Cooperative learning instruction for conceptual change in the concepts of chemical kinetics†
Özgecan
Taştan Kırık
a and
Yezdan
Boz
b
aÇukurova Üniversitesi, Eğitim Fakültesi, İlköğretim Bölümü, Fen Bilgisi Öğretmenliği Anabilim Dalı, Balcalı, Adana/Turkiye. E-mail: ozge.deniz@gmail.com
bMiddle East Technical University, Orta Doğu Teknik Universitesi, Eğitim Fakültesi, Orta Öğretim Fen ve Matematik Alanları, Eğitimi Bölümü, 06531, Ankara/Turkiye
First published on 2nd April 2012
Abstract
Learning is a social event and so the students need learning environments that enable them to work with their peers so that they can learn through their interactions. This study discusses the effectiveness of cooperative learning compared to traditional instruction in terms of students' motivation and understanding of chemical kinetics in a high school chemistry course. Participants were 110 eleventh grade students from two different schools. The researchers administered the Reaction Rate Concept Test to measure the students' understanding of chemical kinetics, the Science Process Skill Test to decide whether the groups were different in terms of their science process skills before instruction, and the Motivated Strategies for Learning Questionnaire to assess students' motivation for a chemistry course. Results of the experiment showed that compared to traditional instruction, cooperative learning enabled better understanding of the concepts of chemical kinetics and improved students' motivation to study chemistry for both schools.
Cooperative learning based on conceptual change to teach chemical kinetics
How science educators should teach has been the central issue of many research studies for decades. The major goal of science teaching is to make students capable of understanding the nature of the world by enabling them to gain knowledge (Hodson, 1992). Contemporary educators have to modify their teaching methods because the “… teaching of higher level reasoning and critical thinking does not depend on what is taught, but rather than on how it is taught” (Ruggiero, as cited in Johnson and Johnson, 1994, p. 57). Thus, we ask the following question as science educators: “what is the best teaching method for my students?” Cooperative learning, which is founded on constructivism, is one of the methods used to foster active student participation during the learning process. When students work in cooperative groups, they more frequently use higher levels of reasoning and critical thinking skills to create new ideas and solutions compared to when they work in competitive and individualistic situations (Johnson and Johnson, 1999a).Besides the cognitive aspects, the motivational components of academic performance are also important to students' classroom learning (Bryan et al., 2011; Pintrich, 2003). Motivational components involve students' perceptions of the classroom environment as well as their own beliefs, such as personal goals, self-efficacy, interests, and value beliefs. Research studies indicated that positive motivational beliefs such as perceptions of high self-efficacy, a focus on the mastery of goals, high value and interest in the task or content, and low levels of test anxiety are positively related to higher academic performance and greater use of cognitive strategies (Bryan et al., 2011; Pintrich and Schrauben, 1992). In the present study, the Student Teams-Achievement Divisions (STAD) method of cooperative learning was intended to promote conceptual understanding in chemical kinetics and to improve students' motivation for a chemistry course in terms of their intrinsic goal orientation, extrinsic goal orientation, task value, the control of learning beliefs, self-efficacy for learning and performance and test anxiety.
Theoretical background
Conceptual change and cooperative learning
Students may have acquired thoughts about some events or concepts before formal instruction in the classroom (Amir and Tamir, 1994). Students' conceptions or ideas that do not match scientific explanations are called alternative conceptions (Horton, 2007). Taber (2009) mentioned the multifaceted property of students' alternative conceptions by stating that they may have different alternative conceptions related to the same concept and may shift between different alternative conceptions to explain the same context. Different arguments about the nature of alternative conceptions have been put forward in the related literature. Alternative conceptions have been found to be consistent and coherent by some researchers (e.g.Driver and Easley, 1978; Vosniadou, 1992), and fragmented, inconsistent and task specific by some other (e.g.BouJaoude, 1991; Solomon, 1992). In this sense, it is important to consider the conditions such as nature of the science topics, ability and the ages of the learners and how the learners encounter with the topic while interpreting about the nature of alternative conceptions (Taber, 2009). Once misinformation is located in a person's knowledge structure, new information is often distorted, and this distortion causes the reinforcing or retaining of the incorrect idea (Vosniadou, 2001). If a scientific concept is not clearly expressed and explained, students are more likely to hold on to an alternative conception that makes sense to them. As a result, every new term or theory will then be incorporated into that flawed framework. For this reason, science educators must investigate students' science related preconceptions.Researchers have identified numerous alternative conceptions in chemistry (Barke et al., 2009; Horton, 2007; Kind, 2004). Chemistry is full of abstract concepts and is difficult to grasp for students most of the time. Chemical kinetics, which covers the concepts of the rate of chemical reactions and factors affecting that rate, is one such topic. Research studies have indicated that students have many alternative conceptions and face great difficulties in understanding chemical kinetics (BouJaoude, 1993; Çakmakçı, 2010; Çakmakçı et al., 2006; Garnett, et al., 1995; Griffiths, 1994; Kousathana and Tsaparlis, 2002). Therefore, it is necessary to devise proper strategies to overcome student alternative conceptions. Table 1 shows common alternative conceptions of chemical kinetics reported in the literature and included in the Reaction Rate Concept Test (RRCT) used in the present study.
| Reaction Rate |
| Students failed to grasp the fact that reaction yield and reaction rate are different concepts that are not directly related to each other (Kousathana and Tsaparlis, 2002). |
| The reaction rate is zero at the beginning. Over time, the interaction of molecules increases, and as a result, the reaction rate increases (Çakmakçı, 2005). |
| As reactants are used up, the formation of product increases, and accordingly, the reaction rate increases until all reactants are consumed where the reaction rate is constant (confusion of the reaction rate and the amount of product) (Çakmakçı, 2005). |
| The rate of the reaction increases at the beginning of the reaction. When reactants are used up, the reaction rate drops and at the end of the reaction, the rate is zero (Çakmakçı, 2005). |
| The reaction rate is the time required for reactants to form products (Nakiboğlu et al., 2002). |
| The reaction rate is the amount of substance turning into products per unit time at a constant temperature and concentration (Nakiboğlu et al., 2002). |
| The forward reaction rate always equals the reverse reaction rate (Garnett et al., 1995). |
| The students made the general statement that the “rate of reactions decreases as reactions progress”. Thus, they tended to make over-generalizations of principles and ignore some variables (e.g., the order of the reaction) (Çakmakçı, 2005). |
| The rate equation is written according to the fast step or net reaction equation (Bozkoyun, 2004). |
| The reaction rate is equal to the product of concentrations of reactants (Nakiboğlu et al., 2002). |
| Reaction rate = ΔHproducts − ΔHreactants so, if the rate of products is greater than the reactants, the reaction rate (ΔH) will be ΔH > 0. If the rate of reactants is greater than the products, the reaction rate will be ΔH < 0 (Çakmakçı, 2005). |
| Factors Affecting Reaction Rate |
| Many of the students assumed that as long as certain factors (e.g., temperature, concentration or catalysts) were not altered, the reaction rate would remain constant or remain the same during a reaction (Çakmakçı, 2005). |
| When the temperature is increased, the rate of the endothermic reaction increases, but the rate of the exothermic reaction decreases (Hackling and Garnett, 1985; Nakiboğlu et al., 2002; İcik, 2003). |
| Exothermic reactions occur faster than endothermic reactions (Çakmakçı, 2005). |
| An increase in temperature (temperature change) does not affect (change) the rate of exothermic reactions. Since exothermic reactions release energy, they do not need energy to proceed, and a rise in temperature would not affect the reaction rate (Çakmakçı, 2005). |
| A rise in temperature would not affect the reaction rate because the reaction rate is independent of temperature. The reaction rate only depends on the rate constant and molarities (Çakmakçı, 2005). |
| Increasing temperature increases the time necessary for a reaction to occur (Nakiboğlu et al., 2002). |
| Increasing the concentration of reactants always increases the rate of reaction (İcik, 2003; Kıngır and Geban, 2006). |
| A change in concentration does not affect the reaction rate (İcik, 2003). |
| There is a linear relationship between the concentration of reactants and the reaction rate (students did not anticipate the order of the reaction or the role of the solid catalyst). They expected a higher rate from increasing concentrations of reactants (Çakmakçı et al., 2006). |
| The volume of a container does not affect the reaction rate (Çakmakçı, 2005). |
| When the volume of a container is decreased, the kinetic energy of molecules increases (Çakmakçı, 2005). |
| A catalyst only speeds up the forward reaction (Voska and Heikkinen, 2000). |
| A catalyst gives energy to a reaction; therefore, it increases the activation energy of the reaction (Çakmakçı, 2005). |
| A catalyst is needed to initiate reaction (Kıngır and Geban, 2006). |
| Most of the students confused reaction intermediate and the catalyst in the reaction mechanism (Bozkoyun, 2004). |
| The catalyst increases the average speed of the molecules (Kıngır and Geban, 2006) |
| When a catalyst is used, more substances react (İcik, 2003). |
| A catalyst facilitates the collision of particles by interposing them (İcik, 2003). |
| A catalyst does not affect/change the mechanisms of the reaction (Çakmakçı, 2005). |
| A catalyst does not react with any of the reactants or products (İcik, 2003; Çakmakçı, 2005). |
| Reaction rates are the same whether the reactant is granulated or powdered since the molarities are equal in both cases (Çakmakçı, 2005). |
| Molecules of granulated MgO(s) are more strongly bonded to each other than those of powdered ones (Çakmakçı, 2005). |
| The surface area of reactants doesn't affect reaction rate (İcik, 2003). |
| Activation Energy |
| Activation energy is the kinetic energy of reactant molecules (Çakmakçı, 2005). |
| The faster a reaction, the more energy is released (Çakmakçı, 2005). |
| Temperature affects activation energy (Kıngır and Geban, 2006). |
| Increasing the temperature increases the activation energy (Kıngır and Geban, 2006; Çakmakçı, 2005). |
| The bigger the activation energy, the faster a reaction occurs (Çakmakçı, 2005). |
| Exothermic reactions have lower activation energy (Çakmakçı, 2005). |
Though students' difficulties are mentioned in the related literature, number of the research studies suggesting a teaching strategy to enhance students' understanding of chemical kinetics have been very limited (Chairam et al., 2009; Çakmakı and Aydoğdu, 2011). At this point, it is important to work on an appropriate teaching strategy to motivate students for meaningful learning and to deal with their alternative conceptions. To promote meaningful learning, a conceptual change approach is an alternative way to address students' alternative conceptions (Tsai, 2000; Vosniadou et al., 2001). Conceptual change is a slow process where a learner actively judges new information with their existing mental models. Learners form initial theoretical framework based on their daily life experiences and when they encounter new information, they actively begin to restructure these initial mental models and they may form synthetic models which is the synthesis of the scientific knowledge and their initial model (Vosniadou et al., 2008). We could say that students may keep holding their existing ideas while grasping a new idea, which also reveals the multifaceted nature of students' conceptions (Taber, 2009). Chi et al. (1994) also claimed that conceptual change is a gradual process, where learners continuously make additions and revisions to their initial mental models. Chi et al. (1994) suggested that concepts are connected with ontological categories. When a concept is learned, it is identified into a special category that will aid the comprehension of its entity and attributes. In further learning, revision of the learner's ontological categorization is necessary. As distinct from the knowledge as theory perspective, DiSessa (2001) stated that fragmented unstructured pieces of knowledge called phenomenological primitives are formed in the mind as a result of the learner's experiences and conceptual change is defined as the reorganization of this knowledge into coherent and stable network.
Thagard (1992) states that conceptual change process can occur through judging the usefulness of different theories. The one that has more value to explain the concept, that has explanatory coherence, should be the one that is preferred over the others. Therefore, it is important to increase the status of the scientifically accepted view. Conceptual change model proposed by Posner et al. (1982) also mentioned that it is necessary to increase the status of a conception by providing conceptual change conditions. Firstly, learners should be dissatisfied with their existing ideas and the new knowledge presented has to be intelligible, plausible, and fruitful. Vosniadou et al. (2008) stated that making students aware of the inconsistencies between their conceptions and the scientifically accepted knowledge is essential in order to promote conceptual change. In addition to metaconceptual awareness, theoretical coherence is also needed for conceptual change (Vosniadou and Ioannides, 1998). For the theoretical coherence, as mentioned above, the scientific knowledge should be made more valuable for learners to explain the concept so that children will prefer to use that scientific knowledge. Similar to the principle of theoretical coherence, Strike and Posner (1985) described the conditions necessary to increase the status of the scientific knowledge as being intelligible, plausible and fruitful.
Besides to cognitive variables, affective variables should also be considered in conceptual change. (Pintrich et al., 1993; Sinatra and Mason, 2008). During the learning process, learners should make an effort and be attentive, and they should be encouraged to be actively involved in the course of action instead of being passive. Pintrich et al. (1993) offered four general motivational constructs including goals, values, self-efficacy and control beliefs influencing conceptual change. Similarly, Gregoire (2003) developed the cognitive-affective model of conceptual change in which the process of conceptual change includes learner's goals, prior beliefs and motivational factors.
Learning is a social event, and students should be provided with learning environments to work with their peers that can also allow for their individual differences. Miyake (2008) claimed that sociocultural factors such as collaboration and discussions were found to influence conceptual change since discussions may make students aware of the need to change their conceptions. Vosniadou (2007) also addressed the importance of creating social environment in class where students have the chance to exchange their ideas for conceptual change. Among the conceptual change strategies, cooperative learning was found to be effective in enhancing students' understandings (Acar and Tarhan, 2008; Doymus, 2007; Gijlers and de Jong, 2005; Graham, 2005; Mori, 2002; Slavin, 1987). Cooperative learning requires students to work together in small groups to support each others' learning and understanding and to accomplish shared goals. According to Vygotsky (1981), children learn through their interactions with other people. They internalize skills and knowledge experienced during these interactions and, ultimately, they use those internalized skills and knowledge to shape their own behaviors. Cooperative learning has also been used successfully as a teaching strategy to help students learn to manage conflict (Stevahn et al., 1997). Moreover, cooperative learning positively affects motivation when high-achievers and low-achievers work together in a small group for group rewards (Gage and Berliner, 1992). Students feel good about making a contribution to the welfare of others. Furthermore, Johnson and Johnson (1987) discovered that when a cooperative learning approach was used more in the classroom, students learned science better, they tolerated differences more and they valued themselves more as science students.
Several cooperative learning methods have been developed and tested over the last 30 years. One of these is the Student Teams-Achievement Divisions (STAD), which is a simple and a good method to start for teachers who are new to the cooperative learning (Slavin, 1995). Correspondingly, teachers participating in the present study were not experienced with cooperative learning, so STAD was a suitable method for them to practice it for the first time. In this method, students are assigned to four-or five-member heterogeneous groups with respect to academic achievement, gender, and ethnic background. The groups should be heterogeneous in terms of gender, academic achievement and ethnicity so that they might represent the position of the classroom in terms of these characteristics. Slavin (1995) suggested five main components for STAD: Class presentation, teams, quizzes, individual improvement scores, and team recognition. The instruction starts with the teacher's presentation. The teacher presents the material by lecturing. The students are told what they are expected to learn and why it is important. The difference between this approach and the class presentations of the traditional teacher-centered method is that students should understand that they must carefully focus on the teacher''s presentation because it will guide them during their group work, quizzes, and team scores, which are determined by quiz scores. After the teacher's presentation, team study begins. During group activities, group members work cooperatively on the worksheets given by the teacher and master the material. After one to two teacher presentations and one to two group work practice sessions, each student is given a quiz to be answered individually. Everyone must understand the material well in order to get high scores on the quizzes. The group scores are calculated through these individual quizzes. The group with the highest score is rewarded, which provides team recognition.
In cooperative groups, students sometimes have to deal with conflict, which leads to conceptual change (Crook, 1994, as cited in Tao and Gunstone, 1999). Most of the models explaining conceptual change underline the role of cognitive conflict as a main condition to establish conceptual change (Limon, 2001; Zohar and Aharon-Kravetsky, 2005). According to Brown and Palincsar (1986), conceptual change is most likely to occur when situations creating dissatisfaction with existing knowledge are provided; by contrast, change is unlikely when the status quo goes unquestioned. Teaching strategies supporting questioning, evaluating, and criticizing are thought to be fruitful breeding grounds for restructuring student thinking. Dissatisfaction enables mental experimentation; evaluation leads to uncertainty, and group settings are amenable to increased questioning and criticism (Hatano, 1982; Inagaki and Hatano, 1983). When one is required to explain, elaborate or guard one's position to others (or sometimes to oneself), change in mental structure is inevitable.
One of the common strategies for conceptual change is to create environments invoking disequilibration or cognitive conflict (Piaget, 1985). Johnson and Johnson (1999b) stated that the more positive interdependence that exists within a cooperative learning group, the greater the likelihood of intellectual disagreement and conflict among group members. When students work in cooperative groups, their different perceptions, information, opinions, and conclusions will cause intellectual disagreement and conflict. When they face such opposition, they may manage the situation constructively, depending on their interpersonal and small-group skills. As a result, students experience better mastery and retention of the material being discussed and the frequent use of higher-order thinking skills. On the other hand, students working in competitive or individualistic situations do not have the chance to encounter such an intellectual challenge, and, consequently, their achievement and quality of reasoning suffer. To encourage group productivity, some conflict may be supportive for comparing ideas to reach solutions or create products.
The motivational aspect of cooperative learning
Motivation is the inner force that drives people to attain personal or organizational goals and objectives (Lindner, 1998); it is highly valued due to its consequences. Teachers, managers and coaches are concerned with motivation because it generates results. The motivational systems enhancing learning within cooperative environments include extrinsic motivation and intrinsic motivation.Extrinsic motivation occurs when the source of motivation is outside of the individual and the task being performed (Ormrod, 1999; Pintrich and Schunk, 1996). Extrinsically motivated individuals work on tasks because they believe that the contribution will result in desirable outcomes such as a reward, teacher praise and so on. Slavin (1995) claimed that cooperative learning improves motivation through the use of group goals and group reward; this is extrinsic motivation. Motivational perspectives on cooperative learning mainly concentrate on the reward or goal structures that students operate (Slavin, 1977, 1983, 1995). From a motivationalist perspective (e.g., Johnson and Johnson, 1992; Slavin, 1995), cooperative goal structures (as opposed to competitive or individualistic goal structures) necessitate that the only way group members can achieve their own personal goals is if the group is successful. Thus, in order to reach his or her own personal goals, a student, as a group member, must support group mates in order to exercise maximum efforts to master the task. Rewarding groups depending on their performance facilitates an interpersonal reward structure in which group members will provide or hold back reinforcers (such as praise and encouragement) in response to the efforts of group members (Slavin, 1995). In cooperative classrooms, in contrast to a traditional environment, students that work hard, attend class regularly and help others learn are praised and appreciated by group mates. Rewards in cooperative learning contribute to the motivation of students to improve their academic success because rewards allow students to value the success of the group; thus, students encourage and assist each other to achieve (Slavin, 1995).
Another type of motivational system that promotes learning within cooperative learning situations is intrinsic motivation (Johnson and Johnson, 1999a; Johnson et al., 1991). Intrinsic motivation exists when the source of motivation lies within the individual and the task; the individual engages in activity for its own sake (Ormrod, 1999; Pintrich and Schunk, 1996). Intrinsically motivated students work on tasks because they find the task enjoyable and valuable. When cooperative learning situations are organized well, the class usually has a positive emotional climate, in which the students are willing to participate in tasks with greater social support, such as assistance, encouragement and caring. These components have an effect on students' learning. Student comprehension of subject matter increases, and they feel more confident about their knowledge. The students develop more positive attitudes toward learning and perceive their learning as interesting, which improves their intrinsic motivation to learn. These aspects influence each other and promote student learning.
Reviews of cooperative learning research (Cohen, 1994; Qin et al., 1995) have indicated that cooperative learning increases and improves achievement, positive attitudes toward the subject area, self-esteem, and conceptual development. According to Johnson and Johnson (1999b), working in cooperative groups and valuing cooperation brings about better psychological health and self-esteem than does competing with classmates or working alone. When students work together to complete assignments, they interact (developing social skills and competencies), encourage each other's success (increasing self-worth), and structure personal and professional relationships (building the base for healthy social development). Working cooperatively improves personal ego-strength, self-confidence, independence, and autonomy. Therefore, students have the opportunity to share and solve personal problems, which enhances an individual's resistance and ability to deal with trouble and stress. Cooperative experiences are necessary for the healthy social and psychological development of individuals who can act independently.
In light of related literature, it can be concluded that students' prior knowledge and alternative conceptions strongly influence their learning, and higher motivation enhances learning. In chemistry, chemical kinetics is important in order for students to understand how reactions occur (collision theory) and by which factors their rates are influenced. In addition, chemical kinetics underlies the concept of chemical equilibrium, which is also among the most difficult topics in chemistry for students to understand. Thus, it is necessary to design a suitable teaching strategy other than the traditional method to deal with these alternative conceptions and promote students' understanding.
Based on the theoretical principles presented in this section, cooperative learning seems to be a reasonable method or strategy to teach a subject and improve students' understanding and motivation. On the other hand, more research studies on the effectiveness of cooperative learning based on conceptual change to enhance students' conceptions and motivation are necessary. Therefore, the aim of the present study is to investigate the effectiveness of cooperative learning based on conceptual change to teach chemical kinetics and motivate the students to learn chemistry. Correspondingly, this study searched for information to answer following questions:
1. Will cooperative learning based on conceptual change strategy enhance secondary school students' learning of chemical kinetics?
2. Will students instructed by cooperative learning based on the conceptual change method be more motivated in their chemistry course?
Materials and methods
Subjects
Four eleventh grade classes, 110 students in total, participated in the study over a period of six weeks. The classes were from two different schools in Turkey; one was an Anatolian high school and the other was an ordinary high school (59 students from Anatolian high school and 51 students from ordinary state high school). Students in the Anatolian school were considered to be brighter than the students in the ordinary high school since they were accepted to that school based on higher grades received on a nationwide exam. These schools were selected based on convenient sampling. The teachers were willing to implement a new strategy in their classes and they were known by the researchers, and their teaching styles were similar. Moreover, reason of including two different schools in the study was to increase the sample size. There were only two classes of 11th grades for each school. Therefore, the study was implemented in different schools. One teacher from each school, who had two classes as one experimental and one control class, taught the students. As a result, there were two experimental classes and two control classes. Two classrooms from each school were randomly assigned to the experimental and control groups. It was not possible to assign the individuals to experimental and control group randomly since the school administration had already formed the classrooms at the beginning of the semester. Therefore, from the Anatolian high school, one class was assigned randomly as experimental group and one class as control group. Similarly, from the other school, one class was assigned randomly as experimental group and one class as control group. The students were ages 16–17. The teachers had nine and 11 years of teaching experience and similar chemistry content backgrounds. The pattern of the intervention and the comparison for each school is given in Fig. 1.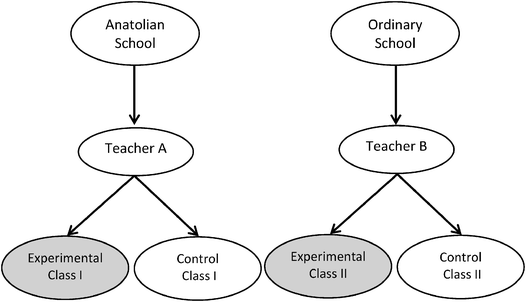 | ||
| Fig. 1 The pattern of the intervention and the comparison for each school. | ||
Instruments
The instrument is composed of two sections; the first section contains 16 two-tier items, and the second section contains 7 multiple-choice items in Turkish. The items of two-tier multiple choice instruments are specifically designed to discover alternative conceptions in a clearly defined content area. In a two-tier item, there are two parts. The first part consists of two or three choices to be selected. In the second part, students are expected to give their reasoning about their answer in the first part by selecting among four alternatives. The alternatives in the second part are prepared based on the students' alternative conceptions. As Treagust (1987) stated, the second part of two-tier tests involves four alternatives consisting of alternative conceptions, incorrect statements and a correct alternative. Correspondingly, the multiple choice items in the second section were prepared based on students' alternative conceptions derived from research studies in the literature (Bozkoyun, 2004; Çakmakçı, 2005; Çakmakçı et al., 2006; Garnett et al., 1995; Haim, 1989; İcik, 2003; Kıngır and Geban, 2006; Nakiboğlu et al., 2002).
The test covered chemical kinetics concepts including reaction rate, collision theory, activation energy, heat of reaction, potential energy diagrams, reaction mechanisms, rate equations and orders and the factors affecting reaction rate (concentration, temperature, surface area, and catalyst). Test items were developed through the examination of related literature (İcik, 2003; Çakmakçı, 2005), chemistry textbooks (e.g., Ebbing and Gammon, 1999) and several high school test books. Each item of RRCT was examined by four chemistry educators and two chemistry teachers in order to assess its content validity, accuracy, and format. RRCT was piloted to 203 students who had already learned the reaction rate concept from different schools during the 11th grade. Based on the reliability analysis, some of the items' alternatives were altered, while some of the items were excluded from the test. It was piloted again in its final form to 251 high school students who had learned chemical kinetics before. The reliability coefficient of the test by the KR-20 Formula was found to be 0.78. Some examples of items from RRCT are given in the Appendix A.
Before implementing the test to the students of experimental and control group, it was piloted to 316 eleventh and twelfth grade students with an age range of 16–17 in different schools of Ankara. Cronbach alpha values of the motivation section are as follows: IGO = 0.75, EGO = 0.65, TV = 0.88, CLB = 0.67, SELP = 0.90, TA = 0.70. During the study, MSLQ was given to both experimental and control group as a pre- and post-test to measure their motivation to learn chemistry in terms of intrinsic goal orientation, extrinsic goal orientation, task value, control of learning beliefs, self-efficacy for learning and performance, and test anxiety.
Procedure
The concept of reaction rate was a part of the curriculum in the chemistry course taught to both the experimental and control group students over six weeks. There were three chemistry class sessions in a week, and each class session lasted 45 min. In the Turkish curriculum, chemical kinetics is taught at 11th grade after the concept of enthalpy. The concepts of chemical kinetics are definition and measurement of reaction rate, change in rate with time, instantaneous rate, average rate, rate law, one-step and multi-step reactions, activation energy, factors affecting reaction rate (concentration, temperature and catalyst) and enzymes as biological catalysts. Unfortunately, laboratory facilities are not enough to make experiments. Therefore, the students learn this topic theoretically.In the experimental group, the STAD method of cooperative learning was applied. The teachers were provided with information about the STAD method of cooperative learning and the application of cooperative learning in the content of reaction rate. All materials, including detailed explanations of cooperative learning, lesson plans, instruments, group work activities, teacher manual providing information about the role of the teacher and quizzes, were given to the teachers to be examined in advance. After a week, two-hour meetings were conducted with the teachers to inform them of and discuss the application of cooperative learning as well as answer related questions, if there were any.
Before instruction, the teacher lectured the students for two class hours about cooperative learning, its implementation, the aim of group work, social skills needed for group work, and what would be expected of students during their instruction. In the next lesson, four-member groups, heterogeneous with respect to achievement and gender, were created by the teacher. Hence, each group included one high achiever two average achievers, and one low achiever. Achievement level was decided based on students' previous chemistry grades. Depending on the number of students in each class, one or two of the groups included five students. The positions of the desks were placed so that students were face to face. Teacher-student and student-student interaction was highlighted during the instruction.
The teacher started the instruction with a class presentation. He presented the concepts and necessary information that would be used by the students during their group activities after the presentation. Teacher presentation sessions took about one hour depending on the topic for each week. After the teacher presentation, group members came together to study worksheets. The worksheets demanded group members to discuss among themselves in order to reach a common solution instead of loading the responsibility onto one or two students. These questions also included those requiring interpretations of events from daily life related to reaction rate. Cooperation and interdependence among group members were crucial. Furthermore, the teacher asked some disequilibrating questions to encourage the discussions during the group activities. He guided students and provided help when necessary. In addition, the teacher assessed each group's work by means of a Group Evaluation Form, which was intended to evaluate groups in terms of students' social skills, participation, and contributions to the completion of the task. The teacher observed the groups while they were working on the task, filled out the form for each group once a week, and provided feedback to increase the performance of the groups for the next week. Since the students were new to the cooperative learning environment, they needed as much feedback as possible from the teacher. Moreover, the teacher oriented students when they had difficulty expressing the social skills necessary for group work including knowing how to share their ideas, acknowledge the contributions of others, deal with discrepancies, manage conflicts, share resources fairly, take turns, and engage in democratic decision making (Johnson and Johnson, 1975).
For example, as a first group activity, students were given the question in Fig. 2. This question was prepared in Turkish by using common alternative conceptions related to the definition of reaction rate and translated in English.
 | ||
| Fig. 2 Worksheet example given to cooperative groups. | ||
Using alternative conceptions in the questions created contradiction or disequilibrium among group members and they started to discuss the different definitions given in the task. The researchers observed the groups. In some groups, some of the students claimed that there was more than one correct definition. Mostly, these students insisted on Serap and Ali's explanations in addition to the correct definition. The teacher guided students to compare the rate of a chemical reaction with the term “speed” in physics and then think over the definitions given in the question again. He also asked the students to think about how the concentrations of reactants change during a reaction. The teacher continuously guided students and provided help and feedback when necessary. The groups discussed their ideas. For example, one of the students stated that “faster reactions occur in a shorter time so Serap's definition may be correct”. Her group mate claimed that “time is not enough by itself to define the rate; we must consider the amount of product, so Murat's definition may be correct”. This discussion indicated that students sometimes contradicted each other, thought over others' perspectives, and argued actively to reach a consensus.
The students were actively involved in their own learning. The idea was that children learn better by teaching something to a peer. The teacher guided the groups by giving the following example: “we define the speed of a car in terms of km/hour, so we must consider the distance to be traveled in addition to time passed”. When the group work was completed, the teacher chose a student from each group randomly to present and discuss his or her group's solutions to the whole class. Three more examples of worksheets are given in the Appendix B.
After two or three group activities, a quiz was given to the experimental group students to answer individually. Group discussion was not allowed during the process of answering the quiz questions. Four quizzes were administered in total. These individual quizzes provided individual accountability since each member had to be ready for the quiz. They were collected, corrected, and graded by the teacher. During the next lesson, the quizzes were given back to the students in order for them to see their in-group performance and improvement and to establish group processing (Johnson et al., 1990). Considering the total points of groups on quizzes, the group with the highest score was rewarded. They got 5 point bonus for their mid-term exam and their names were hung on the clipboard as “the best performers”. The reward was for encouraging and motivating the students in their group activities.
During the instruction, the researcher and a chemistry education Ph.D. student examined the instruction in the experimental group classes once a week by filling out the treatment verification checklist prepared by the researchers in order to decide whether the cooperative learning method was applied as intended. Utilizing this checklist indicated that many of expected characteristics of cooperative learning were provided in experimental group classes.
In the control group, the students were taught the reaction rate concept during a lecture. Before defining reaction rate, teacher asked the students how the concentration of reactants and products change with time. After the whole class discussion, he drew concentration of reactants and products vs. time curves for a given reaction on the board. These curves were used to define instantaneous rate and average rate. He also emphasized that in defining reaction rate, both change in amount of reactants or products and time should be considered together. The teacher calculated average rate of a reaction of which concentration-time data was given. Then he expected the students to calculate average rate of some other reactions by using concentration-time data. The students confirmed that average rate of a reaction decreases with time. In another lesson, they learned to write rate law for one step gaseous reactions. They also discussed why solids and liquids are not written in the rate law equation. The teacher wrote a multi-step reaction on the board and explained the slowest step as the rate-determining step. The teacher used an analogy that the slowest car determines the speed of the other cars behind it in the traffic. He presented “reaction order” concept through the slowest step. Before learning the effect of temperature on reaction rate, the teacher discussed collision theory with the students and expected them to predict the effect of temperature on reaction rate based on this discussion. He concluded that increasing temperature increases the number of molecules having energy greater than the activation energy of the reaction. He drew potential energy diagrams to explain the potential energy of reactants, “activated complex” and the products. He also provided examples of endothermic and exothermic reaction equations to highlight the idea that increasing temperature increases rate of both type of reactions. Relating to the effect of catalyst, the teacher presented the definition of catalyst as “the substance that changes reaction rate without affecting the composition of the products” and gave examples of negative and positive catalysts. He asked them if the catalyst enters the reaction with the reactants or not. A small discussion was conducted and they reached the conclusion that catalyst participates in the reaction with the reactants. He explained the inhibitor and exemplified it from human body mechanism. The teacher designated enzymes as biological catalysts speeding up the biological reactions. He related the working mechanism of catalysts with activation energy and discussed it on potential energy curves. At the end of the chapter, effect of surface area of the reactants was discussed on a heterogeneous phase reaction.
The teacher used questioning and sometimes whole class discussions to remedy the alternative conceptions. He also used analogies to make some abstract points clearer. Worksheets were distributed to the students at the end of each week as homework, and the answers were given in the next lesson. While at home, students were supposed to practice related problems and interpret verbal questions. Similar problems and content were explained in the control group as in cooperative learning class. The instruction was teacher-centered, and student-student interaction was very limited.
Results
Previous to treatment, the researchers conducted an independent-samples t-test by using the Statistical Package for Social Sciences (SPSS) to determine whether there was a significant mean difference between the experimental group and the control group in terms of SPST and pre-RRCT scores for each school.The results for Anatolian high school indicated that there was no significant difference between experimental group (EG) and control group (CG) with respect to previous conceptual understanding measured by pre-RRCT, t(57) = −0.893, p > 0.05, and science process skills measured by SPST, t(57) = 0.660, p > 0.05.
The results for ordinary state high school indicated that there was no significant difference between EG and CG with respect to pre-RRCT scores, t(49) = 0.672, p > 0.05. On the other hand, there was a significant mean difference between the groups with respect to science process skills measured by SPST, t(49) = 3.501, p < 0.05. Therefore it was assigned as covariate in the analysis of post-RRCT scores of ordinary state high school students.
In order to test the first research question, after meeting the assumptions, one-way ANOVA was used for the analysis of post-RRCT scores of the students from Anatolian high school, where treatment was the independent variable and the understanding of the reaction rate concept was the dependent variable for the participants to reveal the effect of cooperative learning.
The results indicated that the cooperative learning group displayed significantly better understanding of chemical kinetics [F(1, 57) = 23.19, p = 0.00, partial η2 = 0.29]. Partial η2 of 0.29 suggests a large relationship between treatment and the dependent variable, implying that the magnitude of the difference among groups was large (Green et al., 2007). In other words, cooperative learning based on conceptual change conditions produced better results in terms of coping with students' alternative conceptions about chemical kinetics compared to traditional instruction in Anatolian high school.
After meeting the assumptions, post-RRCT scores of the students from ordinary high school were analyzed by one-way ANCOVA, where treatment was the independent variable, understanding of reaction rate concept was the dependent variable and SPST scores were the covariate. According to the results, there was a significant mean difference between EG and CG in terms of students' understanding of reaction rate concept when SPST scores were used as covariate [F(1, 48) = 5.21, p = 0.027, partial η2 = 0.09]. Partial η2 of 0.09 suggests a moderate relationship between treatment and dependent variable. This means that, 9% of variance on dependent variable was attributed to treatment in ordinary high school. Descriptive statistics of EG and CG students' pre-RRCT, SPST and post-RRCT scores are given in Table 2 for each school.
| Group | N | Pre-RRCT | SPST | Post-RRCT | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| Anatolian High School | EG | 30 | 9.73 | 3.85 | 23.70 | 4.06 | 21.60 | 2.28 |
| CG | 29 | 10.48 | 2.47 | 23.06 | 3.21 | 18.10 | 1.65 | |
| Ordinary High School | EG | 26 | 7.42 | 2.85 | 22.42 | 2.85 | 16.76 | 3.52 |
| CG | 25 | 6.92 | 2.46 | 19.04 | 2.46 | 13.28 | 2.71 | |
Percentage of the students' selections of the alternatives of RRCT was examined. Results for the items reflecting the alternative conceptions that several students had were presented. Accordingly, Table 3 indicates the percentage of these alternative conceptions identified by RRCT in each school. Item analysis results supported that cooperative learning dealt better with students' alternative conceptions in the experimental group. On the other hand, these results also revealed that some of the alternative conceptions resisted more in the students of ordinary state high school than those of Anatolian high school.
| Alternative conceptions | Anatolian School | Ordinary School | ||
|---|---|---|---|---|
| EG | CG | EG | CG | |
| (1) The reaction rate is the amount of substance turning into products per unit time at a constant temperature and concentration. | 6.7 | 62.1 | 15 | 65.5 |
| (2) The rate of reaction increases at the beginning, decreases with a decrease in the amount of reactants, and becomes zero at the end. | 5.9 | 18 | 13.2 | 20 |
| (3) Exothermic reactions are faster than endothermic reactions. | 6.7 | 17.2 | 15.4 | 24 |
| (4) In exothermic reactions, rate of forward reaction decreases with increasing temperature. | 0 | 0 | 10.5 | 24 |
| (5) Exothermic reactions have lower activation energy than endothermic reactions. | 20 | 41.4 | 7.7 | 28 |
| (6) Kinetic energy of molecules increases by decreasing the volume of the vessel. | 40 | 45 | 60 | 84.6 |
| (7) Since the molecules of granulated MgO(s) are more strongly bonded than those of powdered MgO(s), they hardly react compared to the powdered ones. | 13.3 | 17.2 | 19.2 | 48 |
| (8) Increase in temperature decreases the activation energy and so does the rate of the reaction. | 0 | 17.2 | 0 | 16 |
| (9) Catalyst increases the average speed of the molecules. | 0 | 13.4 | 14 | 19.2 |
| (10) Catalyst increases the reaction rate without changing the mechanism. | 3.3 | 15 | 0 | 20 |
Findings from item analysis may suggest that more than half of the students of control groups from both schools had difficulties in understanding what the reaction rate is. Correspondingly, the first alternative conception in Table 1 was selected as the answer of the related item by 62.1% of CG in Anatolian high school and 65.5% of CG in ordinary state high school. Furthermore, more students in CG from both schools selected the alternative that the rate of reaction increases at the beginning, decreases with a decrease in the amount of reactants, and becomes zero at the end than EG. This shows that more students in traditional groups had problems with understanding how reaction rate changes with time. Table 3 implies that the participants of CGs of both schools confused more with the thermochemical identity of the reactions and the reaction rate than EGs. Specifically, they selected the following alternative conceptions among the alternatives of the related items: (i) exothermic reactions are faster than endothermic reactions; (ii) in exothermic reactions, rate of forward reaction decreases with increasing temperature; (iii) exothermic reactions have lower activation energy than endothermic reactions. Furthermore, several students of both groups from both schools did not understand the effect of volume on reaction rate since many of them from Anatolian high school (40% of EG and 45% of CG) and the majority of them from ordinary state school (60% of EG and 84.6% of CG) selected that kinetic energy of molecules increases by decreasing the volume of the vessel. Likewise, the learners in both groups had problems in understanding the effect of surface area on the reaction rate, although the experimental group students performed better in their answers in each school. Correspondingly, more students in CGs chose the alternative that since the molecules of granulated MgO(s) are more strongly bonded than those of powdered MgO(s), they hardly react compared to the powdered ones. In addition, nobody from EGs of both schools selected the alternative conception that increase in temperature decreases the activation energy and so does the rate of the reaction although 17.2% of CG students of Anatolian high school and 16% of CG students of ordinary state school did so. This result indicates that students in CGs did not understand the effect of temperature on reaction rate completely. Similarly, participants of EGs of each school is superior to CGs in terms of understanding the effect of catalyst on reaction rate since the percentage of students from EGs choosing following alternative conceptions are more less: (i) catalyst increases the average speed of molecules; (ii) catalyst increases the reaction rate without changing the mechanism.
For the analysis of the pre-MSLQ scores to explore motivational characteristics of the experimental and control group students of each school before the instruction, after satisfying the assumptions, MANOVA was conducted on the six variables of intrinsic goal orientation, extrinsic goal orientation, task value, control of learning beliefs, self-efficacy for learning and performance, and test anxiety. The results for the students of Anatolian high school revealed that their motivational characteristics were similar in both groups before the instruction (F[6,52] = 1.31, p > .05). Likewise, motivational characteristics of the students of ordinary state high school were also similar in EG and CG before the instruction (F[6,44] = 0.56, p > .05). Table 4 shows the descriptive statistics of the motivational dependent variables for pre-MSLQ for each school.
| Dependent variable | Anatolian High School | Ordinary State High School | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||
| CG | EG | CG | EG | CG | EG | CG | EG | |
| Intrinsic goal orientation | 18.89 | 19.36 | 4.81 | 4.27 | 19.20 | 20.26 | 5.50 | 4.35 |
| Extrinsic goal orientation | 20.82 | 22.13 | 4.02 | 4.04 | 20.52 | 20.38 | 4.48 | 5.69 |
| Task value | 29.48 | 32.80 | 6.75 | 4.71 | 30.20 | 29.00 | 7.08 | 6.32 |
| Control of learning beliefs | 21.48 | 22.90 | 4.08 | 2.92 | 23.04 | 22.30 | 3.27 | 4.86 |
| Self-efficacy for learning and performance | 42.10 | 47.23 | 10.56 | 5.64 | 39.64 | 38.00 | 8.73 | 3.27 |
| Test anxiety | 18.79 | 18.46 | 5.38 | 5.41 | 20.88 | 21.65 | 5.79 | 5.35 |
For the answer to the second research question, MANOVA was performed on the post-MSLQ scores after satisfying the assumptions to see the effect of cooperative learning on students' motivation for each school. The analysis for Anatolian high school indicated that there was a significant mean difference between the experimental and control group in terms of collective dependent variables after the instruction (F[6,52] = 0.94, p <.05, partial η2 = .09). The Partial Eta Squared (η2) value is medium, meaning that the magnitude of the difference between the groups is moderate (Green et al., 2000). In other words, 9% of the variance of dependent variables was explained by the treatment.
MANOVA results for the post-MSLQ scores of ordinary state high school students revealed that there was a significant difference between EG and CG in terms of their motivation (F[6,44] = 3.25, p <.05, partial η2 = .30). The Partial Eta Squared (η2) value is large, that is the difference between the groups arouse from treatment effect, and this difference had practical value (Green et al., 2000). Table 5 shows the descriptive statistics of the motivational dependent variables for post-MSLQ for each school. It revealed that EG had higher mean scores on task value and self-efficacy for learning and performance in Anatolian high school, and intrinsic goal orientation, extrinsic goal orientation and test anxiety in ordinary high school.
| Dependent variable | Anatolian High School | Ordinary State High School | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||
| CG | EG | CG | EG | CG | EG | CG | EG | |
| Intrinsic goal orientation | 18.06 | 18.66 | 5.07 | 4.24 | 17.40 | 21.88 | 4.67 | 3.94 |
| Extrinsic goal orientation | 21.48 | 21.96 | 4.70 | 3.35 | 20.36 | 22.50 | 4.79 | 5.10 |
| Task value | 29.62 | 31.20 | 6.49 | 6.91 | 30.12 | 30.00 | 7.50 | 7.57 |
| Control of learning beliefs | 21.86 | 22.33 | 4.04 | 3.30 | 24.08 | 23.42 | 3.78 | 3.61 |
| Self-efficacy for learning and performance | 42.13 | 47.50 | 9.34 | 6.66 | 42.40 | 43.73 | 3.13 | 3.93 |
| Test anxiety | 19.24 | 20.30 | 6.37 | 6.30 | 18.96 | 20.15 | 6.39 | 7.16 |
To determine the effect of treatment on each dependent variable, univariate ANOVAs were applied for each school. Table 6 indicates the results of the univariate ANOVAs for the post-test.
| Source | Dependent variable | Anatolian High School | Ordinary High School | ||||
|---|---|---|---|---|---|---|---|
| df | F | p | df | F | p | ||
| a Self-efficacy for learning and performance. | |||||||
| GROUP | Intrinsic goal orientation | 1 | 0.241 | 0.625 | 1 | 8.309 | 0.006 |
| Extrinsic goal orientation | 1 | 0.208 | 0.650 | 1 | 2.379 | 0.129 | |
| Task value | 1 | 0.816 | 0.370 | 1 | 0.003 | 0.955 | |
| Control of learning beliefs | 1 | 0.241 | 0.635 | 1 | 0.433 | 0.514 | |
| Self-effic. for learning and perf.a | 1 | 4.280 | 0.043 | 1 | 0.488 | 0.488 | |
| Test anxiety | 1 | 0.411 | 0.524 | 1 | 0.506 | 0.506 | |
As seen in Table 6, there was a statistically significant mean difference between the groups in terms of intrinsic goal orientation in ordinary state high school and self-efficacy for learning and performance in Anatolian high school. Table 5 also supports that experimental group students had higher scores on those variables than control group students in the schools specified.
Table 7 presents the percentages of agreement with the selected items in the intrinsic goal orientation scale (item 16 and 22) and self-efficacy for learning and performance scale (item 5, 6, 12, 20, 21, 29) across groups.
| Scale | Item no | Groups | 1 (%) | 2 (%) | 3 (%) | 4 (%) | 5 (%) | 6 (%) | 7 (%) |
|---|---|---|---|---|---|---|---|---|---|
| a From 1 to 7, responses represent “not at all true of me” to “very true of me”. | |||||||||
| Intr | 16 | CG | 16 | 12 | 28 | 4 | 20 | 4 | 16 |
| EG | 7.7 | 0 | 7.7 | 15.4 | 19.2 | 34.6 | 15.4 | ||
| 22 | CG | 8 | 0 | 3 | 18 | 8 | 25 | 38 | |
| EG | 0 | 0 | 0 | 11.5 | 12.4 | 30.8 | 45.3 | ||
| Slfef | 5 | CG | 0 | 3.4 | 10.3 | 17.2 | 31 | 20.7 | 17.2 |
| EG | 0 | 0 | 0 | 10 | 23.3 | 26.7 | 40 | ||
| 6 | CG | 6.9 | 13.8 | 0 | 13.8 | 37.9 | 17.2 | 10.3 | |
| EG | 0 | 0 | 3.3 | 16.7 | 23.3 | 23.3 | 33.3 | ||
| 12 | CG | 0 | 3.4 | 0 | 10.3 | 20.7 | 27.6 | 37.9 | |
| EG | 0 | 0 | 0 | 13.3 | 6.7 | 43.3 | 36.7 | ||
| 20 | CG | 3.4 | 3.4 | 3.4 | 17.2 | 31 | 27.6 | 13.8 | |
| EG | 0 | 0 | 0 | 23.3 | 13.3 | 30 | 33 | ||
| 21 | CG | 0 | 3.4 | 0 | 10.3 | 27.6 | 27.6 | 31 | |
| EG | 0 | 0 | 0 | 6.7 | 20 | 36.7 | 36.7 | ||
| 29 | CG | 0 | 6.9 | 10.3 | 3.4 | 31 | 31 | 17.2 | |
| EG | 0 | 3.3 | 6.7 | 13.3 | 20 | 26.7 | 30 | ||
Item 16 and 22 are among the ones contributing to the intrinsic goal orientation scale. Item 16 states that “In chemistry lessons, I prefer course material that arouses my curiosity, even if it is difficult to learn”. Accordingly, 50% of the experimental group students from ordinary state high school rated this item as 6 and 7, while 20% of the control group students rated that as 6 and 7, which indicates the agreement of this statement. Moreover, in the same school, 76.1% of the experimental group students agreed with item 22, stating, “The most satisfying thing for me in chemistry is trying to understand the content as thoroughly as possible”, while 63% of students in the control group agreed with it. In addition, students in the experimental group had higher scores of self-efficacy for learning and performance in Anatolian high school. For instance, item 5 stating, “I believe I will receive an excellent grade in the chemistry lesson”, was rated 6 and 7 indicating agreement by 66.7% of students in the experimental group, while 37.9% of the control group students agreed with it. In addition, 56.6% of the experimental group students agreed with the statement, “I'm certain I can understand the most difficult material presented in the readings for the chemistry lesson” (item 6), whereas 27.5% of the control group students agreed with it. Similarly, 80% of students in the experimental group and 65.5% of the control group students agreed with the statement, “I'm confident I can learn the basic concepts taught in the chemistry lesson” (item 12). Furthermore, 66% of students in the experimental group and 41.4% of students in the control group agreed with item 20 stating, “I am confident I can do an excellent job on the assignments and tests in the chemistry lesson”. Item 21, stating, “I expect to do well in the chemistry lesson” was agreed upon by 73.4% of the experimental group students and by 58.6% of the control group students. In addition, 56.7% of the experimental group students and 48.2% of the control group students agreed with item 29 stating, “I'm certain I can master the skills being taught in the chemistry lesson”.
By contrast, as seen in Table 6, there was no significant mean difference between groups in terms of extrinsic goal orientation, control of learning beliefs, task value and test anxiety when post-MSLQ scores of students were analyzed.
Discussion
The present study investigated the effects of cooperative learning based on conceptual change conditions on 11th grade students' motivation and understanding of chemical kinetics in two different schools. According to the results, cooperative learning group resulted in significantly better acquisition of knowledge related to reaction rate than traditional group in both schools. However, cooperative learning improved students' understanding of reaction rate in Anatolian high school more than it did in ordinary state high school because the magnitude of the difference between EG and CG in Anatolian high school is larger. This may be because of the fact that, the students in Anatolian high school were brighter than the ones in ordinary state high school because they had higher scores on the nationwide exam. Therefore, it was easier for them to grasp the concepts compared to the students in ordinary state high school. As Taber (2009) addressed, learners' knowledge representations is dependent on their ability level. The effectiveness of cooperative learning on students' conceptions was also supported by other studies in the literature (e.g., Acar and Tarhan, 2008; Barbosa et al., 2004; Bilgin and Geban, 2006; Doymuş, 2007; Felder, 1996). On the other hand, post-RRCT scores revealed that the students had some alternative conceptions related to reaction rate even after experiencing the cooperative learning instruction based on conceptual change conditions designed to cope with those alternative conceptions. The most frequent and persistent alternative conceptions, which were in agreement with previous studies (Bozkoyun, 2004; Çakmakçı, 2005; Çakmakçı et al., 2006; Garnett et al., 1995; Haim, 1989; İcik, 2003; Kıngır and Geban, 2006; Nakiboğlu et al., 2002) are as follows:• The reaction rate is the amount of a substance turning into products per unit time at a constant temperature and concentration.
• Students over-generalized, suggesting that the rate of reactions always decreases as the reaction proceeds without considering the order of the reaction.
• The kinetic energy of molecules increases by decreasing the volume of the vessel (The students had difficulty understanding the effect of volume on reaction rate).
• Since the molecules of granulated MgO(s) are more strongly bonded than those of powdered MgO(s), they hardly react compared to the powdered ones.
• The activation energy of exothermic reactions is lower than that of endothermic reactions.
• A catalyst increases reaction rate without changing the mechanism.
These results confirmed that alternative conceptions may be resistant to change even after instruction that differs from a traditional method. If the teacher does not deal with them, they will distort students' further learning. For example, in school curriculum, the chemical equilibrium chapter comes after the reaction rate concept and is strongly based on understanding reaction rate. In other words, if students don't understand reaction rate completely, their learning of chemical equilibrium will be compromised. Thus, the teacher should consider these alternative conceptions while preparing teaching materials for further lessons. Specifically, worksheets activating alternative conceptions were used in experimental groups and teacher directed discussions and lecturing highlighting alternative conceptions were implemented in control groups. Some of them still existed in both groups after the treatment although cooperative learning instruction dealt with them better compared to the traditional one. In this study, a cooperative learning model was designed based on conceptual change conditions suggested by Posner et al. (1982). Posner et al. (1982) instructional theory requires that the learner be dissatisfied with their existing ideas and that the new concept taught should be intelligible, plausible, and fruitful. The group activities were prepared by considering students' alternative conceptions of reaction rate. The worksheets were designed to create dissatisfaction in students since they included alternative conceptions contradicting scientifically accepted knowledge. Moreover, discussions in cooperative groups provided contradiction because the students noticed that their group mates had different ideas or perspectives from their own points of view. In addition, the teacher asked some questions of the groups in order to encourage discussions and to create contradictions. During the group work, students could ask questions of the teacher related to the points or questions that they didn't understand. After the group activity, each group explained their answers to whole class. During this phase, the teacher gave feedback to the groups. After the discussions of the whole class, the teacher provided reasonable explanations of problematic points. This was meant to fulfill intelligibility and plausibility. Furthermore, some of the questions on the worksheets enabled students to make connections between scientific knowledge and daily life. Also, the teacher used some additional everyday life examples while presenting the concept before group work and provided support to the groups during group work to meet the fruitfulness condition.
Contrary to this strategy, which requires the consideration of students' existing knowledge and alternative conceptions to establish conceptual change, traditional instruction was strongly dependent on teacher coordination without taking students' backgrounds and needs into account. Information was directly transmitted from the teacher to the students instead of permitting students to create their own knowledge. In addition, students mostly were not allowed to talk to each other in order to provide silence and authority in the classroom. There was almost no student-student interaction and little student-teacher interaction. However, students usually prefer to ask their peers questions related to a concept that they do not understand before asking the teacher. They hesitate to ask the teacher. Therefore, when the teacher asks the class if there are any questions and gets no answer, this does not mean that everybody understands the related content. That is why the intervention classes experienced conceptual change more and of high quality. Pintrich et al. (1993) criticized the conceptual change model in terms of its lack of attention to affective, situational, and motivational factors. In fact, although dissatisfaction provides students with an affective reason to change their existing knowledge, Strike and Posner (1992) recommended that it was necessary to deal with “motives and goals and the institutional and social sources of them need to be considered” to improve the model (p. 162). In spite of the fact that students may have similar existing knowledge; they may not have the goal of learning the content or motivation to resolve inconsistencies between their knowledge and the new concept. In this study, cooperative learning environment was designed to meet the deficit of conceptual change related to motivation. Motivational perspective emphasizes the importance of rewarding groups to promote individual learning of all group members and favoring active helping of peers. Providing an incentive for group learning efforts is critical to improve the learning outcomes. In the present study, STAD method of cooperative learning, which includes rewarding of high-performed group, was implemented. It was aimed to motivate students to provide their group mates with assistance to improve individual learning of all group members. Correspondingly, the results of this study showed that cooperative learning based on conceptual change conditions improved students' motivation significantly in both schools. On the other hand, effect size values imply that this method was more effective on motivation to chemistry lesson in ordinary state high school. In other words, although their conceptual change level is lower than the students in Anatolian high school, they are more motivated to learn chemistry. Specifically, this strategy increased the students' intrinsic goal orientation in ordinary state high school and self-efficacy for learning and performance in Anatolian high school. This supports the idea that students in ordinary high school instructed by the STAD method of cooperative learning participated in the chemistry lesson for challenge, curiosity, and mastery, and actively devoted themselves to the learning process to satisfy their internal needs for improving their own competency. Moreover, the students in Anatolian high school had a higher perception of their ability to perform a task and expectancy for success than the students taught traditionally. Courtney et al. (1992) stated that cooperative learning improves low-achieving students' level of achievement and self-esteem. Heterogeneous composition of groups provides students with the feeling of being empowered as a result of group support and the pooling of skills. Also, intrinsic motivation of the students is improved since most of them think that working together is more enjoyable than working individually. Courtney et al. (1992) also declared that a student's self-efficacy increases through repeated experiences of success with specific tasks. Schunk (1985) claimed that self-efficacy increases when students are provided with feedback on their progress toward mastery. Students focus on the mastery of a task instead of relative success or failure in comparison to their group mates in a cooperative learning environment (Crooks, 1988). Task mastery is strongly related to self-efficacy and intrinsic motivation (Ames, 1984). In this sense, high-motivation students were willing to make conceptual change by actively solving their conflict while low-motivation students did not take an interest in learning events and passively waited teacher to provide the answers, as a result, poor conceptual change was observed.
The findings of the present study concerning the improvement in motivation were supported by some research studies (Bryan et al., 2011; Blaney et al., 1977, as cited in Sharan, 1980; Courtney et al., 1992; Hancock, 2004; Nicholes, 1996). On the other hand, this study indicated that cooperative learning did not affect extrinsic goal orientation, task value, control of learning beliefs and test anxiety. This result might be because of the fact that the implementation period for cooperative learning was only six weeks. Thus, this limited time may not be enough for participants to be aware of the usefulness or the importance of the task and to develop expectancy for positive outcomes with their own efforts instead of those of the teacher or another external factor. Furthermore, since the present study revealed that students participated in the chemistry lesson for challenge, curiosity, and mastery which imply intrinsic goal orientation instead of grades or evaluation by others that denote extrinsic goal orientation (Garcia et al., 1991), it can be concluded that cooperative learning may have had no significant effect on extrinsic goal orientation. Correspondingly, students having high extrinsic goal orientation concern issues other than those directly related to participating in the task itself (Garcia et al., 1991). In addition, students may worry about their performance on exams, which indicates test anxiety because the method was totally new for them and they were administered more tests than their ordinary classroom. In fact, though it was not statistically significant, post-MSLQ scores of the experimental group students in both schools were higher than that of the control group students, demonstrating a higher level of test anxiety in the cooperative learning group.
Novelty effect which means increased motivation, interest or engagement of the participants since they are doing something new or different, is a possible external threat for this study (Gay and Airasian, 2000). It can be controlled by conducting the study over a sufficient period of time. However, six weeks may not be enough to control for this threat. Therefore, the experimental group students' performance might be affected because they were involved in an application which is out of ordinary. In addition, experimenter effect where the experimenter may unintentionally influence the procedures, the participants or assessment of their performance is another possible threat for the current study (Gay and Airasian, 2000). At the beginning, the teachers were provided all materials including worksheets for group works, lesson plans, the instruments etc. concerning the experimental classes, and materials including lesson plans, example problems to be discussed in the class and homework sheets concerning the control classes. Also, related documents and lesson plans were reviewed with the teachers in each week for both experimental and control groups. However, since the experimental group instruction was new and unordinary for the teachers, more time was spent with them concerning the application compared to the traditional instruction. Nevertheless, we tried to behave similarly to the participants of each group. Experimental group students were not told that they would learn the concept of reaction rate with a different way compared to the other class to prevent the feeling of receiving special attention. Hence, we expect experimenter effect threat to be controlled. Moreover, treatment diffusion that happens when the treatment groups communicate with and learn different aspects from each other, which may lead overlap of treatments, was controlled by requesting the teachers not to permitting the classes to communicate with and to arrange the lessons of experimental and control groups at different days. Finally, selection-treatment interaction is another potential threat for this study. It mainly occurs when already formed groups (instead of selecting individuals randomly) are used for the experiment because the selected groups may in some important way different from each other (Gay and Airasian, 2000). In our study, the experimental and control group students were compared in some variables such as previous knowledge about reaction rate, motivational characteristics and science process skills before the implementation. They were similar in terms of previous knowledge and motivation. The difference on science process skills were controlled by using covariance analysis. Therefore, selection-treatment interaction is minimized. This study is expected to contribute to chemistry education at high schools and to be helpful for chemistry teachers since it provides evidence for the positive effects of cooperative learning on students' conceptual understanding and motivation. It also provides directions and procedures in the context of chemical kinetics to apply this method, which has been found to be helpful to improve students' understanding in science and their motivation (e.g., Eilks, 2005; Gillies, 2008; Sisovic and Bojovic, 2000; Slavin, 1987). Moreover, considering the fact that very limited studies have been conducted about the effect of cooperative learning on students' motivation in chemistry lessons (Shachar and Ficher, 2004), the present study also contributes to the international literature by reporting the positive effects of cooperative learning on students' motivation to learn chemistry.
To summarize, contributing to the progress of science learning is difficult. Student-centered methods, which provide students with the ability to construct their own knowledge, require more efforts than teacher-centered methods. Presenting a new concept or explaining to the learners that their ideas are wrong, as traditional methods do, does not improve students' understanding of scientific knowledge. However, teaching strategies where the students are actively involved in their learning process promote meaningful learning and motivation.
Appendix A: Some example items of the reaction rate concept test
1. The rate of a chemical reaction is calculated by measuring the amount of substance consumed or produced per unit time.*(I) TRUE (II) FALSE
Reason
*(A) The reaction rate is the change in the concentrations of reactants per unit time at a constant temperature.
(B) The reaction rate is the time required for reactants to form products.
(C) The rate of forward reaction is always equal to the rate of reverse reaction.
(D) The reaction rate is the amount of substance turning into products per unit time at a constant temperature and concentration.
2. A → B + C
The rate equation of the reaction above is found experimentally as V = k[A]0 = k. According to this equation, the rate of this reaction;
(I) increases (II) decreases *(III) is constant as the reaction proceeds.
Reason
*(A) The collision frequency of molecules decreases since the number of A molecules decreases with time.
(B) The amount of products (number of B and C molecules) increases over time.
(C) The interaction between molecules increases as the reaction proceeds.
*(D) The rate of this reaction does not depend on the number of A molecules.
3.
Given the plot for the potential energy versus progress of reaction, which of the following is correct?
(A) A → C is the rate determining step.
(B) B and D are catalysts.
*(C) C is the reaction intermediate.
(D) ΔH > 0 for the reaction of A → E.
(E) The reaction takes place in 3 steps.
*Correct Alternative
Appendix B: Some examples of cooperative groups worksheets
Worksheet 1. Digestion begins with chewing in the mouth. Chewing facilitates swallowing by breaking food into small pieces. It provides salivating and improves the effectiveness of saliva and gastric enzymes. Starchy food starts to be digested in the mouth. With reference to this information, please explain how chewing improves the effect of saliva and gastric enzymes?Worksheet 2. Sedef, Ezgi, Didem and Emrah exemplify the effect of temperature on reaction rate below. Whose examples can be accepted as correct? Please explain why they are correct or incorrect.
Sedef: “Water evaporates faster when heated.”
Ezgi: “Sugar dissolves faster in hot water than in cold water.”
Didem: “Batteries can be utilized longer if they are kept in refrigerator when they are not used.
Emrah: “Food is cooked faster in pressure cooker.”
Worksheet 3. A2 and B2 react to form AB. What are the figures given below imply to you? Explain your ideas by using collision theory.
A2 + B2 → 2 AB
References
- Acar B. and Tarhan L., (2008), Effects of cooperative learning on students' understanding of metallic bonding, Research in Science Education, 38, 401–420.
- Ames C., (1984), Competitive, cooperative, and individualistic goal structure: A cognitive-motivational analysis. In R.E. Ames and C. Ames (ed.), Research on motivation in education: Vol. 1. Student motivation (pp. 177–208). Greenwich, CT: Academic Press.
- Amir R. and Tamir P., (1994), In-depth analysis of misconceptions as a basis for developing research-based remedial instruction: The case of photosynthesis, The American Biology Teacher, 56(2), 94–100.
- Barke H. D., Hazari A. and Yitbarek S., (2009), Misconceptions in chemistry: Addressing perceptions in chemical education. Verlag Berlin Heidelberg: Springer.
- Barbosa R., Jofili Z. and Watts M., (2004), Cooperating in constructing knowledge: case studies from chemistry and citizenship, International Journal of Science Education, 26(8), 935-949.
- Bilgin İ. and Geban, Ö., (2006), The effect of cooperative learning approach based on conceptual change condition on students' understanding of chemical equilibrium concepts, Journal of Science Education and Technology, 15(1), 31–46.
- Blosser P. E., (1975), How to ask the right questions. Washington, DC: National Science Teachers Association.
- BouJaoude S. B., (1991), A study of the nature of students' understanding about the concept of burning, Journal of Research in Science Teaching, 28, 689–704.
- BouJaoude S. B., (1993, April 16–19), Students' systematic errors when solving kinetic and chemical equilibrium problems. Paper presented at the Annual Meeting of the National Association for Research in Science Teaching, Atlanta, USA.
- Bozkoyun Y., (2004), Facilitating conceptual change in learning rate of reaction concepts. Unpublished master's thesis, Middle East Technical University, Ankara, Turkiye.
- Brotherton P. N. and Preece F. W. P., (1995), Science process skills: Their nature and interrelationships, Research in Science & Technological Education, 13(1), 5–11.
- Brown A. L. and Palincsar A. S., (1986), Guided, cooperative learning and individual knowledge acquisition (Tech. Rep. No.372.). Urbana, Illinois University, Center for the Study of Reading.
- Bryan R. R., Glynn S. M. and Kittleson J. M., (2011), Motivation, achievement, and advanced placement intent of high school students learning science, Science Education, 95(6), 1049–1065.
- Chairam S., Somsook E. and Coll R. K., (2009), Enhancing Thai students' learning of chemical kinetics, Research in Science & Technological Education, 27(1), 95–115.
- Chi M. T. H., Slotta J. D. and Deleeuw N., (1994), From things to processes: A theory of conceptual change for learning science concepts, Learning and Instruction, 4, 27–43.
- Cohen E. G., (1994), Restructuring the classroom: Conditions for productive small groups, Review of Educational Research, 64, 1–35.
- Courtney D. P., Courtney M. and Nicholson C., (1992, November 11–13), The effect of cooperative learning as an instructional practice at the college level. Paper presented at the Annual Meeting of the Mid-South Educational Research Association, Knoxville, TN.
- Crooks T., (1988), The impact of classroom evaluation practices on students, Review of Educational Research, 58(4), 438–481.
- Çakmakçı G., (2005), A cross-sectional study of the understanding of chemical kinetics among Turkish secondary and undergraduate students. Unpublished doctoral dissertation, The University of Leeds, Leeds, United Kingdom.
- Çakmakçı G., Leach J. and Donnelly J., (2006), Students' ideas about reaction rate and its relationship with concentration or pressure, International Journal of Science Education, 28(15), 1795–1815.
- Çakmakçı G., (2010), Identifying alternative conceptions of chemical kinetics among secondary school and undergraduate students in Turkey, Journal of Chemical Education, 87(4), 449–455.
- Çakmakçı G. and Aydoğdu C., (2011), Designing and evaluating an evidence-informed instruction in chemical kinetics, Chemistry Education Research and Practice, 12, 15–28.
- DiSessa A. A., (2001), Changing Minds: Computers, Learning, and Literacy. USA: MIT Press.
- Doymuş K., (2007), Teaching chemical equilibrium with the jigsaw technique, Research in Science Education, 38(2), 249–260.
- Driver R. and Easley J., (1978), Pupils and paradigms: a review of literature related to concept development in adolescent science students, Studies in Science Education, 5, 61–84.
- Ebbing D. D. and Gammon S. D., (1999), General Chemistry (6th ed.), USA: Houghton Mifflin Company.
- Eilks I., (2005), Experiences and reflections about teaching atomic structure in a jigsaw classroom in lower secondary school chemistry lessons. Journal of Chemical Education, 82(2), 313–319.
- Felder R. M., (1996), Active-inductive-cooperative learning: An instructional model for chemistry? Journal of Chemical Education, 73(9), 832–836.
- Gage N. L. and Berliner D. C., (1992), Educational psychology. Boston, MA: Houghton Mifflin.
- Garcia T., McKeachie W. J., Pintrich P. R. and Smith D. A., (1991), A manual for the use of the Motivated Strategies for Learning Questionnaire (Tech. Rep. No. 91-B-004). Ann Arbor, MI: The University of Michigan, School of Education.
- Garnett P. J., Garnett P. J. and Hackling M. W, (1995), Students' alternative conceptions in chemistry: A review of research and implications for teaching and learning, Studies in Science Education, 25, 69–95.
- Gay L. R. and Airasian P., (2000), Educational Research: Competencies for analysis and application. New Jersey: Prentice-Hall Inc.
- Geban Ö., Aşkar P. and Özkan İ., (1992), Effects of computer simulated experiments and problem solving approaches on high school students, Journal of Educational Research, 86, 5–10.
- Gijlers H. and de Jong T., (2005), The relation between prior knowledge of students' collaborative discovery learning processes, Journal of Research in Science Teaching, 42, 264–282.
- Gillies R. M., (2008), The effects of cooperative learning on junior high school students' behaviours, discourse and learning during a science-based learning activity, School Psychology International, 29, 328–347.
- Graham, C. D., (2005), Cooperative learning methods and middle school students. Dissertation, Capella University.
- Green S. B., Sulkind N. J. and Akey T. M., (2000), Using SPSS for windows: Analyzing and understanding data (2nd ed.). New Jersey: Prentice-Hall, Inc.
- Gregoire M., (2003), Is it a challenge or a threat? A dual-process model of teachers' cognition and appraisal processes during conceptual change, Educational Psychology Review, 15(2), 147–179.
- Griffiths A. K., (1994), A critical analysis and synthesis of research on students' chemistry misconceptions. In H.-J. Schmidt (Ed.), Proceeding of the international seminar at Dortmund University. Problem solving and misconceptions in chemistry and physics (pp. 70–99). Hong Kong: ICASE.
- Hackling M. W. and Garnett P. J., (1985), Misconceptions of chemical equilibrium, International Journal of Science Education, 7(2), 205–214.
- Haim A., (1989), Catalysis: New reaction pathways, not just a lowering of the activation energy, Journal of Chemical Education, 66, 935–937.
- Hancock D., (2004), Cooperative learning and peer orientation effects on motivation and achievement, The Journal of Educational Research, 97(3), 159–168.
- Harlen W., (1999), Purpose and procedures for assessing science process skills, Assessment in Education, 6, 129–144.
- Hatano G., (1982), Cognitive consequences of practice in culture specific procedural skills, Quarterly Newsletter of the Laboratory of Comparative Human Cognition, 4, 15–18.
- Hodson D., (1992), In search of a meaningful relationship: An exploration of some issues relating to integration in science and science education, International Journal of Science Education, 14, 541–562.
- Horton C., (2007), Student alternative conceptions in chemistry, California Journal of Science Education, 7(2), 1–78.
- İcik H, (2003), Lise II. sınıf öğrencilerinin reaksiyon hızı konusunu kavrama düzeyleri ve kavrama düzeylerine öğrencilerin bilişsel ve duyuşsal özelliklerinin etkisi. [Effect of students' cognitive and affective characteristics on their level of understanding of reaction rate concept at 10th grade]. Unpublished master's thesis, Gazi University, Ankara, Turkiye.
- Inagaki K. and Hatano G., (1983), Collective scientific discovery by young children, The Quarterly Newsletter of the Laboratory of Comparative Human Cognition, 5, 13–18.
- Johnson R. T. and Johnson D. W., (1987), How can we put cooperative learning into practice? The Science Teacher, 54, 46–50.
- Johnson D. W. and Johnson R. T., (1992), Positive interdependence: Key to effective cooperation. In R. Hertz- Lazarowitz and N. Miller (ed.), Interaction in cooperative groups: The theoretical anatomy of group learning (pp. 145–173). New York: Cambridge University Press.
- Johnson D. W. and Johnson R. T., (1994), Learning together and alone: Cooperative, competitive, and individualistic learning (4th ed.). Boston, MA: Allyn and Bacon.
- Johnson D. W. and Johnson R. T., (1999a), Learning together and alone: Cooperative, competitive, and individualistic learning (5th ed.). Boston, MA: Allyn and Bacon.
- Johnson D. W. and Johnson R. T., (1999b), Making cooperative learning work, Theory into Practice, 38(2), 67–73.
- Johnson D. W., Johnson R. T., Holubec E. and Roy P., (1990), Circles of learning (3rd ed.). Edina, MN: Interaction Book Company.
- Johnson D. W. and Johnson R. T. and Smith K., (1991), Cooperative Learning: Increasing College Faculty Instructional Productivity, (Tech. Rep. No. 4). Washington, D.C.: The George Washington University, School of Education and Human Development.
- Kıngır S. and Geban Ö., (2006, November 22–24), Analysis of students' misconceptions in rate of reaction. Paper presented at the International Science Education Conference, Singapore..
- Kind, V., (2004), Beyond Appearances: Students' misconceptions about basic chemical ideas (2nd ed.). Retrieved February 10, 2012, from http://www.rsc.org/images/Misconceptions_update_tcm18-188603.pdf.
- Kousathana M. and Tsaparlis G., (2002), Students' errors in solving numerical chemical-equilibrium problems, Chemistry Education: Research and Practice in Europe, 3(1), 5–17.
- Limon, M., (2001), On the cognitive conflict as an instructional strategy for conceptual change: A critical appraisal, Learning and Instruction, 11, 357–380.
- Lindner, J., (1998), Understanding employee motivation, Journal of Extension, 36(3), 37–41.
- Miyake N., (2008), Conceptual change through collaboration, In S. Vosniadou (Ed.), International handbook of research on conceptual change (pp. 453–478). New York: Routledge.
- Mori J., (2002), Task design, plan and development of talk-in-interaction: An analysis of small group activity in a Japanese language classroom, Applied Linguistics, 23, 323–347.
- Nakiboğlu C., Benlikaya R. and Kalın Ş., (2002, September 16–18), Kimya öğretmen adaylarinin “kimyasal kinetik” ile ilgili yanlış kavramalarının belirlenmesinde v-diyagraminin kullanilması. [Use of V-diagrams to identify pre-service chemistry teachers' misconceptions related to chemical kinetics]. Paper presented at V. National Science and Mathematics Conference, Ankara, Turkiye.
- Nicholes J. D., (1996), The effects of cooperative learning on student achievement and motivation in a high school geometry class, Contemporary Educational Psychology, 21(4), 467–476.
- Okey J. R., Wise K. C. and Burns J. C, (1982), Test of Integrated Process Skills (TIPS II). Athens: University of Georgia, Department of Science Education.
- Ormrod J. E., (1999), Human learning (3rd ed.). Upper Saddle River, NJ: Prentice Hall.
- Piaget J, (1985), The equilibrium of cognitive structure. Chicago: Chicago University Press.
- Pintrich P. R., Marx R. W. and Boyle R. A., (1993), Beyond cold conceptual change: The role of motivational beliefs and classroom contextual factors in the process of conceptual change, Review of Educational Research, 63(2), 167–199.
- Pintrich P. R. and Schrauben B., (1992), Students' motivational beliefs and their cognitive engagement in classroom academic tasks. In D. Schunk and J. Meece (ed.), Student perceptions in the classroom (pp. 149–183). Hillsdale, NJ: Lawrence Erlbaum Associates.
- Pintrich P. R. and Schunk D. H., (1996), Motivation in education. Theory, research, and applications. Englewood Cliffs, NJ: Prenctice Hall.
- Pintrich P. R., Smith D. A. F., Garcia T. and McKeachie W. J., (1991), A Manual for the use of the Motivated Strategies for Learning Questionnaire (MSLQ) (Tech Rep. No. 91-B-004). Ann Arbor, MI: National Center for Research to Improve Postsecondary Teaching and Learning, The University of Michigan.
- Pintrich P. R., (2003), A motivational science perspective on the role of student motivation in learning and teaching contexts, Journal of Educational Psychology, 95, 667–686.
- Posner G. J., Strike K. A., Hewson P. W. and Gertzog W. A., (1982), Accommodation of a scientific conception: toward of conceptual change, Science Education, 66, 211–227.
- Qin A., Johnson D. W. and Johnson R. T., (1995), Review of Educational Research, 65, 129–143.
- Schunk D., (1985), Self-efficacy and classroom learning, Psychology in the Schools, 22, 208–223.
- Shachar H. and Ficher S., (2004), Cooperative learning and the achievement of motivation and perceptions of students in 11th grade chemistry classes, Learning and Instruction, 14, 69–87.
- Sharan S., (1980), Cooperative learning in small groups: Recent methods and effects on achievement, attitudes, and ethnic relations, Review of Educational Research, 50(2), 241–271.
- Sinatra G. and Mason L., (2008), Beyond knowledge: Learner characteristics influencing conceptual change. In S. Vosniadou (Ed.), International Handbook of Research on Conceptual Change (pp. 560–582). New York: Routledge.
- Sisovic D. and Bojovic S., (2000), Approaching the concepts of acids and base by cooperative learning, Chemistry Education: Research and Practice in Europe, 1(2), 263–275.
- Slavin R. E., (1977), Classroom reward structure: An analytic and practical review, Review of Educational Research, 47, 633–650.
- Slavin R. E., (1983), Cooperative learning. New York: Longman.
- Slavin R. E., (1987), Development and motivational perspectives on cooperative learning: A reconciliation, Child Development, 58, 1161–1167.
- Slavin R. E., (1995), Cooperative learning: Theory, research, and practice (2nd ed.). Boston: Allyn & Bacon.
- Solomon J, (1992), Getting to know about energy in school and society. London: Falmer Press.
- Stevahn L., Johnson D., Johnson R., Green K. and Laginski A., (1997), Effects On high school students of conflict resolution training integrating into English literature, Journal of Social Pschology, 137, 302–315.
- Strawitz B. M., (1989), The effects of testing on science process skill achievement, Journal of Research in Science Teaching, 26(8), 659–664.
- Strike K. A. and Posner G. J., (1985), A conceptual change view of learning and understanding. In L. H. T. West and A. L. Pines (ed.), Cognitive Structure and Conceptual Change (pp. 211–231), London: Academic Press Inc.
- Strike K. A. and Posner G. J., (1992), A revisionist theory of conceptual change. In R. A. Duschl and R. J. Hamilton (Eds). Philosiphy of Science, Cegnitive Psychology, and Educational Theory and Practice. Albany, N.Y: State University of New York Press.
- Sungur S., (2004), The implementation of problem based learning in high school biology courses. Unpublished doctoral dissertation, Middle East Technical University, Ankara, Turkey.
- Taber K. S., (2009), Progressing Science Education: Constructing the scientific research programme into the contingent nature of learning science. Dordrecht: Springer.
- Tao P. K. and Gunstone R. F., (1999), Conceptual change in science through collaborative learning at the computer, International Journal of Science Education, 21(1), 39–57.
- Thagard P., (1992), Conceptual Revolutions. Oxford: Princeton University Press.
- Treagust D. F., (1987), Development and use of diagnostic tests to evaluate students' misconceptions in science, International Journal of Science Education, 10(2), 159–169.
- Tsai C., (2000), Enhancing science instruction: the use of ‘conflict maps’, International Journal of Science Education, 22, 285–302.
- Vosniadou S., (1992), Knowledge acquisition and conceptual change, Applied Psychology: an International Review, 41, 347–357.
- Vosniadou S., (2001), What can persuasion research tell us about conceptual change that we did not already know? International Journal of Educational Research, 35, 731–737.
- Vosniadou S., (2007), Conceptual change and education, Human Development, 50(1), 47–54.
- Vosniadou S, (Ed.), (2008), International Handbook of Research on Conceptual Change. London: Routledge.
- Vosniadou S. and Ioannides C., (1998), From conceptual development to science education: A psychological point of view, International Journal of Science Education, 20(10), 1213–1230.
- Vosniadou S., Ioannides C., Dimitrakopoulou A. and Papademetriou E., (2001), Designing learning environments to promote conceptual change in science, Learning and Instruction, 11, 381–419.
- Vosniadou S., Vamvakoussi X. and Skopeliti I., (2008), The framework theory approach to the problem of conceptual change. In S. Vosniadou (Ed.), International handbook of research on conceptual change, (pp. 3–34). New York, NY: Routledge.
- Voska K. W. and Heikkinen H. W., (2000), Identification and analysis of student conceptions used to solve chemical equilibrium problems, Journal of Research in Science Teaching, 37(2), 160–176.
- Vygotsky L. S., (1981), The genesis of higher mental functions. In J. V. Wertsch (Ed.), The concept of Activity in Soviet Psychology (pp. 144–188). White Plains, NY: Sharpe.
- Zohar A. and Aharon-Kravetsky S., (2005), Exploring the effects of cognitive conflict and direct teaching for students of different academic levels, Journal of Research in Science Teaching, 42(7), 829–855.
Footnote |
| † A part of this study was presented at XIV. Symposium of the International Organization for Science and Technology Education (IOSTE) 2010, Bled, Slovenia, June 13–18. |
| This journal is © The Royal Society of Chemistry 2012 |


