A continuum of learning: from rote memorization to meaningful learning in organic chemistry
Nathaniel P.
Grove
*a and
Stacey
Lowery Bretz
b
aDepartment of Chemistry and Biochemistry, University of North Carolina Wilmington, Wilmington, NC 28403, USA. E-mail: groven@uncw.edu
bDepartment of Chemistry and Biochemistry, Miami University, Oxford, OH 45056, USA
First published on 6th March 2012
Abstract
The Assimilation Theory of Ausubel and Novak has typically been used in the research literature to describe two extremes to learning chemistry: meaningful learning versus rote memorization. It is unlikely, however, that such discrete categories of learning exist. Rote and meaningful learning, rather, are endpoints along a continuum of learning. This paper reports the results of a qualitative investigation of students' experiences in a spiral, organic chemistry curriculum, specifically describing additional positions along the learning continuum between meaningful learning and rote memorization.
Introduction
Much of life can be understood in rational terms if expressed in the language of chemistry. It is an international language, a language for all of time, and a language that explains where we came from, what we are, and where the physical world will allow us to go. Chemical language has great aesthetic beauty and links the physical sciences to the biological sciences. Arthur Kornberg (Kornberg, 1987).The above quote from Arthur Kornberg, the Nobel Prize-winning biochemist, speaks eloquently to the important role that chemistry plays in unifying the sciences. Although the sciences have changed a great deal during the last several decades, a recent co-citation analysis by Balaban and Klein (2006) continues to support the idea of chemistry as the “central science.”
Similar to more traditional classification schemes (for example, see the work of Auguste Comte (1830)), the relationship among the mathematics and physical sciences is linear with mathematics serving as the framework for physics and physics in turn serving as the framework for chemistry. This model differs from older arrangements, however, in suggesting that chemistry serves as the underlying structure for not only the biological sciences but also the xeno-sciences (cosmology, astronomy, and planetary science) and the nano-sciences. As a result of their analysis, Balaban and Klein suggest that chemistry as a discipline is presently in the midst of an internal realignment—a realignment that has thrust organic chemistry and biochemistry center stage as the predominant nexus points among the sciences. Organic chemistry is a rich, vibrant discipline and given its central importance to science, it is imperative that students develop a sound, conceptual understanding of the subject.
The teaching and learning of organic chemistry
Organic chemistry has garnered a reputation for being a “killer course” with attrition rates at some universities ranging as high as 30–50% (Grove et al., 2008). Because of these high attrition rates, and the essential role that organic chemistry plays in the science curriculum as noted above, numerous curricular reforms have been proposed and implemented in an effort to better support student learning and success (see Grove et al., 2008 for an extensive listing of these reforms). Concurrently, several research studies have identified topics that present students with particular difficulties in organic chemistry. Such studies have included investigations regarding the use of the curved-arrow notation to convey electron flow during mechanistic processes (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008; Grove et al., 2012a; Grove et al., 2012b), the construction and perceived utility of structural representations (Cooper et al., 2010), the essential features of organic acids and bases (Cartrette and Mayo, 2011), and students' meaningful understanding of organic chemistry reactions (Vachliotis et al., 2011). While it is undeniable that these studies provide value for the practitioner, we believe that more holistic accounts of students' experiences in organic chemistry are also of value. Our previous work in this area (Grove and Bretz, 2010) focused on understanding student difficulties in learning organic chemistry through the lens of Perry's scheme of intellectual and ethical development. This work re-examines the same set of data, this time analyzed through the lens of the Assimilation Theory of Ausubel and Novak.The Assimilation Theory of Ausubel and Novak: meaningful and rote learning
For years, educational psychology was dominated by the work of behaviorists such as B. F. Skinner whose theory of learning advocated that all knowledge, and any subsequent behavior associated with it, was ultimately of external origin. In other words, the learner was viewed as a blank slate—devoid of any preconceived ideas—passively waiting for some external source to imprint new knowledge upon the mind. While such theories may sufficiently explain why dogs salivate at the ringing of a bell, they fail to adequately explain the complex interactions that occur in most educational settings.Consider, for example, chemical misconceptions—a subject of intense interest in the chemistry education research community for the last several decades (Driver and Easley, 1978; Driver, 1983; Treagust, 1988; Bodner, 1991; Nakhleh, 1992; Mulford and Robinson, 2002; Galley, 2004). Some misconceptions are now known to be didaskalogenically induced (Cooper et al., 2010), i.e., they are misconceptions that arise as a direct result of instruction. For example, introductory chemistry textbooks frequently depict atoms with fixed electron orbits and many biology or biochemistry textbooks suggest that energy is released when bonds are broken. Other misconceptions, however, do not appear to stem from direct instruction. Certainly, students are never taught that the bubbles in boiling water consist of hydrogen and oxygen gas, yet a significant minority believes this to be the case (Bodner, 1991). Clearly chemistry students do not enter classrooms as “blank slates,” nor is the knowledge of chemistry experts transferred intact from one individual to the next. With regard to explaining the challenges of learning chemistry, the Assimilation Theory of Ausubel and Novak (Ausubel, 1968; Novak, 1977; Novak, 1998; Bretz, 2001) offers a more powerful framework.
In the preface to Educational Psychology: A Cognitive View, author David Ausubel wrote (Ausubel, 1968), “If I had to reduce all of educational psychology to just one principle, I would say this: The most important single factor influencing learning is what the learner already knows. Ascertain this and teach him [sic] accordingly.” This profound statement speaks to the central role of prior knowledge in Assimilation Theory. According to Ausubel and Novak, the learner is anything but a blank slate, instead, bringing a diverse collection of prior ideas to their study of chemistry. We previously reported how prior knowledge, especially as it relates to the nature and scope of chemistry, impacted students' learning of organic chemistry (Grove and Bretz, 2010). Meaningful learning will occur when three key requirements are satisfied:
1. As alluded to in the quote from Ausubel, students must first possess relevant prior knowledge with which to situate and anchor the new knowledge.
2. The knowledge to be learned in and of itself must be perceived by the student as “relevant to other knowledge and must contain significant concepts and propositions” (Novak, 1998).
3. The learner must choose to learn meaningfully. That is, the learner must “consciously and deliberately choose to relate new knowledge to knowledge the learner already knows in some nontrivial way” (Novak, 1998).
In cases where one or more of these conditions is not met, students may use rote learning techniques. That is, rather than connect the new knowledge in any substantive manner to the pre-existing cognitive structure, the learner chooses to simply memorize information.
The contrast between meaningful and rote learning would suggest that these two approaches are opposites, and indeed it is presented as such in many published research studies (Clary and Wandersee, 2007; Tsai and Wen, 2005; Lewis et al., 2009). However, it is important to note that purely meaningful or rote learning does not exist and that the idea of a dichotomy ought to be more appropriately discussed as that of a continuum (Novak, 1977):
Except perhaps in a newborn infant, absolute rote learning probably never occurs. […] It is very important to recognize that rote → meaningful is a continuum and not a dichotomy. This is a fundamental idea, since we doubt if any school learning occurs in an absolutely rote fashion. The real issue in school learning is not whether new information will be learned meaningfully or by absolute rote; the problem centers on the extent of meaningfulness in new learning. (p. 80)
Research goal
We believe that many of the difficulties that students encounter in learning organic chemistry ultimately stem from an over-reliance on rote memorization without using more meaningful techniques. The research reported herein was conducted as part of a larger study to better understand the difficulties that students encountered in learning organic chemistry in the context of a spiral curriculum. Specifically, this research sought to understand the learning strategies that students employed to learn organic chemistry and to document how those strategies evolved throughout the course. This paper expands upon our previously published work (Grove et al., 2008; Grove and Bretz, 2010) by describing additional positions along the continuum between meaningful learning and rote memorization. Just as organic chemists study the transition states and mechanism of a new chemical reaction in order to optimize its use in the lab, this research study sought to characterize the “transition states” and “mechanism” by which students move among the various positions on the meaningful/rote learning continuum, in hopes of identifying possible strategies to help students transition toward more meaningful techniques while learning organic chemistry.Methodology
The research context: the spiral, organic chemistry curriculum at Miami University
In order to assist readers with determining the transferability of the results of this inquiry to their own classrooms and students, a detailed description (Geertz, 1973) of the educational environment is provided. This study was conducted in the context of a two-semester spiral, organic chemistry sequence (a detailed description of this implementation can be found in Grove et al., 2008), largely populated by undergraduates who intend to pursue advanced professional degrees, for example, in medicine or dentistry. Each lecture course is three credit hours and accompanied by a two-credit laboratory course. More than 75% of the students enrolled in these courses are second year undergraduates, and all have completed at least one year of general chemistry either at the university-level or through advanced placement credit from a high school chemistry course.Although the spiral curriculum retained functional groups as an organizational scheme, the level of detail associated with the initial introduction of each group is minimized. During the first semester students learn how to name organic compounds, discuss defining physical and chemical properties of the different types of organic compounds, and explore basic mechanistic processes for the reactions of alkanes and cycloalkanes, alkenes, alkynes, alkyl halides, alcohols, benzene and benzene derivatives, amines, aldehydes, ketones, and carboxylic acids and their derivatives. During the second semester, students revisit these major categories of organic compounds, focusing this time around on more advanced, current reactions, mechanisms, and synthetic strategies.
During lecture, the instructor expanded upon ideas using online notes that students could download before class and provided additional examples to clarify salient points. Students were assessed during both semesters based upon their performance on four, one hour exams (worth 66% of the course grade), a comprehensive final (worth 17% of the course grade), and online homework assignments that students were required to complete outside of class (worth 17% of the course grade).
Data collection
Rather than merely describe the difficulties that students encountered in learning organic chemistry, we sought to better understand the reasons why students had the difficulties that they did. Therefore, we used a qualitative, grounded theory approach (Creswell, 2007; Corbin and Strauss, 2008). Given the nature of qualitative research, the 18 participants in our study were purposefully selected (Patton, 2002) to ensure diversity in gender (10 females, 8 males), ethnicity (10 white/Caucasian, 8 minority), academic level (1 first year, 11 second years, 3 third years, 3 fourth years), and academic performance in general chemistry (8 high performing, 5 average performing, 5 low performing). Students were selected from a pool of volunteers and were compensated $25 for each semester that they participated in the study. All phases of the research were reviewed and approved by Miami University's Institutional Review Board. All research participants were provided with information detailing their rights as human subjects; informed consent was obtained from all of the participants. Throughout this report, pseudonyms are used in place of the students' real names.Data were collected through reflective essays and interviews. Throughout the year-long study, participants were asked to respond to three sets of essay questions. These questions initially focused on exploring their goals and expectations for learning organic chemistry, while later asking the students to reflect upon their experiences after completing both courses. These essay questions were administered to students via email at the start of organic chemistry I, after completing organic chemistry I, and after completing organic chemistry II. Responses were submitted using a secure online form. Semi-structured interviews (Patton, 2002; Bretz, 2008) were conducted with students twice during the study—at midterm in both fall and spring semesters—and focused on exploring topics from the reflective essays in more detail. Specifically, the students were asked to comment on how they approached learning organic chemistry and how they prepared for class, quizzes, and exams. This provided an opportunity to explore the students' views about what they felt was successful about their study habits, what was not, and the reasons for using such approaches. Further, students were asked to describe any changes to their studying throughout the year and the impetus for those changes.
Research design: towards a grounded theory approach
Both the students' responses to the essay prompts and the interview transcripts were analyzed for themes and trends. Subsequent interviews and essay prompts were modified to capture opportunities to better understand these emerging trends. Accordingly, our findings were fundamentally “grounded” in the data and continually refined as more data was collected. We began initially by grouping students with similar approaches to learning organic chemistry. During our analysis, we noted many similarities between these emerging approaches and the Assimilation Theory of Ausubel and Novak. As such, we postulated that Assimilation Theory could potentially provide a useful framework for understanding the approaches that students used to learn organic chemistry. At the same time, it is important to note that a fully realized, grounded theory study was not possible. As will be expounded upon later in this report, the theoretical model developed based upon students' experience suggests additional student groupings. Because of time and structural limitations, it was not possible to explore these hypothesized groups, and therefore, the study likely did not reach saturation. This does not necessarily invalidate the results of this research nor the theoretical model developed; however, it does point to the need for continued research in this area.Data analysis was further informed by the first author's experiences in directly working with the professor and students of the course. During the fall semester, he observed the classroom setting at least once a week and assisted the course professor in grading the hour-long exams. In his capacity as teaching associate, he taught three sections of the laboratory portion of the course during the spring semester. The use of such tacit knowledge (Lincoln and Guba, 1985) was vital in shaping the collection and analysis of data. (None of the students who participated in this research were assigned to the first author's laboratory sections nor did he have any influence over their grades.)
Finally, we must comment on issues surrounding the trustworthiness of the results. These issues are important to all research; yet, the conventional trustworthiness criteria of reliability and validity are not appropriate for most qualitative research. Rather, Lincoln and Guba argue that the criteria of transferability, credibility, dependability, and confirmability are more appropriate measures (Lincoln and Guba, 1985). It is beyond the scope of this manuscript to describe these measures in detail; however, member checks, overlapping methodological approaches, and triangulation were all employed to address these trustworthiness criteria (Kirk and Miller, 1986; Denzin, 2006; Oliver-Hoyo and Allen, 2006; Bretz, 2008).
Results
The experiences of 12 of the 18 students were included in the final analysis; the remaining participants either did not complete all required research activities or dropped out of the inquiry mid-study. Analysis of the results suggested that the participants of this study could be identified as belonging to one of four groups with regard to their learning: Meaningful Learners, Transitional Learners, Unaware Learners, or Indifferent Learners. These four groups are described in more detail below using the students' own experiences and words.Group A. Meaningful Learners: Students recognize that developing a sound, conceptual understanding of organic chemistry requires a meaningful approach and are generally using such techniques.
Four students—Charlie, Ginny, Lily, and Luna—were characterized as meaningful learners based upon their epistemological views of learning organic chemistry. While it would be disingenuous to say that these students were unconcerned with their grades, it was striking how important it was for them to leave the course with a sound, conceptual understanding of the material. As Ginny remarked during her fall interview, it was important that she “actually learn for the process of learning, not just for an exam.” Tied to this strong commitment was a belief that organic chemistry was essential to their development as future professionals. For example, Charlie felt that organic chemistry would provide him with a foundation to eventually understand
…the natural functions of life…at a very basic level. I mean organic chemistry—those sorts of reactions are played out every second of your life. Within your body, different sorts of reactions and so understanding those at a basic, molecular level…you can build on that sort of understanding, like more complex things that your body does that you might actually use as a doctor…if there's some disease that causes certain proteins not to synthesize, is that because of some chemical reaction that isn't occurring?
Luna echoed these thoughts when she said that “[o]rganic chemistry is the study of carbon, which is the basis of life…Understanding the chemical reactions that occur in medicine, their effects, and why it occurs is crucial.” Although not quite sure how organic chemistry would specifically prepare her to be a dentist, Ginny was willing to accept at face value that the relevance of organic chemistry eventually would become apparent.
These students' commitments to learning in a meaningful manner also influenced how they approached studying for the course. In describing her typical study routine, Lily frequently relied upon the use of flashcards. While more commonly associated with simple memorization—and indeed, Lily did use them for this purpose when she needed to remember large amounts of factual information such as lists of protic or aprotic solvents—Lily used her flashcards in an active manner by asking herself questions about the concepts on the cards and by exploring the connections among them. In other words, Lily used her flashcards to connect the information she was studying. George frequently created his own study guides to synthesize the important concepts from the textbook and lecture notes while Luna made sure that she could explain key concepts to her study partner in her own words. Afterwards, she and her study partner created reaction/synthesis problems for each other and exchanged them for additional practice. Although these students prepared for quizzes and exams quite differently from one another, they all shared a common perspective on learning, i.e., they ultimately focused upon internalizing course material to make key concepts more personally meaningful.
An integral component of each of these meaningful techniques was frequent, strategic meta-cognition. For example, George typically began his daily studying by reviewing what he had covered the previous day and asking, “Okay, what did I keep? What did I retain from my studying yesterday and what type of material do I need to keep going over?” Ginny kept a running list of concepts she did not understand and used the list to frame the conversations she had with the course professor or her organic chemistry tutor. She also kept track of what she gauged to be “good questions,” i.e., those questions that she thought might be similar to those on the exam. Luna did something similar, taking it one step further and using her list as a guide when she created additional practice problems for her study partner.
Group B. Transitional Learners: Students recognize that developing a sound, conceptual understanding of organic chemistry requires a meaningful approach and are in the process of changing (or trying to change) to the use of more meaningful techniques. Because these students have not yet fully achieved this transition, they may occasionally decide to use rote memorization techniques, especially when a more meaningful approach is not apparent, or during periods of stress.
Of the 12 students included in the final analysis, Hannah, Marietta, and Parvatti, were three students who could best be described as Transitional Learners. Unlike their classmates in Group A, these students were less certain about organic chemistry's relevance to their chosen professions. Although Marietta felt that organic chemistry served as “the basis for a lot of medicine,” neither Hannah nor Parvatti were sure why they were required to study the subject. In conversations with future colleagues, Hannah said that
…everyone I've talked to, you know, that works with animals or…even in research, they're like, “yeah, we had to take [organic chemistry] but we never use it.” No one has really explained why zoology majors need organic chemistry.
Hannah's comments were all the more interesting given that she was repeating the course for the second time. Parvatti's views, based mainly on conversations she had had with a number of doctors, were similar. In fact, she described several “arguments” she had had with friends in the class:
…they [referring to her friends] say that we need to take organic chemistry because it's really important. You have to understand the basis of what's going on—the reactions. I can see that. I know everything is organic and all of that, but the detail of reactions we do in class…I just can't understand why I need to do that for medical school.
Parvatti was ultimately able to justify her study of organic chemistry given that the material would eventually appear on the Medical College Admission Test.
While Transitional Learners were not initially focused on concepts and meaningful learning at the beginning of their study of organic chemistry, they did undergo significant changes in their approach to learning over the year and transitioned to more meaningful approaches. Consider, for example, the changes that occurred in Parvatti's approach to her study of organic chemistry:
Parvatti (talking about her experiences during the spring semester): There are three of us—we study together…we quiz each other back and forth, back and forth. We'll be walking to class or eating and we'll just turn around and like randomly quiz each other so you're like put on the spot and you have to think about it. So, we do that and then…we go over [the professor's] notes and we always study in that group—just the three of us. We go over his notes so if someone doesn't understand something, usually the other person has the answer and then we do the [online homework]. We make sure we understand the [online homework] back and forth…we go over the practice exam separately and then we go over it together, the note cards, and his notes…and quizzing each other really helps. We've done that for every exam since the first and it's worked really well.
Interviewer: So, how does that compare then to, to what you did last semester because didn't you study in a group last semester as well?
Parvatti: Yeah, we did. We kind of studied on our own and just looked at each other. We never actually took the time to sit down for hours and quiz each other and go over things together to see whether we really understood or asked each other questions. We kind of just sat in the same room and studied together. We didn't really interact that much as we did this semester
Parvatti described the impetus for this change as a deep-seated dissatisfaction with her performance during the first semester and her desire to improve her understanding of the course material, and as a consequence, her grade.
This notion of dissatisfaction also served as the catalyst for the changes made by Hannah and Marietta during the course of the academic year. It is important to note, however, that the process of transition was not always smooth for students and was frequently characterized by false starts and backtracking. In particular, Marietta found it difficult to develop an approach to learning organic chemistry that satisfactorily met her needs. After a disappointing first exam during the fall semester, Marietta realized that she could not simply memorize, but instead had to develop a more conceptual understanding of the material. Despite this early recognition, Marietta struggled with how to actually learn concepts, even at the end of the spring semester. When it was not immediately clear to her how to best proceed, or in instances when she felt pressed for time, Marietta reverted back to memorization despite the fact that she knew she ought not to:
Interviewer: Is there anything that you feel is ineffective or needs improvement in what you do to learn organic chemistry? [referring to her experiences during the spring semester]
Marietta: Maybe too much memorization…where in all of the stuff you're memorizing, you forget, you know, the concept behind it. That's probably something that I do because it's a lot easier to memorize and say, “Ok, I know what this is” but what does it do? “Oh shoot. I don't know.” Honestly, though, sometimes it's hard to maybe find the time I need to learn the concept and it's just easier to memorize and worry about really learning the concept later.
Group C. Unaware Learners: Unaware students generally use rote memorization techniques to learn organic chemistry and are not cognizant of the fact that more meaningful techniques exist.
The third position on the continuum, labeled as Unaware Learners, included Angelina, George, and Katie. All three students were characterized by their dependence upon rote memorization techniques, coupled with a lack of recognition that more meaningful approaches to learning were possible. Furthermore, these students were also unsure why organic chemistry was a required course for their major and/or held unrealistic beliefs as to what they would learn from it. Katie predicted that organic chemistry would eventually enable her to better understand what differentiated living organisms from non-living objects. As the year progressed, however, and this perspective failed to materialize, she found it increasingly difficult to maintain her interest in the course. Both Angelina and George felt that the course was unnecessary, and in the words of George, “hyped up a bit more than it should be.”
In describing his approach to learning organic chemistry, George admitted that he typically did little to prepare for the course until the evening before the exam when he would “start looking at the stuff heavier.” Given the sheer amount of material that he was responsible for learning, he usually ended up “cramming.” While George realized that this approach was not effective (and that was certainly reflected in his grades), it never occurred to him to do anything differently. When asked about these difficulties during his spring semester interview, George refused to take personal responsibility for his own learning. Instead, he blamed his poor performance on the fact that he was not an organic chemistry “wiz.”
During the fall semester, Katie employed a strategy similar to George's, i.e., waiting until a day or two before the exam and then simply memorizing as much material as she could. Although Katie indicated at the end of the fall semester that she intended to start her preparations for exams and quizzes sooner in the spring, she continued to rely upon memorization. In other words, although she intended to change the frequency and duration of her preparations, her approach still focused on memorization of the material rather than the development of a conceptual understanding. Much like George, Katie—even at the end of the spring semester—had not considered any other approach to learning organic chemistry except pure memorization:
Katie (talking about her experiences during the spring semester): …there was a lot to memorize and I didn't take enough time to memorize everything well enough as I learned it. I tried to memorize it all too close to the exams…I was unsuccessful trying to cram for exams.”
Group D . Indifferent Learners: Indifferent learners generally use rote memorization techniques to learn organic chemistry despite the knowledge that more meaningful ways to learn exist.
Much like the Unaware Learners described above, the Indifferent Learners relied upon rote memorization techniques to learn organic chemistry. What differentiated these two groups of students, however, was the recognition by Indifferent Learners that more meaningful approaches did exist, yet they chose not to use them. Ernie and Fred were two such students.
When starting organic chemistry, Ernie recognized that it would be a very time intensive course that would require he spend a little bit of time preparing each day:
Ernie: …it's just a matter of kids applying themselves and like taking the time to study the material. I don't think the course material is difficult and I don't believe that it's impossible. It's not impossible to get an A.
Interviewer: So, a lot of people don't do that well and a lot of people say it's very difficult. What do you think is the biggest problem for them?
Ernie: They just don't take the time to…like study, do the problems, and go to the review sessions. I mean, it's their own fault!
Paradoxically, this was not the approach that Ernie took in his own preparations, especially during the spring semester when he had particular trouble keeping up with the increased pace and difficulty of the course. By that point in the semester, however, Ernie was firmly set in his ways and refused to modify his approach to learning organic chemistry.
In reflecting upon the mistakes that he had made during the fall semester, Fred felt that he had not spent enough time working with the material. Further, the time he had spent focused primarily on “memoriz[ing] as many example problems as I could in hopes that I would see the same or similar problems on exams.” He realized this approach was not particularly effective as it left him with little understanding of “how and why reactions took place.” Although he indicated at the end of the fall semester that he intended to correct these deficiencies in his study habits, no changes actually materialized:
Interviewer: So what changes, if any, have you made in how you study since last semester?
Fred: I think none.
Interviewer: I guess I'm curious then…you said that if you had made any mistakes [during the fall semester] that it was that you hadn't spent enough time on things. So you recognize the fact that that was a problem but you haven't changed it. So why do you think that is?
Fred: Well, I think it's kind of like a vicious cycle that I really don't enjoy organic chemistry at all so then I don't want to spend more time with it and so I don't get any better at it so I enjoy it less and it just keeps compounding and getting worse.
Discussion
Ausubel and Novak's Assimilation Theory emphasizes the central role of the learner in constructing knowledge. That is to say, relevant prior knowledge and meaningful curriculum are necessary, but not sufficient, conditions for learning to take place (Bretz, 2001). The learner must consciously choose to learn meaningfully by seeking connections and not default to rote memorization. Although the results of this inquiry suggest that students' experiences in organic chemistry are influenced by a complex set of factors, the students' approaches to learning organic chemistry can be depicted using a model that characterizes the four groups along two dimensions (Fig. 1). The first dimension describes the extent to which students actually use rote or meaningful learning techniques. The second dimension describes the students' meta-cognitive awareness. Using this model, the Meaningful Learners (Group A) exist within quadrant I, quadrant III contains the Unaware Learners (Group C), and the Indifferent Learners (Group D) are in quadrant II. The Transitional Learners of Group B can theoretically exist within any of the four quadrants; however, the defining feature of this group is that the students exist in a state of flux and are on a trajectory towards quadrant I.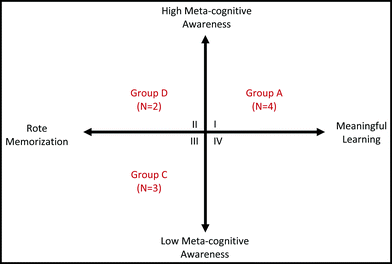 | ||
| Fig. 1 Theoretical model describing students' approaches to learning organic chemistry. | ||
In looking at the learning strategies that the students developed and chose to use, two patterns emerged: (1) strategies were successful only if they were integrated into the students' everyday studying routine and (2) the most successful strategies were those that actively engaged the students in their learning of organic chemistry. Successfully adapting to constantly evolving challenges required students to remain flexible and cognizant of the need to modify their learning strategies during the year. As such, it was important for students to self-monitor their learning and to actively and regularly reflect upon which strategies worked and which did not. The results of this study strongly suggest that meaningful learners, and those who are making the transition to more meaningful techniques, are particularly meta-cognitive in their approach to learning. This thesis is fundamentally important as recognition of more meaningful learning techniques emerged as the defining feature between Unaware Learners and Indifferent Learners.
One of the more significant ideas to emerge from analysis of the experiences of the organic chemistry students in this research study was the perceived relevancy of the course. Katie, Marietta, and Parvatti were not certain why organic chemistry was relevant. They were, however, able to convince themselves that it would become apparent at some point in the future, or that the material would be an important component of the professional school exams that many of the students were required to take. As time passed and this relevance did not emerge, their belief in the importance of organic chemistry diminished, thereby simultaneously eroding one of the three necessary requirements for meaningful learning—the commitment to choose to make connections between that which is known and that which needs to be learned (Novak, 1998). Indeed, for some students like George and Hannah who had initially heard from doctors and researchers that organic chemistry was not useful, or that its importance was “hyped,” such a commitment may never have existed at all.
Labeling organic chemistry as irrelevant may also explain why Indifferent Learners like Ernie and Fred remained so inflexible with regard to modifying their approaches to learning organic chemistry and refused to commit to more meaningful approaches. It seems reasonable to conclude that some students were unlikely to expend considerable time and effort to design and implement new learning strategies when they perceived little importance for the material to be learned. The factors that shaped this decision were complex. However, significant issues surrounding time management and motivation emerged for many of the students who were interviewed.
Conclusions and implications for practice
The results of this research suggest that there are identifiable, intermediate positions on the continuum connecting meaningful learning and rote learning. These positions are differentiated not only by the usage of meaningful/rote learning techniques but also by such issues as organic chemistry's perceived relevancy and the use of meta-cognitive strategies. The theoretical model presented in Fig. 1 hints at additional student groupings that were not identified in the current study. For example, no students in this study were categorized within quadrant IV. Is this simply a reflection of the lack of saturation during data collection, or are more meaningful learning techniques and low meta-cognitive awareness mutually exclusive? We believe that the latter is more likely; however, more results are necessary to substantiate that claim. It is probable that additional, intermediate positions exist within this model and future research must focus not only on characterizing these positions, but also on describing the mechanisms by which transitions are likely to occur between these positions on the continuum. In general, this model provides a framework from which future researchers can design new instructional materials and approaches that seek to move students towards more meaningful learning techniques.Because perceived relevancy plays an important role in the process of meaningful learning, it is essential that professors make every effort to highlight the vital role that organic chemistry plays in connecting the sciences by providing students not only with numerous examples from many different contexts—for example, medicine, biology, industry, and biochemistry—but also pertinent non-examples that may help students better define the conceptual boundaries. Although many textbooks provide students with examples of the uses for organic chemistry in other science disciplines, this material is frequently presented as a tangent that many students ignore. Given the typical population of organic chemistry students, it would also be helpful for organic chemistry professors to better understand why pre-professional students are required to take organic chemistry. While many may offer hypotheses, only those who are actually involved in the training of future professionals can answer with any degree of certainty. Therefore, it is essential that a dialogue be initiated among those with a vested interest in the education of pre-professional students and that the year-long organic chemistry sequence be brought into better alignment with what professional schools expect students to learn from the subject. In the United States, the beginnings of such a dialogue between the American Chemical Society and the Association of American Medical Colleges is currently underway (Committee on Professional Training, 2012). The results of these discussions may lead to curricular revisions that convince more students that organic chemistry is worthy of their time and effort and offers something essential to their education.
While the issue of relevancy may partially explain why Indifferent Learners ultimately decided to continue relying on rote memorization techniques, we hypothesize that these students also possessed significant misconceptions about the very nature of learning organic chemistry, which may have lead them to maintain their unsuccessful approaches. Our previous work has lead to the development of a valid and reliable instrument to measure these misconceptions (Grove and Bretz, 2007) and has documented how they change over time. We are currently beginning work to better understand why these misconceptions change and to identify the factors that influence this process, specifically as they relate to the groups of learners described herein. Finally, the results of this inquiry strongly suggest that meta-cognition plays a crucial role in not only helping Transitional Learners navigate the pathway to becoming Meaningful Learners, but also may be essential in helping Unaware Learners recognize that more meaningful ways to learn organic chemistry exist. This connection warrants further study, focusing in particular on the development, and subsequent assessment, of instructional strategies that explicitly prompt meta-cognition use.
Epilogue
The results of this study highlight the value of utilizing multiple theoretical frameworks during the course of research. Our prior reports detailing results of this research (Grove and Bretz, 2010) were guided by Perry's scheme of intellectual and ethical development as an analytical tool, enabling us to characterize student difficulties for learning organic chemistry. By relying upon a distinctly different theoretical framework, Ausubel and Novak's Assimilation Theory, the same data were analyzed to yield an orthogonal, and yet complementary, set of findings, namely additional positions connecting the continuum between meaningful learning and rote memorization.Acknowledgements
The authors would like to acknowledge the support and assistance of Dr James Hershberger, without whom, this research would not have been possible. This manuscript is dedicated to his memory.References
- Ausubel D., (1968), Educational Psychology: A Cognitive View, Holt, Rinehart, and Winston: New York.
- Balaban A. and Klein D., (2006), Is chemistry “The Central Science”? How are different sciences related?, Scientometrics, 69, 615–637.
- Bhattacharyya G. and Bodner G., (2005), “It gets me to the product”: How students propose organic mechanisms, J. Chem. Educ., 82, 1402–1407.
- Bodner G., (1991), I have found you and argument: The conceptual knowledge of beginning chemistry graduate students, J. Chem. Educ., 68, 385–388.
- Bretz S.L., (2001), Novak's theory of education: Human constructivism and meaningful learning, J. Chem. Educ., 78, 1107.
- Bretz S.L., (2008), Qualitative research design in chemistry education research, in D. Bunce and R. Cole (ed.), Nuts and bolts of chemical education research, American Chemical Society: Washington, D. C., 79–100.
- Cartrette D. and Mayo P., (2011), Students' understanding of acids/bases in organic chemistry contexts, Chem. Educ. Res. Pract., 12, 29–39.
- Clary D. and Wandersee J., (2007), A mixed methods analysis of the effects of an integrative geobiological study of petrified wood in introductory college geology classrooms, J. Res. Sci. Teach., 44, 1011–1035.
- Committee on Professional Training, (2012), ACS Task Force on Scientific Foundations for Future Physicians, CPT Newsletter Winter 2011, 9, 4.
- Comte A., (1830), Cours de philosophie positive, Bachelier: Paris.
- Cooper M., Grove N., Underwood S. and Klymkowsky M., (2010), Lost in Lewis structures: An investigation of student difficulties in developing representational competence, J. Chem. Educ., 87, 869–874.
- Corbin J. and Strauss A., (2008), Basics of qualitative research: Techniques and procedures for developing grounded theory, (3rd ed.). Sage Publications: Thousands Oaks, CA.
- Creswell J., (2007), Qualitative inquiry and research design: Choosing among five approaches, (2nd ed.). Sage Publications: Thousand Oaks, CA.
- Denzin N., (2006), Sociological methods: A sourcebook, Transaction Publishers: Edison, NJ.
- Driver R. and Easley J., (1978), Pupils and paradigms: A review of literature related to concept development in adolescent science students, Stud. Sci. Educ., 5, 61–84.
- Driver R. and Erickson G., (1983), Theories-in-action: Some theoretical and empirical issues in the study of students' conceptual frameworks in science, Stud. Sci. Educ., 10, 37–60.
- Ferguson R. and Bodner G., (2008), Making sense of the arrow-pushing formalism among chemistry majors enrolled in organic chemistry, Chem. Educ. Res. Pract., 9, 102–113.
- Galley W., (2004), Exothermic bond breaking: A persistent misconception, J. Chem. Educ., 81, 523–525.
- Geertz C., (1973), Thick description: Toward an interpretive theory of culture, Basic Books: New York.
- Grove N. and Bretz S.L., (2007), CHEMX: An instrument to assess students' cognitive expectations for learning chemistry, J. Chem. Educ., 84, 1524–1529.
- Grove N., Hershberger J. and Bretz S. L., (2008), Impact of a spiral curriculum on student attrition and learning, Chem. Educ. Res. Pract., 9, 157–162.
- Grove N. and Bretz S. L., (2010), Perry's Scheme of intellectual and epistemological development as a framework for describing student difficulties in learning organic chemistry, Chem. Educ. Res. Pract., 11, 207–211.
- Grove N., Cooper M. and Rush K., (2012), Decorating with arrows: Towards the development of representational competence in organic chemistry, J. Chem. Educ., accepted.
- Grove N., Cooper M., and Cox E., (2012), Does mechanistic thinking improve success in organic chemistry?, J. Chem. Educ., accepted.
- Kirk J. and Miller M., (1986), Reliability and validity in qualitative research, Sage Publications: Newbury Park, CA.
- Kornberg A., (1987), The two cultures: Chemistry and biology, Biochem., 26, 6888–6891.
- Lewis S., Shaw J., Heitz J. and Webster G., (2009), Attitude counts: Self-concept and success in general chemistry, J. Chem. Educ., 86, 744–749.
- Lincoln Y. and Guba E., (1985), Naturalistic inquiry, Sage Publications: Thousand Oaks, CA.
- Mulford D. and Robinson W., (2002), An inventory for alternative conceptions among first-semester general chemistry students, J. Chem. Educ., 79, 739–744.
- Nakhleh M., (1992), Why some students don't learn chemistry: Chemical misconceptions, J. Chem. Educ., 69, 191–196.
- Novak J., (1977), A theory of education, Cornell University: Ithaca, NY.
- Novak J., (1998), Learning, creating, and using knowledge, Lawrence Erlbaum Associates, Inc.: Mahwah, NJ.
- Oliver-Hoyo M. and Allen D., (2006), The use of triangulation methods to validate results of qualitative education research, J. Coll. Sci. Teach., 35, 42–47.
- Patton M., (2002), Qualitative research and evaluation methods, Sage Publications: Thousand Oaks, CA.
- Treagust D., (1988), Development and use of diagnostic tests to evaluate students' misconceptions in science, Int. J. Sci. Educ., 10, 159–169.
- Tsai C. and Wen L., (2005), Research and trends in science education from 1998 to 2002: a content analysis of publication in selected journals, Int. J. Sci. Educ., 27, 3–14.
| This journal is © The Royal Society of Chemistry 2012 |
