The use of microcomputer based laboratories in chemistry secondary education: Present state of the art and ideas for research-based practice
Montserrat
Tortosa
Universitat Autònoma de Barcelona, Facultat Ciències de l'Educació, Edifici G5, Campus de la UAB, 08193 Bellaterra (Cerdanyola del Vallès), Spain. E-mail: montserrat.tortosa@uab.cat
First published on 28th June 2012
Abstract
In microcomputer based laboratories (MBL) and data loggers, one or more sensors are connected to an interphase and this to a computer. This equipment allows visualization in real time of the variables of an experiment and provides the possibility of measuring magnitudes which are difficult to measure with traditional equipment. Research shows that the advantages of using this technology go further than simply motivating students as they can improve other abilities, such as interpretation of graphs, and it can help to develop several competencies and higher order learning skills in students. The aims of this study are to learn about the potential of MBL in chemistry classrooms and to present a framework for research based lab sheets. In this work, research reporting significant learning in secondary school chemistry laboratory using an inquiry approach related to microcomputer based laboratory experiences is reviewed. Instructional effectiveness of the technology, research based materials for students, ideas for practice and opinions of teachers and students when using this technology are reviewed.
Introduction
Significant learning in the chemistry laboratory
The idea that meaningful learning is possible in the laboratory if students are given opportunities to manipulate equipment and materials in a suitable environment (Tobin, 1990) is widely accepted, but research in didactics has not found simple relationships between laboratory experiences and the learning outcomes of students (Hofstein and Lunetta, 1982, 2003). The science laboratory is a unique learning environment as it has the potential to provide science teachers with opportunities to vary their instructional techniques and to avoid a monotonous classroom learning environment. Although it has been demonstrated that traditional teaching methods do not solve students' learning difficulties, even for those who wish to become scientists, there are various opinions on how to teach or how to apply the results of the research on science education into school laboratories. It is generally accepted that meaningful learning takes place when students not only remember but also make sense of and are able to apply what they have learned (Anderson and Krathwohl, 2001), and that there is a considerable amount of evidence collected by researchers of science teaching that traditional instructional methods, largely lectures and undertaking exercises, are not effective methods for all learners. Sufficient data do exist to suggest that laboratory instruction is an effective and efficient teaching medium to attain some of the goals for teaching and learning science and that appropriate laboratory activities have a great potential in promoting positive attitudes and in providing students with opportunities to develop skills regarding cooperation and communication (Hofstein, 2003; Hofstein and Mamlok-Naaman, 2007).To improve science competencies in citizenship, inquiry-based science education (IBSE) has been proposed by many science researchers and educators. IBSE has proven its efficacy at both primary and secondary level increasing students' interest and attainment level while motivating the teacher at the same time (Hofstein et al., 2005; Fortus et al., 2006; Rocard et al., 2007; Barnea et al., 2010). There is agreement (Rocard et al., 2007) that inquiry is a good way of presenting laboratory work, and that to improve scientific literacy (PISA OECD, 2003) learners must have opportunities to practice selected skills. The analysis regarding the students' perceptions clearly demonstrates (Hofstein, 2003) that students who were involved in inquiry-type investigations found the laboratory learning environment to be more open-ended and more integrated with a conceptual framework than those students in a control group. They also found that the gap between the actual and the preferred learning environment on various levels was significantly smaller in the inquiry group than in the control group. Students perceived that they were more involved in the learning process and found the procedures more open-ended. The integration of laboratory experience with other pedagogical interventions and classroom instructional techniques was associated with a significant reduction in the magnitude of the differences between ratings of actual versus preferred features of a laboratory learning environment. In other words, the inquiry group found that its learning environment was significantly more aligned with their preferred environment compared with the control group. Fortus et al. (2006) created middle school curriculum materials to help students to develop a deep understanding of key learning goals through engaging in inquiry and completing complex tasks such as constructing scientific explanations and modeling. Westbroek et al. (2005) developed, and refined in successive versions of teaching materials based on three potential strategies: using relevant contexts, offering content on a need-to know basis and making students feel that their input matters. They propose that to strengthen the relationship between the three strategies, it is relevant that students know, or are emotionally involved in something about the context, even if they do not know exactly the chemical processes implied in the teaching sequence.
Moreover a main goal of chemistry education is to guide students in building mental models of chemical phenomena, ensuring close congruence to scientifically accepted models by challenging students' higher-order thinking skills (Aksela, 2005). To serve the needs of the diverse range of students studying science, activity-based, computer-supported interactive learning environments were suggested two decades ago (Thornton, 1999).
Microcomputer based laboratory in chemistry laboratories, introductory aspects
Microcomputer based laboratories (MBL) are one of the ICT applications in science laboratories in schools; they allow real time visualization of the variables of an experiment. The basic mode of operation of MBL, or probe ware and data loggers, is that one or more sensors are connected to an interface and this to a computer. The interface is an analogue-digital converter. Pertinent software allows programming the frequency of measures, and the data format (table, kind of graph) to be presented on the computer screen (Fig. 1). | ||
| Fig. 1 Picture and schematic of microcomputer based laboratory (MBL) equipment. | ||
Students can use this technology to obtain data and the time-scale of data-capture can be very short in comparison with traditional equipment. This feature can leave time in the classroom to implement other activities such as discussion and interpretation of the results or working with variables. Using this technology, predictions and hypothesis formulated by learners can have easy, quick and precise feedback ratification.
The name “Microcomputer Based Laboratory” was invented by Tinker, one of the first authors working with this technology thirty years ago, as he explains in his work about the history of this device: “By 1980, the idea of real-time data acquisition for educational purposes needed a name. I wanted the name to capture not only the technique, but also an open-ended educational approach that would distinguish it… I decided to name our approach Microcomputer Based Labs, or MBL for short” (Tinker, 2009).
Apart from the way of presenting the work to students, microcomputer based laboratory equipment has clear advantages with respect to the traditional approaches. Among them the following are highlighted: (1) there are variables that are difficult to measure with traditional school equipment, but very easy to measure with a sensor, for example pressure variation of gasses produced or consumed in a process, or vapour pressure of a liquid. (2) Presenting the graph on screen in real time prevents students spending class time plotting results. (3) Students work with real graphs obtained instantly, which can be more or less similar to ideal graphs from books or from those obtained using predetermined animations or interactive simulations previously prepared by the programmer. (4) There is the potential advantage that the time of capturing data, if the design is accurate can be very short, and students can have time to practice other competencies. (5) The contrary is also true. Very long experiments can be done by programming the equipment to capture them. Data can then be dumped to the computer and students can work with them after data collection is complete.
There are also potential disadvantages that must be stated. One of them is the price: MBL equipment can be more expensive than traditional equipment, and some spare kit to replace faulty sets of equipment is needed. This could be solved sharing equipment among several schools. Another potential disadvantage is that preparing the laboratory for MBL is time consuming: this factor is especially relevant in schools or countries where no laboratory technicians are available and the preparation is extra-work for teachers.
Objectives and methodology
This paper has two objectives: one is to explore the potential of real time experiments in chemistry classrooms and the other one is to present a framework for research based lab sheets that can be used by teachers to design teaching materials.This work aims to answer questions like: What is the potential of microcomputer based laboratories at schools? What does research say on its efficiency in chemistry classrooms? Which are the difficulties that teachers face with when using MBL? What do students say?
Research on the use of sensors in Chemistry classrooms is presented and classified, and so is done with the learning of students using this technology. We will have a picture of what is it known on the topic, and on which conditions have been some researches made. This will give us an idea of which ways would be interesting or useful to continue making researches, from the author's view, to have enough information to bring the results of research to real classrooms to help to improve scientific competencies of citizenship.
Although the perspective of many studies in didactics conclude that the teacher is a key factor in the students learning outcomes, this paper assumes that learning materials given to students and class management are also relevant factors that influence learning. Having an understanding of what can be done with this technology in the classroom, and of what does not work as well, is crucial. It can provide tools to teachers when designing or adapting their teaching materials; it can also help teachers decide which configuration is needed for teachers to implement MBL successfully. This information is also crucial to education managers at the different levels, including from politicians at Education Departments, to heads of Chemistry or of Science Departments at schools. It is also essential for the research in the field to identify possible gaps that would need to be focused on.
Results and discussion
(a) Features of microcomputer based laboratories relevant in science education
Improving lab-work through the use of MBL technology has been strongly recommended by many science educators who adopt a constructivist approach to education (Borghi et al., 2001; Bernhard, 2003; Russell et al., 2003; Ambrose, 2004; Pintó et al., 2004; Sassi et al., 2005).Many science education researchers state that using this technology presents clear advantages with respect to a classical laboratory, both in collecting and in visualising data. (Nakhleh and Krajcik, 1994; Hake, 1997; Redish et al., 1997; Euler and Müller, 1999; Svac, 1999; Marcum-Dietrich, 2002; Russell et al., 2003, 2004). Performing real time experiments is an activity that allows students to work out many features of science competencies, it allows expressing both orally and in writing the knowledge that they acquire, and a quick and continuous interaction with new learning.
MBL allows working on and improving the abilities of students to interpret graphs (Mokros and Tinker, 1987; McDermott et al., 1987; Brasell, 1987; Testa et al., 2002). The fact that the graph is obtained simultaneously with the observed phenomena has been shown to favour its comprehension and interpretation. It allows stress to be placed on the epistemological problem that students face when they obtain a real graph and they need to relate it to an ideal graph from a theoretical model (Sassi et al., 2005). MBL can promote the capability of reasoning (Friedler et al., 1990) and conceptual understanding (Fernández et al., 1996; McRobbie, 2002; Marcum-Dietrich, 2002; Saez et al., 2005). This technology has proven to be useful for some disabled students (Bernhard and Bernhard, 1998). Real time experiments stimulate the process of modelling as building concepts as it promotes its transfer to more general and abstract situations (Thornton and Sokoloff, 1990). A complete research report on affordance of ICT in science learning written by Webb (2005) states that data logging provides affordance in (i) collecting data and graphing data, (ii) interpreting the results of experiments and (iii) making links between observations and their graphical representation. The elements that may increase the degree of affordance are rapid data presentation and graphing by software in the three aspects mentioned, and the prompts made by the teacher or from other students in the last two aspects.
It is worth noting that few research studies have been undertaken to analyse the relevance of MBL technology in chemistry courses in comparison with a number of studies undertaken in other scientific disciplines, mainly physics (Hogarth et al., 2006). In chemistry learning, the pioneer work of Nakhleh and Krajcik (1994), recommends that working with MBL technology can contribute to a deeper understanding of acids, bases and pH. Now, nearly twenty years after this excellent and frequently quoted work (undertaken in-depth with a sample of 15 students), we still await research with sufficiently large samples of students to test the effectiveness of approaches statistically. Other studies conclude that data loggers have been shown as an efficient way to work higher-order learning skills and chemistry competencies in the design of experiments (Aksela, 2005; Tortosa, 2007, Tortosa et al., 2008; Tortosa and Pinto, 2010).
As a conclusion we can say that use of MBL can support a constructivist view of education, it presents clear advantages in collecting and visualizing data and in working with graphs. Data loggers allow work higher-order learning skills. More research should be conducted to know their potentialities in the understanding of concrete chemical concepts.
(b) Instructional effectiveness of real time experiments
Since the implementation of MBL in the eighties, much research has been carried out about the efficient use of it in the laboratory to improve the learning of the sciences (Thornton and Sokoloff, 1990; Rogers and Wild, 1996; Barton, 1997; Harrison, 1997; Millar et al., 1998; Pintó et al., 2004, 2007, 2010; Hamne and Bernhard, 2001; Kennedy, 2001; Kwon, 2002; Newton, 2002, Aksela, 2005; Tortosa et al., 2008; Dori and Sasson, 2008; Kaberman and Dori, 2009).Research suggests that MBL technology, with its intrinsic potential towards experimental work, is not sufficient by itself to ensure the learning of scientific concepts: rather a good design of teaching materials and an appropriate pedagogical approach are also needed (Pintó and Aliberas, 1996). A good design of laboratory activities must ensure and promote suitable interactions. In this sense student–computer, student–student and student–teacher interactions have proven essential to working student competencies and to improving learning. Taking this concept, Redish et al. (1997) compared the results of eleven lecture classes in introductory university physics taught by six different teachers with and without tutorials, they showed that the MBL tutorials resulted in a significant improvement compared to traditional recitations. It would certainly be useful to know at what extent this could be applied to other science disciplines and school levels.
MBL can provide numerous opportunities to enhance the ways we teach chemistry to support the goals of chemistry. It offers new possibilities to integrate experiments in the chemistry classroom as there is a need for laboratory work which helps students think and reflect on chemical phenomena. Moreover, with sensitive sensors, it is possible to reduce the amounts of chemicals needed and provide opportunities for students to easily investigate. It is also taken into account that some magnitudes, for example pressure, that are difficult to measure with traditional equipment, can be easily measured with a sensor, and this fact broadens the range of experiments and the associated thinking made by students in the classroom.
Good design of lab sheets and suitable implementation in classrooms allowing students to practice selected competencies is important for effective teaching with MBL. Design of lab sheets to be used by students should therefore avoid being a cookbook of laboratory recipes encouraging students to act without any personal initiative, just blindly following some perhaps meaningless indications. Students' practice must be carefully structured. Several authors (Pintó et al., 1999, 2010; Borghi et al., 2003) have studied the structure of laboratory instructions for students when using MBL equipment. Their proposal belongs to a didactic vision in which several conditions are considered important:
(i) The need for the experimental work performed by students be a consequence of answering a question or problem – so students are clear what are they looking for and not simply following some rules or a recipe.
(ii) It is important that students, before handling any laboratory glassware, build a mental representation of the phenomena to be analysed.
(iii) The significance of the fact that students incorporate by themselves the goals that the teacher intends with the activity (self-regulation of learning).
(iv) The importance of peer-verbalisation through oral, written and graphical language, expressing what they observe through the experimental equipment.
(v) The need for each student to express their views related to the evolution of the process or phenomenon observed. In doing this, after having obtained the data and graphs, each student should be aware of the correctness of his/her views and of the theoretical model used to describe them.
(vi) The benefits of implementing the ideas and concepts used in this situation to other areas, in order to expand and to broaden its significance. Using real-world problems could be a motivating factor which might help to encourage the transfer of knowledge and skills.
The proposal of MBL instructions presented as an inquiry-guided activity and structured as a learning cycle has revealed to be effective in achieving significant learning. The benefits of using inquiry centred tasks in secondary chemistry as a way to improve learning significantly have been widely demonstrated generally (Hofstein and Lunetta, 1982, 2004), and particularly with the use of sensors and MBL technology (Pintó et al., 2004; Aksela, 2005; Tortosa et al., 2007; Espinoza and Quarless, 2010). More concretely it is stated that when properly developed, inquiry-centred laboratories have the potential to enhance students' meaningful learning (Minster and Krauss, 2005). It has been demonstrated that after performing a contextualized inquiry-based student-centred MBL activity, students could make sense of buffer solutions, a concept which they had not studied previously (Tortosa et al., 2008).
Another approach to using this technology in the classroom is described by Lavonen et al. (2003), who have worked on the design of a ‘user friendly’ MBL package with the objective of obtaining a rich learning environment in which the teaching and learning processes are supported in multiple ways. A practical quality of such an MBL package must have at least the following four qualities: (i) User-friendly hardware based on the “plug-and-play” concept, (ii) User-friendly and well-structured software, with easy set-up and data processing, (iii) User-friendly support, including instruction guides, training and on-line help, and (iv) Versatile and flexible features, including among others high-quality curriculum materials and a teacher's guide to chemistry.
We can conclude that the way work is presented to students is relevant in MBL. A context-based inquiry-type activity has shown to be efficient and a classroom management style that promotes student verbalization and interaction is desired. It is also important to minimize technical complications assuring a user-friendly hardware.
(c) Microcomputer based laboratories in chemistry education: Opinion of teachers and experiences in teacher-training courses
In this section several studies exploring the points of view of teachers who use microcomputer based laboratories are reviewed. The research on the use of MBL equipment by “Chemistry” and “Physics and Chemistry” teachers reveals that it is very important that teachers and designers of MBL lab-sheets to be used in real classrooms work together closely.Lavonen et al. (2003) identified which factors are essential to allow MBL to be used by chemistry teachers: the most important features are the versatility of the tool and the user interface, and also important is both the data presentation and acquisition given by the equipment.
Research on teachers' use of MBL in relation to beliefs about teaching and learning (Pintó et al., 2007), revealed that beliefs about the role of practical work in a science course and issues referring to school constraints and limitations play an important role in the results of the use of MBL. Unsatisfactory results can lead to not implementing this technology. An important affordance of MBL technology is not being fully grasped in science learning, when teachers do not see an improvement in the students' learning experience when using inquiry-based science and the promotion of different skills. This can result from lack of teacher skills and experience in teaching with MBL. Teachers should be provided with more time, consistent access to resources, they should have encouragement and support, and more significantly teachers should receive specific guidance for appropriate and effective use of MBL. Research about the use of MBL in Finland (Aksela, 2005), revealed that only 7% of chemistry teachers used MBL tools when teaching chemistry. The most commonly mentioned reasons to use it were that it was easy to handle, fast in completing experiments and saved time. The reasons that those teachers surveyed gave for not using MBL were the unavailability of both resources (57% of responses) and in-service training (33%). Some chemistry teachers (12%) thought that MBL was unnecessary for junior secondary-level students, or they reported that they did not know how to use it at that level. This research concluded that chemistry teachers needed help in using one specific MBL package, and a large internet library of school experiments was created for this purpose.
Other study (Sáez, 2006) shows that teachers positively value the use of MBL equipment, even when its use can present some difficulties that can be overcome with practice. The main difficulties mentioned by teachers about the organisation of the experimental work with MBL can be classified in four categories:
(i) The number of students in the laboratory: a number greater than four students in each group makes it difficult for all students to access the equipment in order to carry out the laboratory experience.
(ii) Material infrastructure: a suitable amount of MBL sets, adjusted to the number of students that work at the laboratories in each school, makes it easy to distribute the students in small groups, therefore increasing the management of the kit and the group discussion about the data collected. Also it is important to have sensors and replacement pieces in case of failure during a practical session.
(iii) The fact that teachers lack the time to prepare the computer and the equipment before starting the practice can reduce their use.
(iv) The frequency of classes devoted to laboratory work: This factor can influence the selection of topics and how they are faced in practice. Depending on the time devoted to laboratory lessons, data can be collected in the lab, but other actions such as discussion of the results and conclusions can be left for classroom sessions.
Another fact to be considered is teacher training in the use of MBL equipment: inadequate teacher training on this kind of technology is one of the main reasons leading to the equipment not being used. A number of studies (Sassi et al., 2005, Tan et al., 2005) have shown that many of the difficulties arise from a lack of training to get the basics to use it effectively.
In a study conducted to determine the most relevant aspects of MBL activity to achieve its effective use, and how teacher training strategies promote an improvement of the use of MBL in the classroom (Guitart and Tortosa, 2010), 16 secondary school teachers participated in a training course where they were encouraged to reflect on their own methods of practice. Teachers were videotaped in their classrooms, in order to later analyse their own performance. Teachers were also interviewed. The factors that in the opinion of the teachers were relevant for the efficient use of MBL are: (i) the design of worksheets for students, using an inquiry approach (ii) the management of the classroom (good distribution of computers, leaving enough time for students to predict and to discuss results, etc.) and (iii) a teacher training course that promotes the reflection about a teacher's own practice, teachers stressed the importance of the fact that as they were videotaped; they could observe themselves and they could discuss their own practice, this gave them important clues for improvement.
Difficulties related to school constraints are the most stated by teachers as reasons for a little use of MBL. Teacher training courses should include a common design of teachers and researchers (or teacher-trainers) of classroom activities. A reflective diary and formative-sessions of reflection on their own practices, improve the awareness of teachers using MBL.
(d) Students' performance and opinion
The opinion of students when working with MBL in chemistry classes is an important factor to take into account when designing teaching materials. Studies show that it varies to a great extent. Most of students declare that the use of this technology motivates them, and learners have positive opinions about its use. Some students, however, seem not to be interested in computers, or even express their difficulties when interpreting the results obtained. From the diverse student opinions we conclude that, two aspects are really important in order to achieve allowing the all students to take advantage of the use of MBL: the learning materials and the management of the classroom.It is important to take into account the work of Atar (2002) who interviewed high school chemistry students using MBL, to find out what their views were regarding the use of MBL as a learning tool. Although he obtained positive feedback, some slow learners stated that the immediacy of the data did not give them time “to think” about the experiment. His results suggest that MBL is better for students who already have an idea of both the content and the handling of sensor technology. These results are in accordance with research conducted on a high school chemistry group of Finnish students (Aksela, 2005) working with MBL in inquiry guided tasks: students were asked after the experience and they responded that they liked using MBL because (a) “it visualised the phenomena”, (b) “it showed the results clearly”, (c) “I do not have to draw the graphs or write”, and (d) “it was interesting and different from what we are used to doing”. Their opinions on the inquiry approach were positive. In the same research it was found that some students did not like to use MBL because (a) “it was complex”, (b) “it was incoherent”, (c) “the notes were not clear enough”, (d) “to use a computer was difficult”, (e) “it was difficult to start to work with it” and (f) “it was not interesting”.
Students need little time to get familiar with MBL equipment. Visual observations (Tortosa, 2007) on six groups (n = 66) of different Catalan secondary school students who had never used these devices, revealed that the first time they were faced with MBL, one third of them (22 students) could configure the computer program in order to obtain data following written instructions without any teacher help. Another 39% (26 students) were able to do so following a short teacher instruction. Altogether, over 70% of students could configure the software program the first time they worked with MBL equipment. On the other hand, 5 students (8%) could not configure the equipment, and the teacher had to do it for them, while 10 of them (15%) did not attempt to configure the program. The opinions of secondary and high school students belonging to various Catalan schools after joining an inquiry guided chemistry task using MBL at the University was obtained through open written questionnaires. In them students express their satisfaction for MBL and doing experiments in general, and that they found this device motivating.
Recent research on students' perceptions of the automation of manual operation in a high school chemistry laboratory found that it motivates them (Verner and Revzin, 2010).
We can state that in most of the research performed regarding the views of student on the use of MBL in chemistry classes, it is generally thought that this is easy-to-use technology, that it motivates them and that it helps them improve their understanding of science. Nevertheless, some research identifies some students that disagree with this view. More research should be done to know if it is possible to overcome the obstacles that they express.
(e) Laboratory approach and structure of research-based lab sheets: some examples
The second objective of this paper is to give examples of lab sheets useful for teachers. When preparing lab sheets for students, it needs to be recognised that previous knowledge and the abilities of students are relevant to whether they can take advantage of the potential of this technology, as it has been verified that mature students in a suitable atmosphere working with MBL in inquiry guided tasks have acquired higher-order skills of applying, analysing, evaluating and creating, while younger students in the same situation have only developed the applying skill (Aksela, 2005). When using research based lab-sheets and suitable guidance, students give more priority to the focal scientific concepts than to the tools to capture data, considering MBL apparatus as another measuring device of the laboratory equipment (Tortosa et al., 2008).As expressed above, several authors have suggested that the best way to present an MBL task to students is by doing so as an inquiry guided structured learning cycle. Students must not only handle the MBL equipment, but must carry out laboratory experiences in which they are guided to develop their initial ideas, and where data capturing and analysis allows them to see that theoretical models are similar to the regularities, and graphical representations that are obtained by traditional techniques. Students need to use their higher-order thinking to understand complex concepts and processes, for example neutralization, pH, acid–base reactions, pressure of gases and its variation, vapour pressure of a liquid, conductivity, titration, etc..
Developing the learning cycle by introducing tasks to give more support to meaningful chemistry thinking and higher-order thinking is strongly recommended. Sensors should only substitute other simple traditonal equipment when doing so offers advantages.
In these lessons, a problematic question or situation is presented to students at the beginning of the exercise. The actions that students must perform should start with activities to recall their previous ideas, and in which they had to make predictions about the results that they will obtain, then they should prepare the MBL equipment to capture data, and finally they should compare the results obtained with their predictions and with the theoretical models (Fig. 2).
 | ||
| Fig. 2 Every occasion in which students take experimental data using MBL (Fig. 3, 4 and 5), they are guided in a four-step sequence: Prediction, Experimental preparation, Observation, Explaining. | ||
As interactions have proven to be essential for understanding and learning, maximum interaction between student–computer and student–student should be promoted in the design of lab sheets and management of the classroom. Even these recommendations are sometimes difficult to follow within the classroom dynamics, they should at least be kept in mind to optimise the process.
As examples, the structure of chemistry lab-sheets designed under this perspective is presented (Fig. 3–5). In them we can see the structure of a four phase learning-cycle; on the horizontal axis the activity goes from simple to complex, and on the vertical from concrete to abstract. At the top of the figures, the arrow indicates the time in the laboratory (or classroom) sessions. The activity starts presenting a real problem, and the laboratory instruments used to model it are introduced, then students are guided through the learning cycle, following the phases of exploration, introduction of new concepts, structuration and application to new situations. The tasks are contextualised, that is, they resemble real-world situations to motivate students to transfer their knowledge and skills. Every time they have to take measurements, students are instructed to first make predictions, then to prepare and to configure the measuring system, to collect data and finally to compare the results obtained with the theoretical model and with the predictions, as shown in Fig. 2.
 | ||
| Fig. 3 Structure of a lab-sheet designed as an inquiry-guided learning cycle for secondary and high school. It is contextualised in an issue relating to human health. Students use pH sensors to obtain data. When students obtain experimental data using MBL they are guided in the four-step sequence: Prediction, Experimental preparation, Observation, Explaining, as described in Fig. 2. | ||
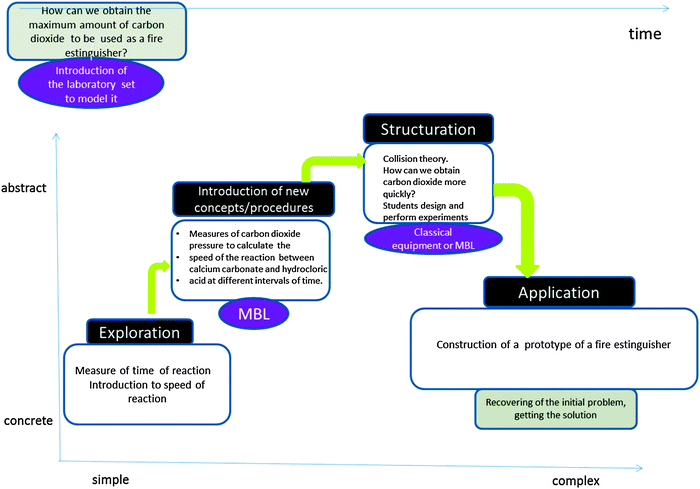 | ||
| Fig. 4 Structure of a lab-sheet designed as an inquiry-guided learning cycle for secondary and high school. Its context is laboratory safety. Students use pressure and temperature sensors to obtain data. Students obtain data using Microcomputer Based Laboratory MBL (and optionally classical equipment). When obtaining experimental data they are guided in a four-step sequence: Prediction, Experimental preparation, Observation, Explaining, as described in Fig. 2. | ||
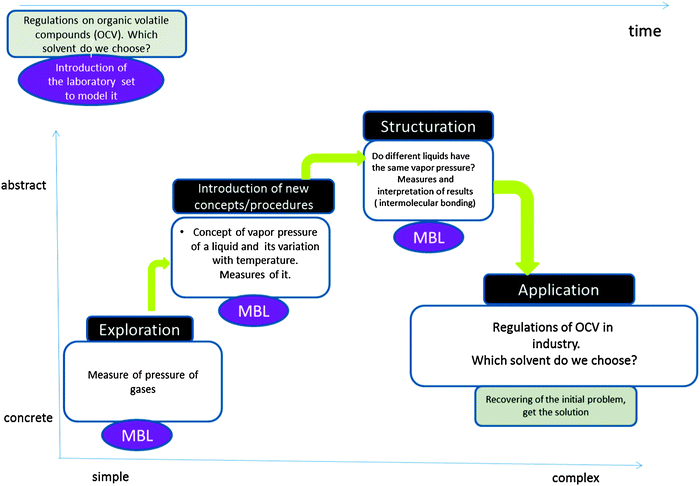 | ||
| Fig. 5 Structure of a lab-sheet designed as an inquiry-guided learning cycle for secondary and high school. Its context is industrial hazards and environment pollution. Students use pressure and temperature sensors in a Microcomputer Based Laboratory (MBL) to obtain data. When students obtain experimental data, they are guided in a four-step sequence: Prediction, Experimental preparation, Observation, Explaining, as described in Fig. 2. | ||
In Fig. 3 and in Table 1, the schema of a lab-sheet used to work in depth with the concepts of pH and equilibrium and its displacement in acids and bases so as to introduce in a functional way the concept of a buffer solution. The context of the activity is human health, and students are guided on how to answer, from a chemical point of view, the question: How can we explain why the pH of plasma blood is constant? The activities that are proposed to be carried out by students are (i) to measure the pH of several common solutions (by doing this activity using a predict-observe-explain approach as described in Fig. 2, students put in motion their previous ideas about pH, and they can learn how to use the MBL equipment to measure), (ii) students are asked to change the equilibrium of a weak acid, and to measure such a change by using a pH sensor, (iii) after this, students are introduced to what a buffer solution is, after which they prepare a buffer solution, and test it to observe how it maintains a constant pH even when adding an acid or alkali, (iv) students investigate the buffer behaviour of natural solutions, and (v) finally, students are given a graph with the ionic composition of human plasma, from which they should then be able to answer the initial question. In Table 1, activities performed by students and teaching objectives of the sequence are presented.
| Sequence of activities in which students were guided to answer the initial question | Learning objectives (teacher) |
|---|---|
| (i) pH measurement using MBL and classifying substances as: acid/base/neutral. | To elicit students' previous ideas on acids, bases and pH. |
| To learn MBL usage/configuration. | |
| (ii) Design an experiment to displace the equilibrium state of a weak acid. | To explore changes in pH with equilibrium displacement. |
| (iii) Preparation of a buffer solution in the laboratory and observation of its pH evolution when adding acid/base. | Introduction of new concepts (buffer solutions). |
| To experience the behaviour of buffer solutions when adding acid/base. | |
| To configure MBL with two sensors. | |
| (iv) Investigation of the buffer behaviour of natural solutions (carbonated water and natural orange juice). | To justify this behaviour by relating to its chemical composition. |
| To determine in the lab if a solution is a buffer. | |
| (v) Generalization: How chemistry can explain invariability of blood plasma pH using theoretical data (bar graph with ionic composition of plasma blood). | To make inferences to new situations. |
In Fig. 4 and in Table 2, an activity is presented in which the concept of the rate of reaction is explained along with how to use the collision theory to change it. The context is safety in the laboratory, and students are guided to answer the question: How can we quickly obtain carbon dioxide to be used as a fire extinguisher? In the activity, students are guided to (i) observe several reactions measuring their time of reaction and classifying them as “quick” or “slow”, (ii) students obtain data of pressure variations and they calculate the rates of reaction at different intervals of time of the reaction between calcium carbonate and hydrochloric acid, (iii) after which students are taught the collision theory and about efficient collisions, then (iv) students must design an experiment, controlling variables, to obtain quickly carbon dioxide, after (v) students perform the experiment and (vi) draw their conclusions to answer the initial question, finally (vi) they design a prototype of a fire extinguisher. In Table 2, the activities performed by students and teaching objectives are detailed.
| Sequence of activities in which students were guided to answer the initial question | Learning objectives (teacher) |
|---|---|
| (i) Observation of chemical reactions and classification in slow/fast | To emerge previous ideas of chemical change and of speed of reaction. |
| (ii) Students obtain variation of pressure in a chemical reaction. They calculate the speed in several intervals | To learn MBL usage/configuration. |
| To interpret graphs of pressure-time and to relate them to the speed of reaction. | |
| (iii) Dialogic lecture on collision theory | Introduction of new concepts (collision theory). |
| (iv) Students design and perform experiments to increase speed of a reaction | To relate hands on experiments with theory. To practice the competency of designing experiments. |
| (v) To build a prototype of a fire extinguisher | To make inferences to new situations |
Research-based lab-sheets as described above, and in Fig. 3 and 4, have been implemented in the REVIR (reality-virtuality) activities (http://crecim.uab.cat/revir/) at the Universitat Autònoma de Barcelona since 2004. REVIR activities consist of workshops that take place at the University that are provided for secondary school pupils. In them, learners come to university to perform a school curricular science activity using new technologies, mainly sensors. This action provides data on students' performance when using MBL equipment in activities on chemistry, physics and biology (Saez et al., 2005; Tortosa et al., 2008; Pintó et al., 2010). The author of this work has designed the lab sheets of the REVIR chemistry workshops and has implemented them over four years (2005–2009) with students from different Catalan schools. Preliminary results of the analysis of the behaviour and productivity of students show that in general pupils can satisfactorily use MBL equipment the first time they are faced with it, and that students using research-based lab-sheets and guidance, integrate the MBL as a tool for measurement, as they give more importance to the chemistry concepts worked on than to the equipment used.
In Fig. 5, students deal with Organic Volatile Compounds and the concept of vapour pressure. The context is safety in industry.
MBL can be very useful in the learning processes of high school chemistry, as it allows students to deal with concepts that are difficult to measure and to model with classical equipment. MBL can be a useful tool for teachers to demonstrate experiments to large groups of students.
Discussion and summary
In this paper a review of the research into the use of Microcomputer based laboratories (MBL) in chemistry classrooms has been performed. It has been found that few studies have been done on the use of this technology in Chemistry, in comparison with its use in the learning of other scientific disciplines. MBL activities broaden the range of phenomena that can be worked on in chemistry laboratories of primary and secondary schools; this technology has objective advantages as allowing the measurement of variables that are difficult to measure with classical equipment, or obtaining the graph in real time.Research about the best way of presenting teaching materials using MBL to students have also been reviewed. The way of presenting the work to students and the management of the classroom are key factors to improving students' learning. Investigators suggest laboratory instructions should be designed to provide an inquiry-guided learning cycle. The students are guided from initial activities, intended to refresh previous ideas, to the final activities in which students solve a complex problem and apply the concepts worked to new situations. It has been found that in doing so, students give more importance to the scientific concepts than to the microcomputer based laboratory technology as they integrate it as a tool to capture data.
There are few studies about the effectiveness of this technology on the learning of chemical concepts and science competencies. Most of the studies are on secondary education. Some positive results are pointed out, but more researches to obtain statistically significant data are desired. Some research reveals that mature students take more advantage of this technology than slow learners, this fact should be analysed looking for possible solutions.
The use of this technology by science teachers is not extensive; the use of MBL in chemistry education is uneven in different secondary and high schools. Teachers are faced with several material constraints, such as a lack of equipment in relation to the number of students, and insufficient replacement parts in case of breakages. Many teachers perceive they have insufficient information about the topic: teachers do not know the potentialities and advantages of the technology and they find it too time consuming. Experiences of teacher training in which teachers can reflect on their actions have been found to encourage the use of MBL.
Not many examples of teaching materials are available for the moment. In this review, examples of research-based teaching materials structured as an inquiry based learning cycle have been presented. These materials have been implemented in chemistry laboratories with secondary school students, and preliminary analyses on student learning outcomes have been positive.
References
- Aksela M., (2005), Supporting meaningful chemistry learning and higher-order thinking through computer-assisted inquiry: A design research approach, Academic Dissertation, University of Helsinki, p. 204.
- Ambrose B. S., (2004), Investigating student understanding in intermediate mechanics: identifying the need for a tutorial approach to instruction, Am. J. Physics, 72(4), 453–459.
- Anderson L. W. and Krathwohl D. R. (ed.), (2001), A taxonomy for learning, teaching, and assessing: A revision of Bloom's Taxonomy of Educational Objectives, New York: Longman.
- Atar H. Y., (2002), Chemistry students' challenges in using MBL's in science laboratories, Proceedings of Association for the Education of Teachers in Science, AETS International Conference, Charlotte, 2002.
- Barnea N., Dori Y. D. and Hofstein A., (2010), Development and implementation of inquiry-based and computerized-based laboratories: reforming high school chemistry in Israel, Chem. Educ. Res. Pract., 2010, 11, 218–228.
- Barton R., (1997), Does data-logging change the nature of children's thinking in experimental work in science? In B. Somekh and N. Davis (ed.), Using Information Technology Effectively in Teaching and Learning (pp. 63–72). London: Routledge.
- Bernhard K. and Bernhard J., (1998), Science for all. Using microcomputer based tools for students with physical disabilities, Paper presented at Int. Conf. Practical work in Science Education, Copenhagen.
- Bernhard J., (2003), Physics learning and microcomputer based laboratory (MBL) learning: effects of using mbl as a technological and as a cognitive tool In Psillos et al. (ed.), Science education research in the knowledge based society, (pp. 313–321). The Netherlands: Kluwer Academic Publishers.
- Borghi L., De Ambrosis A., Lunati E. and Mascheretti P., (2001), In service teacher education: An attempt to link reflection on physics subjects with teaching practice, Physics Education, 36(4), 299–305.
- Borghi L., De Ambrosis A. and Mascheretti P., (2003), Developing relevant teaching strategies during in-service training, Physics Education, 38, 41–45, http://www.iop.org/EJ/abstract/0031-9120/38/1/307.
- Brasell H., (1987), The effects of real-time laboratory graphing on learning graphic representations of distance and velocity, J. Res. Sci. Teach., 24(4), 385–395.
- Dori Y. J. and Sasson I., (2008), Chemical understanding and graphing skills in an honors case-based computerized chemistry laboratory environment: the value of bidirectional visual and textual representations, J. Res. Sci. Teach., 45(2), 219–250.
- Espinoza F. and Quarless D., (2010), An inquiry-based contextual approach as the primary mode of learning science with microcomputer-based laboratory technology, J. Ed. Tech. Syst., 38(4), 407–426.
- Euler M. and Müller A., (1999), Physics learning and the computer: A review, with taste of meta-analysis, Proceedings of Second International ESERA Conference of the European Science Education Research Association, Kiel, Germany.
- Fernández C., Oro J. and Pintó R., (1996), Profile evolution in the interpretation of kinematics graphs using MBL technology, Proceedings of Girep Intenational Conference, Ljubljana, Slovenia.
- Friedler Y., Nachmias R. and Linn M. C., (1990), Learning scientific reasoning skills in microcomputer based laboratories, J. Res. Sci. Teach., 27(2), 173–191.
- Fortus D., Hug B., Krajcik J. S., Kuhn L., McNeill K. L., Reiser B., Rivet A., Rogat A., Schwarz C. and Schwartz Y., (2006), Sequencing and supporting complex scientific inquiry practices in instructional materials for middle school students, Paper presented at the annual meeting of the National Association for Research in Science Teaching, NARST, San Francisco, p. 48.
- Guitart J. and Tortosa M., (2010), Towards an effective use of MBL through analyse of video films of an activity with conductivity sensor in a teacher training course, In I. Maciejowsja and P. Ciesla (ed.), 10th European Conference on Research in Chemistry Education. Book of abstracts, Krákow, Poland, pp. 110–111.
- Hake R. R., (1997), Interactive-engagement vs. traditional methods: a six-thousand-student survey of mechanics test data for introductory physics courses, Am J. Phys., 66, 64–74.
- Hamne P. and Bernhard J., (2001), Educating pre-service teachers using hands-on and microcomputer based labs as tools for concept substitution, In R. Pinto and S. Surinach (ed.), Physics Teacher Education Beyond 2000, pp. 663–666. Paris: Elsevier.
- Harrison G., (1997), Data-logging – A learning tool? Computer Education, 85, 7–12.
- Hofstein A. and Lunetta V. N., (1982), The role of laboratory in science teaching: Neglected aspects of research, Review of Educational Research, 52(2), 201–217.
- Hofstein A. and Lunetta V. N., (2004), The laboratory in science education: Foundations for the twenty-first century, Sci. Ed., 88, 28–54.
- Hofstein A., (2003), The laboratory in chemistry education: Thirty years of experience with developments, implementation and research, Chem. Ed. Res. Pract., 5(3), 247–264.
- Hofstein A., Navon O., Kipnis M. and Mamlok-Naaman R., (2005), Developing students' ability to ask more and better questions resulting from Inquiry-Type Chemistry Laboratories, J. Res. Sci. Teaching, 42(7), 791–806.
- Hofstein A. and Mamlok-Naaman R., (2007), The laboratory in science education. The state of the art, Chem. Ed. Res. Pract., 8(2), 105–107.
- Hogarth S., Bennett J., Lubber F., Campbell B. and Robinson A., (2006), The effect of ICT teaching activities in science lessons on students' understanding of science ideas, In Research Evidence in Education Library, London: Eppi-Centre, Social Institute of Education.
- Kaberman Z. and Dori Y. J., (2009), Question posing, inquiry and modeling skills of chemistry students in the case-based computerized laboratory environment, International Journal of Sci. Maths Ed., 7, 597–625.
- Kennedy D., (2001), Datalogging: What's it all about? Science, 36(2), 24–31.
- Kwon O. N., (2002), The effect of calculator-based ranger activities on students' graphing ability, School Science and Mathematics, 102(2), 57–67.
- Lavonen J., Aksela M., Juuti K. and Meisalo V., (2003), Designing user-friendly datalogging for chemical education through factor analysis of teacher evaluations, Int. J. Sci. Ed., 25(12), 1471–1487.
- Marcum-Dietrich N., (2002), An action research study: Investigating the effective use of computer probe-ware in high school biology, The NARST Annual Meeting, Philadelphia.
- McDermott L. C., Rosenquist M. L. and van Zee E. H., (1987), Student difficulties in connecting graphs and physics: Examples from kinematics, American Journal of Physics, 55(6), 503–513.
- McRobbie C., (2002), Investigating students' learning about gases & kinetic theory using microcomputer-based labs (MBL), NARST Annual Meeting, Philadelphia.
- Millar R., Le Maréchal J.-F. and Tiberghien A., (1998), A map of the variety of labwork. Working Paper 1. European Project: Labwork in Science Education (Contract No. ERB-SOE2-CT-95-2001).
- Minster J. and Krauss P., (2005), Guided inquiry in the science classroom, In M. S. Donovan and J. D. Bransford (ed.) How students learn. Science in the classroom, pp. 107–146.
- Mokros J. R. and Tinker R. F., (1987), The impact of Microcomputer Based Labs on children's ability to interpret graphs, J. Res. Sci. Teach., 24(4), 369–383.
- Nakhleh M. B. and Krajcik J. S., (1994), The influence of level of information as presented by different technologies on students' understanding of acid, base, and pH concepts, J. Res. Sci. Teach., 31, 1077–1096.
- Newton L., (2002), Data-logging in the science classroom: approaches to innovation, In Proceedings of the 3rd ESERA Conference: Research in Science Education: Past, Present and Future, Thessaloniki, Greece.
- OECD, Programme for International Student Assessment. PISA 2003 Assessment Framework in Mathematics, Reading, Science and Problem Solving Knowledge and Skills, (Available at: http://www.pisa.oecd.org).
- Pinto R. and Aliberas J., (1996), Approaches to the use of MBL in the secondary school: New Ways of teaching Physics, Proceedings of the GIREP Conference, Ljubljana 1996.
- Pintó R., Pérez O. and Gutiérrez R., (1999), Implementing MBL (Microcomputer Based Laboratory) technology for the laboratory work in compulsory secondary school science classes, STTIS Spanish National report on WP1, Universitat Autònoma de Barcelona.
- Pintó R., Fernández C., Oro J. and Aliberas J., (2004), Educational approach for CBL. Subwork Packadge 1.3. European Project: Integrating Knowledge for the Use of Informatics Tools in Science Education (IKUITSE), (Contract No. HPSE-CT-2002-60055).
- Pintó R., Fernández C., Oro J. and Saez M., (2007), Teaching patternts in real-time experiments at secondary school. Communication presented at ESERA 2007, Malmöe, Sweden.
- Pintó R., Couso D. and Hernández M., (2010), An inquiry-oriented approach for making the best use of ICT in the classroom, eLearning Papers, 13(20), 1887–1542 (Available at: www.elearningpapers.eu).
- Redish, E. F., Saul, J. M. and Steinberg R. N., (1997), On the effectiveness of active-engagement microcomputer-based laboratories, Am. J. Phys., 65, 45–54.
- Rocard M., Csermely P., Jorde D., Lenzen D., Walwerg-Henriksson H. Y. and Hemmo V., (2007), Science education now: A renewed pedagogy for the future of Europe, European Commission. (Available at: http://ec.europa.eu/research/science-society/document_library/pdf_06/report-rocard-onscience-education_en.pdf).
- Rogers L. and Wild P., (1996), Data-logging: effects on practical science, Journal of Computer Assisted Learning, 12(3), 130–145.
- Russell D. W., Lucas, K. B. and McRobbie C. J., (2003), The role of the microcomputer-based laboratory display in upporting the construction of new understandings in kinematics, Res. Sci. Ed., 33, 217–243.
- Russell D. W., Lucas K. B. and McRobbie C. J., (2004), Role of the microcomputer-based laboratory display in supporting the construction of new understanding in thermal physics, J. Res Sci. Teach., 41(2), 165–185.
- Saez M., Pintó R. and Garcia P., (2005), Interconnecting concepts and dealing with graphs to study motion, In R. Pintó and D. Couso (ed.), Proceedings of the Fifth International ESERA Conference on Contributions of Research to Enhancing Students' Interest in Learning Science, pp. 1229–1232.
- Saez M., (2006), Estat de la implantació de l'aula de noves tecnologies per al treball experimental a Catalunya en el curs 2004–05, [Master Research]. Departament of Didactics of Mathematics and of Experimental Sciences, UAB, Barcelona.
- Sassi E., Monroy G. and Testa I., (2005), Teacher training about real-time approaches: Research-based guidelines and training materials, Sci. Ed., 89(1), 28–37.
- Svac M., (1999), Improving graphing interpretation skills and understanding of motion using micro-computer based laboratories, Electr. J. Sci. Ed., 3(4), 1087–3430.
- Tan D. K. C., Herberg J. G., Koh T. S. and Seah W. C., (2005), Datalogging. A unique affordance unrealized. ASERA, 2005, p. 9. (Available at http://repository.nie.edu.sg/jspui/bitstream/10497/2737/1/datalogging_ASERA.pdf).
- Testa I., Monroy G. and Sassi E., (2002), Students' reading images in kinematics: The case of real-time graphs, Int. J. Sci. Ed., 24(3), 235–256.
- Thornton R. K. and Sokoloff D. R., (1990), Learning motion concepts using real-time microcomputer-based laboratory tools, Am. J. Phys., 58, 858–867.
- Thornton R. K., (1999), Using the results of research in science education to improve science learning. Keynote address to the International Conference on Science Education, Nicosia, Cyprus, p. 9.
- Tinker R., (2009), A history of probeware. (available at http://www.concord.org/work/software/ccprobeware/probeware_history.pdf).
- Tobin K., (1990), Research on science laboratory activities: in pursuit of better questions and answers to improve learning, Sch. Sci. Math., 90, 403–418.
- Tortosa M., (2007), Manejo por parte del alumnado de los equipos de captación automática de datos en el aprendizaje de la química. Proceedings of II Jornadas Nacionales sobre la enseñanza de la química. Murcia, Spain, p. 10.
- Tortosa M., Saez M. and Pintó R., (2007), Experimentos en tiempo real para los cursos de ciencias en secundaria. In P. Membiela (coord.) Experiencias innovadoras de utilización de las NTIC. Educación Editora, Vigo, Spain, pp. 139–157.
- Tortosa M., Pintó R. and Saez M., (2008), The use of sensors in chemistry lessons to promote significant learning in secondary school students, Current Trends in Chemical Curricula. Proceedings of the International Conference, Prague, pp. 135–139.
- Tortosa M. and Pintó R., (2010), How students use their knowledge on collision theory to design, perform and explain experiments about the rate of reaction in an inquiry-based task, In I. Maciejowsja and P. Ciesla (ed.), 10th European Conference on Research in Chemistry Education. Book of abstracts, Krákow, Poland, pp. 269–270.
- Verner I. M. and Revzin L. B., (2010), Automation of manual operations i a high school chemistry laboratory: characteristics and students' perceptions, The Chemical Educator, 15, 1–5.
- Webb M. E., (2005), Affordances of ICT in science learning: Implications for an integral pedagogy, Int. J. Sci. Ed., 27(6), 705–735.
- Westbroek H., Klaassen K., Bulte A. and Pilot A., (2005), Characteristics of meaningful chemistry education, In K. Boersma, M. Goedhart, O. de Jong and H. Eijkelhof (ed.), Research and the Quality of Science Education, Part 2, pp. 67–76.
| This journal is © The Royal Society of Chemistry 2012 |
