrices was achieved by the electropolymerization of the Au NPs in the presence of the analytes (or analogs of the analytes) that formed π-donor–acceptor affinity complexes,1 or electrostatic attractive interactions2 with the thioaniline modifying groups and/or the resulting bis-aniline bridging units. The subsequent removal of the imprinting molecules from the matrices led to the formation of molecular contours that revealed high affinity and selectivity towards the binding of the imprinted substrates. Surface plasmon resonance (SPR) spectroscopy was then used as the readout signal for monitoring the binding processes of the analytes to the respective imprinted sites. As the SPR spectrum is sensitive to dielectric changes occurring on the surface, the binding of the analytes to the surface and the resulting dielectric changes are anticipated to shift the SPR spectra. These shifts are, however, small, particularly at low coverage of the analytes. Nonetheless, we made use of the fact that the electronic coupling between the localized plasmon of the Au NPs and the surface plasmon wave induces a strong shift in the SPR spectrum. Thus, changes in the SPR spectra caused by minute changes in the dielectric properties of the sensing interface can be amplified through the coupling between the localized plasmon of the Au NPs in the composite and the surface plasmon wave. In fact, many reports demonstrated an amplified SPR biosensing (e.g.detection of DNA,6antigen–antibody complexes,7aptamer–substrate complexes8 or biocatalytic processes9) using Au NPs (or Ag NPs) as amplifying labels. Using this imprinting paradigm, different ultrasensitive SPR sensor systems for explosives, such as TNT,1RDX,2 PETN or nitroglycerin,3 for electron acceptor bipyridinium salts4 and for amino acids5 were prepared. In cases where the analyte lacked affinity interactions with the thioaniline (or bis-aniline) units capping the Au NPs, a third component that included a specific ligand for the analyte, was incorporated into the capping layer of the NPs. The primary formation of the complex between the ligand and the analyte was followed by the electropolymerization of the NPs on the Au surface to form the Au NPs composite. The subsequent cleavage of the ligand–analyte complex and removal of the analyte yielded imprinted molecular sites in which the ligand acted cooperatively in binding the analyte to the imprinted sites. This approach was implemented for the development of stereoselective, and even chiroselective, SPR sensors for saccharides10 and selective sensors for a variety of antibiotics11 using a mercaptophenyl boronic acid as a co-ligand associated with the electropolymerizable Au NPs. In the present study we report on the electrosynthesis of imprinted sites for alkaline-earth metal ions. We demonstrate the selectivity of the imprinted sites and discuss future potential applications of such metal ion-imprinted matrices.The imprinting of specific recognition sites for molecular substrates, ions or anions in organic or inorganic polymer matrices has been extensively studied.12 For example, polymerizable 1,3-diketone ligand units were used as templates for the association of Ca2+ ions.13Copolymerization of the complexes consisting of N,N′-dimethyl-N,N′-bis-(4-vinylphenyl)-3-oxapentanediamide monomers, under crosslinking conditions, yielded, after the removal of the calcium ions, imprinted polymer matrices for the selective binding of Ca2+. The matrix was then used for the chromatographic separation of Ca2+ from Mg2+ ions. Also, different thiolated ligands were used for the electrochemical14 or optical15–17detection of metal ions through the modification of Au electrodes or Au NPs. For example, electrochemical detection of alkaline-earth metal ions by dihydroxy-thiolated ligands associated with a Au surface was demonstrated using impedance spectroscopy.14 Also, the aggregation of crown ether-modified Au NPs in the presence of alkali-metal ions or alkaline-earth metal ions, with the accompanying red-to-blue color transition of the nanoparticles, was used for the colorimetric detection of the ions. Similarly, the aggregation of phenanthroline-modified Au NPs in the presence of Li+ ions was used for the colorimetric detection of the ions.15–17 In this study, we implemented the dithiothreitol ligand to imprint specific recognition sites for the alkaline-earth ions Mg2+, Ca2+, Sr2+ and Ba2+ in electropolymerized Au NPs composites crosslinked by bis-aniline bridging units, and associated with Au surfaces.
Results and discussion
Au NPs (ca. 4 nm) were functionalized with a mixed monolayer that included thioaniline (1), dithiothreitol (2) as a coordination ligand, and mercaptoethane sulfonic acid (3), as a stabilizing agent. The resulting functionalized NPs were interacted with the imprinting ion (the counter anion Cl− was used in all experiments), and were electropolymerized on thioaniline-modified Au-coated glass surfaces, Scheme 1. Following the electropolymerization of the Au NPs composite, the imprinting ion was washed off to yield the imprinted matrix. The electropolymerization of the bis-aniline-crosslinked Au NPs aggregate and the release of the imprinted ions were followed by SPR spectroscopy, as exemplified in Fig. 1A for the imprinting of Mg2+ ions. The formation of the Au NPs composite is associated with a large shift of the minimum reflectivity angle of the SPR curve to higher values. The release of Mg2+ ions results in a shift of the minimum reflectivity angle to lower values. AFM measurements, Fig. S1 (ESI†), reveal the formation of a non-homogeneous film of Au NPs exhibiting spikes of aggregated Au NPs as high as 70 nm. Quartz crystal microbalance (QCM) measurements indicate that the weight associated with the Au NPs composite is ca. 2.2 × 10−5 g cm−2, which corresponds to a surface coverage of ca. 4.6 × 1013Au NPs cm−2. By a similar electropolymerization procedure, but excluding the metal ions, non-imprinted Au NPs composites were synthesized on Au slides. Fig. 1B, depicts the SPR spectrum of the Mg2+-imprinted Au NPs composite before interaction with Mg2+ ions, curve (a), and after the treatment of the matrix with Mg2+ ions, 1 nM, curve (b). The interaction of the matrix with the ions results in a shift of the minimum reflectivity angle of the SPR spectrum, consistent with the uptake of the ions by the composite. For comparison, Fig. 1C shows the SPR spectra of the non-imprinted Au NPs composite before, curve (a), and after treatment with Mg2+, 1 nM, curve (b). In this case, the SPR curves are indistinguishable, suggesting the Mg2+ does not bind, or only associates inefficiently to the non-imprinted matrix.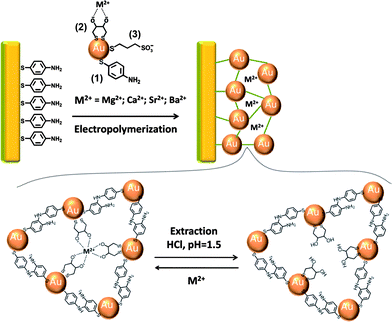 | ||
| Scheme 1 Schematic presentation for the electropolymerization of a bis-aniline-crosslinked Au NPs composite on a Au-coated electrode for the sensing of Mg2+, Ca2+, Sr2+ or Ba2+ using dithiothreitol as a ligand for the imprinted metal ions. | ||
 | ||
| Fig. 1 (A) SPR curves corresponding to: (a) the thioaniline-modified Au surface before electropolymerization; (b) the bis-aniline-crosslinked Au NPs composite electropolymerized on the Au surface in the presence of Mg2+, 10 mM; and (c) the Mg2+-imprinted bis-aniline-crosslinked Au NPs matrix, following the removal of the imprint ion. All measurements were performed in triply-deionized water. (B) SPR curves corresponding to the Mg2+-imprinted bis-aniline-crosslinked Au NPs composite: (a) before, and (b) after the addition of Mg2+, 1 nM. (C) SPR curves corresponding to the non-imprinted bis-aniline-crosslinked Au NPs composite (a) before, and (b) after the addition of Mg2+, 1 nM (the non-imprinted measurements were performed in a 10 mM tetrabutylammonium acetate solution, pH = 6.2). | ||
Fig. 2A, curve (a), shows the sensogram (reflectance changes at a fixed angle of θ = 63.5°) of the Mg2+-imprinted matrix upon interaction with variable concentrations of Mg2+ ions. As the concentration of Mg2+ ions increases, the reflectance changes are intensified. Fig. 2B, curve (a), depicts the resulting calibration curve. An impressive sensitivity for analyzing Mg2+ ions is observed with a linear response in the range of 20–100 fM, Fig. 2B, inset. The reflectance changes level off to a saturation value at a concentration of Mg2+ ions corresponding to ca. 200 pM. Fig. 2C, curve (b), shows the interaction of the non-imprinted composite with variable concentrations of Mg2+ ions. Evidently, the non-imprinted Au NPs composite does not respond in the concentration range where significant reflectance changes are observed for the imprinted surface, curve (a), and only minute reflectance changes are observed for Mg2+ concentrations up to 50 pM. These results indicate that the non-imprinted Au NPs matrix lacks affinity for binding Mg2+.
 | ||
| Fig. 2 (A) Sensograms corresponding to the reflectance intensities changes of the Mg2+-imprinted Au NPs matrix, at θ = 63.5°, upon the addition of variable concentrations of: (a) Mg2+: (a1) 20, (a2) 40, (a3) 60, (a4) 80, (a5) 100 fM, and (a6) 2, (a7) 5, (a8) 20, (a9) 50, (a10) 100, (a11) 250 pM. (b) Ca2+: (b1) 80, (b2) 100 fM, and (b3) 2, (b4) 5, (b5) 20, (b6) 50, (b7) 100, (b8) 250, (b9) 500 pM. (c) Sr2+: (c1) 50, (c2) 100, (c3) 250, (c4) 500 pM, and (c5) 1, (c6) 5 nM. (d) Ba2+: (d1) 50, (d2) 100, (d3) 250 pM, and (d4) 1, (d5) 5, (d6) 50 nM. The inset shows a magnification of one concentration of the sample analyzed. (B) Calibration curves relating the reflectance changes to the concentrations of (a) Mg2+, (b) Ca2+, (c) Sr2+ and (d) Ba2+, on the Mg2+-imprinted matrix. The inset shows the lower concentration region of the Mg2+ calibration curve. (C) Calibration curves relating the reflectance changes to the concentrations of Mg2+ on: (a) the Mg2+-imprinted, and (b) the non-imprinted matrices (the non-imprinted electropolymerization was performed in a 10 mM tetrabutylammonium acetate solution, pH = 6.2). Reflectance changes values presented in the calibration curves were taken after 6 min of measurement. All measurements were performed in triply-deionized water. Error bars correspond to a set of N = 5 measurements. | ||
A further aspect that was addressed relates to the selectivity of the imprinted sites. Fig. 2A, curves (b), (c) and (d), show the reflectance changes of the Mg2+-imprinted Au NPs composite upon interaction with the alkaline-earth ions Ca2+, Sr2+ and Ba2+, and Fig. 2B depicts the resulting calibration curves. It should be noted that the reflectance changes, upon each addition of the Mg2+ ions, were recorded for 10 min. Within this interval, the reflectance reached a saturation value (see Fig. 2(A) inset, demonstrating the expansion of one of the measurements). This implies that the addition of the Mg2+ ions was performed after the reflectance reached a constant value. Furthermore, one may realize that after ca. 6 min, the reflectance changes reached ca. 85% of the saturation value, and this time-interval was used in the extraction of the calibration curves. The time-dependent increase in the reflectance change is presumably controlled by the diffusion of the ions to the imprinted sites. Clearly, the Sr2+ and Ba2+ ions are not sensed by the Mg2+-imprinted Au NPs composite, while Ca2+ ions reveal only low affinity for the matrix, as reflected by the substantially lower reflectance changes as compared to Mg2+. The ultrasensitive detection of the ions by means of SPR spectroscopy is attributed to the coupling between the localized plasmon associated with the Au particles and the surface plasmon wave. The changes in the dielectric properties of the Au NPs matrix and eventually, the changes in the inter-particle distances, due to binding of the ions, alter the coupling phenomenon between the localized plasmon and the surface plasmon wave. This leads to the SPR spectral shifts that allow the sensitive detection of Mg2+ ions. Realizing that the ultrasensitive detection of Mg2+ ions was achieved by the Mg2+-imprinted matrix, we examined the possibility to imprint ionic recognition sites for the other alkaline-earth ions: Ca2+, Sr2+ and Ba2+.
The goal of this study was to evaluate the sensitivity and selectivity, which are achieved by the imprinting process. Accordingly, imprinted Au NPs composites for each of the ions were prepared by the electropolymerization of the functionalized Au NPs in the presence of the different ions, following Scheme 1.
We find that all imprinted matrices exhibit high reflectance changes upon the interactions with the imprinted ions, while the non-imprinted composites do not exhibit significant reflectance changes (see Fig. S2, ESI†). Furthermore, we find that the imprinting process leads to selectivity of the resulting sensing matrices. Fig. 3A shows the calibration curves corresponding to the analysis of the different ions by the Ca2+-imprinted Au NPs composite. The reflectance changes for analyzing Ca2+ by the Ca2+-imprinted matrix are high. In the concentration range of Ca2+ of 40–100 fM, a linear dependence between the reflectance changes and the concentration of Ca2+ ions is observed, and the reflectance changes level off to a saturation value at a concentration of Ca2+ ions that corresponds to ca. 100 pM. The interaction of the Ca2+-imprinted Au NPs composite with Mg2+ ions leads to substantially lower reflectance changes, Fig. 3A, curve (b), implying lower affinity for the binding of Mg2+ to the Ca2+-imprinted sites. Similarly, we find that the Ca2+-imprinted matrix reveals little affinity for the association of Sr2+ or Ba2+ ions, as reflected by the low reflectance changes at the entire concentration range (for the respective sensograms, see Fig. S3, ESI†). The experimental results reveal two important conclusions: (i) the imprinted recognition sites for Mg2+ or Ca2+ in the Au NPs composites led to ultrasensitive matrices for the sensing of the imprinted ions. (ii) The Mg2+- and Ca2+-imprinted matrices demonstrate selectivity, and Sr2+ and Ba2+ can be discriminated by these matrices. It should be noted that the observed selectivity is affected by the formation of sterically imperfect cavities for the association of the two similar sized ions.
 | ||
| Fig. 3 (A) Calibration curves relating the reflectance changes to the concentrations of: (a) Ca2+, (b) Mg2+, (c) Ba2+ and (d) Sr2+ on the Ca2+-imprinted matrix. The inset shows the lower concentration region of the Ca2+ calibration curve. (B) Calibration curves relating the reflectance changes to the concentrations of: (a) Sr2+, (b) Ca2+, (c) Mg2+ and (d) Ba2+ on the Sr2+-imprinted matrix. The inset shows the lower concentration region of the Sr2+ calibration curve. (C) Calibration curves relating the reflectance changes to the concentrations of: (a) Ba2+, (b) Ca2+, (c) Sr2+ and (d) Mg2+ on the Ba2+-imprinted matrix. The inset shows the lower concentration region of the Ba2+ calibration curve. All measurements were performed in triply-deionized water. Error bars correspond to a set of N = 5 measurements. | ||
Significantly improved selectivity is demonstrated for Sr2+ or Ba2+-imprinted Au NPs composites. The calibration curves corresponding to the reflectance changes of the Sr2+ and the Ba2+-imprinted matrices upon analyzing the different ions are shown in Fig. 3B and C, respectively (the sensograms corresponding to the analyses of the respective ions are depicted in Fig. S4 and Fig. S5, ESI†). For the Sr2+-imprinted Au NPs composite, high reflectance changes in the Sr2+ concentration range of 20–100 fM are observed, whereas in this concentration range the other ions do not show any significantly detectable values. The calibration curve for analyzing Sr2+ by the Sr2+-imprinted matrix levels off to a saturation value at a Sr2+ concentration of ca. 200 pM. The other ions, Ca2+, Mg2+ and Ba2+ show lower reflectance changes up to a concentration of 1 nM. Similarly, the Ba2+-imprinted Au NPs matrix reveals impressive sensitivity and selectivity for the detection of Ba2+ ions, Fig. 3C. The reflectance changes reach a saturation value at 25 pM of Ba2+. Within this concentration range, the other ions Mg2+, Ca2+ and Sr2+ yield only minute reflectance changes, and these remain very low even at higher concentrations. It should be noted that the dynamic range for analyzing the different metal ions by the imprinted Au NPs matrices is 20–100 fM. At higher concentrations, the readout signals level off to a saturation value, due to the saturation of the imprinted sites by the respective metal ions. Thus, a possibility to increase the dynamic range for the sensing of the ions would involve the increase in the population of the imprinted sites. This might be accomplished by either increasing the roughness of the support, or by increasing the thickness of the sensing matrix.
The different ion-imprinted Au NPs composites revealed an unaffected sensing performance for at least six days. Furthermore, we find that the sensing matrices can be regenerated by elimination of the ions bound to the imprinted sites by rinsing the composite with an acidic aqueous solution, pH = 1.5, followed by washing the composite with distilled water. In this context, it is worthwhile to address the mechanism of the binding of the ions to the imprinted sites and the ability to sense a specific imprinted ion in the presence of an excess of foreign ions. We find that the regeneration of the sensing matrices and the elimination of the ions bound to the imprinted sites can be achieved by rinsing the composite with pure distilled water (see Fig. S6, ESI†). This suggests that the association of the ions and their dissociation to and from the imprinted sites, are reversible processes. It should be noted, however, that the regeneration of the sensing matrices with pure water is substantially slower in comparison to using an acidic aqueous solution, pH = 1.5. This may be attributed to the protonation of the dithiothreitol ligands, a process that facilitates the elimination of the ions. The reversibility of the binding of the ions to the matrices, and the ability to detect the specific ion in the presence of an excess of foreign ions, were demonstrated by analyzing Ba2+ ions by the Ba2+-imprinted Au NPs matrix, in the presence of an excess of Mg2+, Ca2+ and Sr2+ ions, Fig. 4. In this experiment, the Ba2+-imprinted Au NPs matrix was subjected to a mixture of the ions Mg2+, Ca2+, Sr2+, each 10 pM, which resulted in a reflectance change of ca. ΔR = 70 a.u. Subsequently, the matrix was treated with different concentrations of Ba2+. This resulted in a maximum total reflectance change of ca. ΔR = 200 a.u. These results suggest that Ba2+ ions at a concentration of 60 fM can be sensed at a background signal of Mg2+, Ca2+ and Sr2+ at a concentration of 10 pM each. Furthermore, upon subjecting the surface first to Ba2+ ions, 3 pM, a total reflectance change of ca. ΔR = 200 a.u. was observed, and the subsequent stepwise addition of each of the ions Mg2+, Ca2+, Sr2+, each 10 pM, or as a mixture of the ions (each 10 pM), did not affect the reflectance change of the system. Also, analyzing a mixture of Ba2+ ions, 3 pM, and Mg2+, Ca2+, Sr2+, each 10 pM, resulted in a reflectance change of ca. ΔR = 200 a.u. These results indicate that Ba2+, at a concentration of 3 pM, saturates the imprinted sites and excludes the binding of the other ions that exhibit lower affinity for the sites. On the other hand, the primary binding of the ions exhibiting low affinity to the Ba2+-imprinted sites is followed by an exchange process that saturates the matrix with the Ba2+ ions (exhibiting high affinity towards the imprinted sites). The fact that the sensogram corresponding to the sensing of Ba2+ ions saturates at a reflectance change value of ΔR = 200 a.u. at an added concentration of 3 pM, implies that the Mg2+, Sr2+ and Ca2+ ions were exchanged by the Ba2+ ions, exhibiting higher affinity to the imprinted sites. Without such an exchange mechanism, and in the presence of the Mg2+, Sr2+ and the Ca2+ ions, the reflectance changes would have reached the saturation value at a Ba2+ concentration corresponding to ca. 80 fM.
 | ||
| Fig. 4 Sensogram corresponding to the reflectance intensities changes of the Ba2+-imprinted Au NPs matrix, at θ = 63.5°, upon the addition of: (a) Mg2+, Ca2+ and Sr2+, 10 pM, each, and upon the subsequent addition of Ba2+ ions: (b1) 60, (b2) 600 fM, and (b3) 1.2, (b4) 2, (b5) 3 pM. All measurements were performed in triply-deionized water. | ||
Conclusions
In conclusion, the present study has demonstrated the imprint of specific recognition sites for alkaline-earth ions, and the use of surface plasmon resonance as an effective method to probe the association of the ions to the imprinted sites. While the non-imprinted Au NPs composites did not show significant affinities for the ions to the matrices, the imprinted matrices revealed high affinity for the association of the ions, and an ultrasensitive detection in the fM range was achieved. It should be noted that this sensitivity is ca. 10- to 100-fold more sensitive than the ICP-MS method. This ultrasensitive detection of the ions was facilitated by an amplified SPR readout process, in which minute changes in the dielectric properties of the Au NPs matrix due to the low coverage of the ions were amplified by the coupling between the localized plasmon of the NPs and the surface plasmon wave. Also, the spatial condensation of the NPs upon binding of the ions may affect the electronic coupling of their localized plasmon with the surface plasmon wave, leading to pronounced shifts in the SPR spectra. Besides the impressive sensitivity, we also demonstrated the selective association of the ions to the imprinted sites within the series of alkaline-earth ions. Previous studies have demonstrated that the dithiothreitol ligand could be bound, also, to other metal ions, such as Cr6+ or As3+/5+, and lacked affinity towards binding to other ions, such as Pb2+, Cd2+, Hg2+, Co2+, Fe3+, and others.18,19 These results suggest that the imprinting method can be extended to develop other ultrasensitive and selective sensors for toxic metal ions.Experimental section
Synthesis of the functionalized Au nanoparticles
Au nanoparticles (NPs) functionalized with dithiothreitol, 2-mercaptoethanesulfonic acid and p-aminothiophenol were prepared by mixing a 10 mL solution of 197 mg gold(III) chloride hydrate, HAuCl4, in methanol with a 5 mL solution containing 14 mg of dithiothreitol, 30 mg of 2-mercaptoethanesulfonic acid and 6 mg of p-aminothiophenol in methanol. The two solutions were stirred in the presence of 2.5 mL glacial acetic acid in an ice-bath for 1 h. Subsequently, 7.5 mL of an aqueous solution of 1 M sodium borohydride, NaBH4, was added dropwise, resulting in a dark-colored solution associated with the presence of the Au NPs. The suspension was stirred for 14 additional hours in an ice bath. The resulting Au nanoparticles were successively washed and centrifuged (twice in each solvent) with methanol, ethanol and diethyl ether. A mean particle size of ca. 4 nm was estimated using TEM.Chemical modification of the electrodes
p-Aminothiophenol-functionalized electrodes were prepared by immersing the Au-coated glass slides for 24 h in an ethanolic solution that contained 10 mM p-aminothiophenol. 2 mg ml−1 of the functionalized Au NPs were dissolved in a 10 mM tetrabutylammonium acetate solution (pH = 6.2) and electropolymerized on the p-aminothiophenol-modified Au electrode. The electropolymerization was performed at a constant potential, E = 0.8 V vs.Ag QRE, for 2 h. The resulting films were then washed with the background tetrabutylammonium acetate solution to exclude any residual monomers from the electrode. Similarly, metal ion-imprinted bis-aniline-crosslinked films were prepared using 10 mM solution of the selected metal ion and 2 mg ml−1 of the functionalized Au NPs. The extraction of the metal ions from the films was carried out by immersing the electrodes in 0.03 M HCl, pH = 1.5, for 5 min. The removal of the metal ions from the electropolymerized films was verified by SPR.Analysis of the different metal ions by SPR
The respective metal ion-imprinted or non-imprinted Au NPs-modified surfaces were mounted in an SPR cell of a total volume of 400 μL. Stock solutions of the respective ions at concentrations corresponding to: 2 × 10−5, 1 × 10−5, 2 × 10−6, 2 × 10−7, 2 × 10−8, 1 × 10−8, 5 × 10−9, 2.5 × 10−9, 1 × 10−9, 1 × 10−10, 5 × 10−12, 2 × 10−12 and 1 × 10−12 M, were prepared by the appropriate dilutions of the respective stock solutions. The concentration of the 1 × 10−12 M solution was verified using an ICP-MS analysis. The variability in the concentration according to the ICP-MS, upon analyzing five stock samples, corresponded to ±5%. The appropriate volume of the stock solution was then injected into the SPR cell for analysis. The experimental sensograms were recorded, for each added concentration of the different ions, for a time-interval of 10 min. Within this time interval, the reflectance changes reached a constant value after ca. 6 min, and this value was used in the derivation of the respective calibration curves.Instrumentation
A surface plasmon resonance (SPR) Kretschmann type spectrometer NanoSPR 321 (NanoSPR devices, USA), with a LED light source, λ = 650 nm, and a prism of refraction index, n = 1.61, were used. The SPR measurements were performed using a home-built fluid cell. Au-coated semi-transparent glass plates (Mivitec GmbH, Analytical μ-systems, Germany) were used as working electrodes. For the electropolymerization, a Pt wire (d = 0.5 mm) counter electrode and a Ag wire quasi-reference electrode (d = 0.5 mm) were installed in the cell (cell volume: 0.5 mL, working electrode area: 0.2 cm2). A PC-controlled (Autolab GPES software) electrochemical analyzer potentiostat/galvanostat (μAutolab, type III) was employed. Nanopure (Barnstead) ultrapure water was used in the preparation of the different solutions.Acknowledgements
This research is supported by NanoSensoMach ERC Grant No. 267574 under the EU FP7/2007-2013 program.Notes and references
- M. Riskin, R. Tel-Vered, O. Lioubashevski and I. Willner, J. Am. Chem. Soc., 2009, 131, 7368 CrossRef CAS.
- M. Riskin, R. Tel-Vered and I. Willner, Adv. Mater., 2010, 22, 1387 CAS.
- M. Riskin, Y. Ben-Amram, R. Tel-Vered, V. Chegel, J. Almog and I. Willner, Anal. Chem., 2011, 83, 3082 CrossRef CAS.
- M. Frasconi, R. Tel-Vered, M. Riskin and I. Willner, J. Am. Chem. Soc., 2010, 132, 9373 CrossRef CAS; D. Balogh, R. Tel-Vered, M. Riskin, R. Orbach and I. Willner, ACS Nano, 2011, 5, 299 CrossRef.
- M. Riskin, R. Tel-Vered, M. Frasconi, N. Yavo and I. Willner, Chem.–Eur. J., 2010, 16, 7114 CAS.
- L. He, M. D. Musick, S. R. Nicewarner, F. G. Sallinas, S. J. Benkovic, M. J. Natan and C. D. Keating, J. Am. Chem. Soc., 2000, 122, 9071 CrossRef CAS.
- L. A. Lyon, M. D. Musick and M. J. Natan, Anal. Chem., 1998, 70, 5177 CrossRef CAS; E. Mauriz, A. Calle, L. M. Lechuga, J. Quintana, A. Montoya and J. J. Manclus, Anal. Chim. Acta, 2006, 561, 40 CrossRef.
- E. Golub, G. Pelossof, R. Freeman, H. Zhang and I. Willner, Anal. Chem., 2009, 81, 9291 CrossRef CAS.
- M. Zayats, S. P. Pogorelova, A. B. Kharitonov, O. Lioubashevski, E. Katz and I. Willner, Chem.–Eur. J., 2003, 9, 6108 CrossRef CAS.
- Y. Ben-Amram, M. Riskin and I. Willner, Analyst, 2010, 135, 2952 RSC.
- M. Frasconi, R. Tel-Vered, M. Riskin and I. Willner, Anal. Chem., 2010, 82, 2512 CrossRef CAS.
- G. Wulff, Angew. Chem., Int. Ed. Engl., 1995, 34, 1812 CrossRef CAS; K. Haupt and K. Mosbach, Chem. Rev., 2000, 100, 2495 CrossRef; B. Sellergren, TrAC, Trends Anal. Chem., 1997, 16, 310 CrossRef; K. Haupt, Analyst, 2001, 126, 747 RSC; F. Lanza and B. Sellergren, Anal. Chem., 1999, 71, 2092 CrossRef; M. J. Whitcombe and E. N. Vulfson, Adv. Mater., 2001, 13, 467 CrossRef; A. Katz and M. E. Davis, Nature, 2000, 403, 286 CrossRef; T. L. Panasyuk, V. M. Mirsky, S. A. Piletsky and O. S. Wolfbeis, Anal. Chem., 1999, 71, 4609 CrossRef.
- T. Rosatzin, L. I. Andersson, W. Simon and K. Mosbach, J. Chem. Soc., Perkin Trans. 2, 1991, 1261 RSC.
- D. Burshtain and D. Mandler, Phys. Chem. Chem. Phys., 2006, 8, 158 RSC.
- S. Y. Lin, C. H. Chen, M. C. Lin and H. F. Hsu, Anal. Chem., 2005, 77, 4821 CrossRef CAS.
- R. A. Reynolds, A. H. Haines and D. A. Russell, Langmuir, 2006, 22, 1156 CrossRef.
- S. O. Obare, R. E. Hollowell and C. J. Murphy, Langmuir, 2002, 18, 10407 CrossRef CAS.
- J. R. Kalluri, T. Arbneshi, S. A. Khan, A. Neely, P. Candice, B. Varisli, M. Washington, S. McAfee, B. Robinson, S. Banerjee, A. K. Singh, D. Senapati and P. C. Ray, Angew. Chem., Int. Ed., 2009, 48, 9668 CAS.
- F. Tan, X. Liu, X. Quan, J. Chen, X. Li and H. Zhao, Anal. Methods, 2011, 3, 343 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: An AFM image showing the morphology of the electropolymerized Au aggregated composite, calibration curves relating the reflectance changes to the concentrations of the different ions on the imprinted and non-imprinted matrices, and sensograms corresponding to the changes in the reflectance intensities of the metal ions-imprinted Au NPs matrices, upon the addition of variable concentrations of the metal-ion analytes. See DOI: 10.1039/c1sc00403d |
| This journal is © The Royal Society of Chemistry 2012 |
