N-type organic semiconductor bisazacoronene diimides efficiently synthesized by a new type of photocyclization involving a Schiff base†
Youdi
Zhang
a,
Zhenbo
Zhao
a,
Xiaomei
Huang
a,
Yuanpeng
Xie
a,
Chenxi
Liu
b,
Jun
Li
b,
Xin
Guan
a,
Kaichen
Zhang
a,
Chuanhui
Cheng
*b and
Yi
Xiao
*a
aState Key Laboratory of Fine Chemicals, Dalian University of Technology, 2 Linggong Rd., Dalian, 116012, China. E-mail: xiaoyi@dlut.edu.cn; Fax: +86-411-84986252; Tel: +86-411-84986251
bSchool of Physical and Optoelectronic Engineering, Dalian University of Technology, 2 Linggong Rd., Dalian, 116012, China. E-mail: cheng_ch79@yahoo.com.cn; Fax: +86-411-84707865; Tel: +86-411-84707865
First published on 16th October 2012
Abstract
Novel bisazacoronene bisimides (bisaza CBIs) were efficiently synthesized via a new type of photocyclization involving a Schiff base. One of the bisaza CBIs self-assembles into nanobelts and shows reasonable electron mobility in a space-charge-limited current device.
Perylene bisimides (PBIs) are important n-type semiconducting materials which find many applications in organic electronics.1 As the sisters of PBIs, coronene bisimides (CBIs) have also attracted considerable research interest.2 On the one hand, similar to PBIs, CBI derivatives and analogues show lower LUMO orbital energy favouring electron acceptance and transport. On the other hand, compared with PBIs, CBIs have larger planar cores and more extended conjugation structures which may have significant impact on the properties of electron-distribution and the capability of self-assembly into ordered supramolecular structures3 which help in the improvement of device performance.4 Actually, CBI-based liquid crystals5 exhibit better mobility in organic field-effect transistors2b and higher power conversion efficiency in organic solar cells.6 Thus, CBIs and analogues show particular importance in building rigid planar conjugated aromatic core systems. As shown in Scheme 1, there are two major synthetic approaches: (1) cyclization of bisalkynyl substituted PBIs in the presence of strongly alkaline reagents, such as DBU,5b (2) photoinduced cyclization of bisaromatic group substituted PBIs.6,7 Development of novel n-type organic semiconductors is essential and still challenging in the field of organic electronics. Meanwhile, similar N-decorated CBIs were synthesized by the Wang research group via a Pictet–Spengler reaction,8 however, the electron mobility of the compounds was not characterized using space-charge-limited current (SCLC) one-electric devices or organic field-effect transistors. For this reason, herein, we designed novel bisaza CBIs, which, obviously, could not be obtained via previous procedures. We expected to adopt a photocyclization to fuse the aza-ring to the perylene bay sites.9
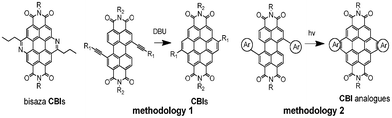 | ||
| Scheme 1 Structure of bisaza CBIs and previous synthetic approaches to CBIs and analogues. | ||
As an initial attempt, we synthesized model compound 4, by a two-step procedure as illustrated in Scheme 2. Firstly, light-promoted condensation of 1-amino-3,4,9,10-tetra(n-butoxycarbonyl)perylene 5 with butyraldehyde yielded corresponding Schiff base 6 which was isolated and characterized. And then, compound 6 underwent photoinduced cyclization in the presence of a catalytic amount of iodine, and was transformed into compound 4. Since both of the above two-steps required light-irradiation, we wondered if they could be combined into a more concise one step procedure. Fortunately, refluxing a mixture of 5, butyraldehyde, I2 and chloroform solvent under adequate sunlight gave 4 in high yields of up to 93%.
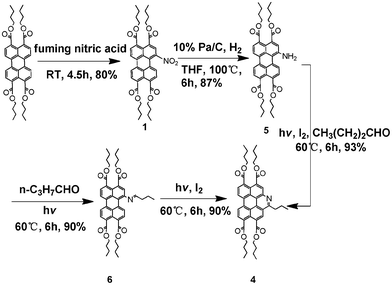 | ||
| Scheme 2 Syntheses of model compound 4. | ||
To gain more insight into the process and mechanism of this one-step synthesis, the reaction kinetics were tracked by recording the UV-vis absorption spectra during the reaction, as shown in Fig. 1 and 2. At the beginning, the color of the solution was orange and the absorption spectrum showed a band centered at 493 nm, which could be ascribed to compound 5. From 0 to 2.5 h, with the extension of the sunlight-irradiation time, the absorption band in the visible region gradually broadened and finally the maximum red-shift was seen at 551 nm. Simultaneously, in the short-wavelength region, there appeared new and structured absorption bands with peaks at 420, 390 and 350 nm. The solution at 2.5 h showed a purple color and an absorption spectrum similar to that of compound 6. From 2.5 to 6 h, the solution's color decayed to light-yellow. The long-wavelength band gradually decreased and finally disappeared, while the short wavelength peaks became very sharp. The absorption spectrum at 6 h was the same as that of compound 4. The above experiments clearly indicated that during the 1-step process, compound 5 was transformed to 4 through an indirect way via intermediate 6.
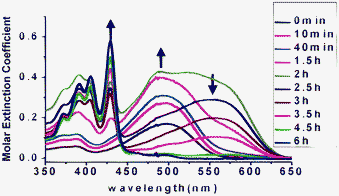 | ||
| Fig. 1 Conversion kinetics of compound 6 under sunlight in CHCl3, tracked by recording UV-vis absorption spectra of the reaction solution at different times. | ||
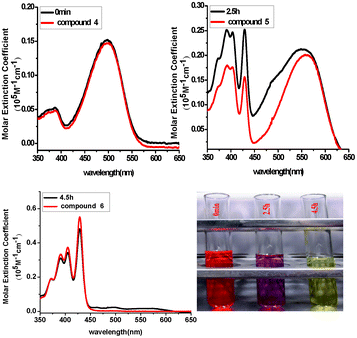 | ||
| Fig. 2 The kinetics of conversion of compound 6 to 5 under sunlight in chloroform: absorption spectra of the reaction solution at different times (in black) and absorption spectra of compounds 6, 4 and 5 in chloroform (in red). | ||
The success of the one step synthesis of model compound 4 inspired us to extend this methodology to develop the targeted bisaza CBIs 3 (3′). As shown in Scheme 3, bisamino perylene tetraesters 7 react with butyraldehyde under sunlight to produce the corresponding bisaza coronene tetraesters 8 (8′) in good yields. Compound 8 (8′) was hydrolyzed completely by chlorosulfonic acid and quantitatively generated bisaza coronene dianhydride 9 (9′). Compound 9 (9′) acted as a versatile intermediate whose condensation with different amines gave corresponding bisaza CBIs. Such a versatile idea to develop novel PBI analogs, with perylenetetraesters as starting materials, via perylenedianhydrides as key intermediates, has proven reasonable and efficient, and thus it should be popularized.10
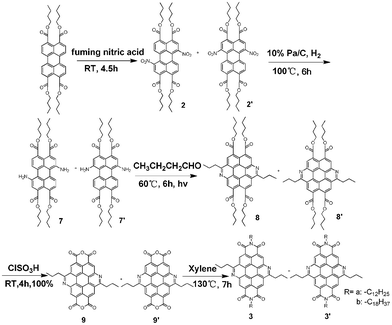 | ||
| Scheme 3 Synthesis of bisaza CBIs3 (3′). | ||
The fundamental photophysical properties of bisaza CBIs 3a (3a′) and 3b (3b′) were studied. In short, they show almost identical absorption spectra of multiple bands, sharp peaks and absorption maxima at around 477 nm. And they have extremely small Stokes shifts of 2–4 nm (Fig. 3 and Table 1). The electrochemical properties of bisaza CBI3 (3′) were investigated by cyclovoltammetry and differential pulse voltammetry (DPV). (see ESI†). Bisaza CBIs 3 (3′) show two pairs of reversible reduction peaks, and thus they can accept two electrons, and their LUMO energy levels are around 3.70 eV. These data indicated that bisaza CBIs 3 (3′) might be potential n-type materials.
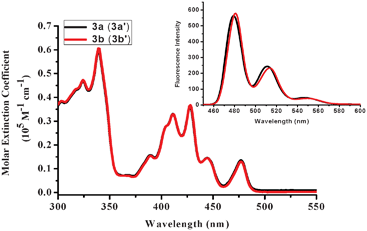 | ||
| Fig. 3 UV-vis absorption and fluorescence emission (inset) spectra of bisaza CBI3a (3a′) and bisaza CBI3b (3b′) in dichloromethane. | ||
| λ abs (nm) | λ em (nm) | E g (eV) | E 1r (V) | LUMO (eV) | HOMO (eV) | |
|---|---|---|---|---|---|---|
| a Measured in 0.05 M solution of Bu4NPF6 in CH2Cl2 with a scan rate of 100 mV s−1. b Based on the assumption that the energy of Fc/Fc+ is 5.08 eV relative to vacuum.11 | ||||||
| PBI | 522 | 575 | 2.33 | −0.69 | 3.94 | 6.27 |
| 4 | 429 | 442 | 2.86 | −1.26 | 3.37 | 6.23 |
| 3a (3a′) | 477 | 479 | 2.66 | −0.91 | 3.70 | 6.36 |
| 3b (3b′) | 475 | 479 | 2.67 | −0.93 | 3.69 | 6.36 |
Bisaza CBI3a (3a′) with 12-carbon side chains has good solubility, up to 20 mg ml−1 in dichloromethane. But 3b (3b′), having a longer side chain of 18 carbons shows much lower solubility, less than 5 mg ml−1 in dichloromethane. Considering the good solubility of 3a (3a′), we preliminarily studied its self-assembly behavior which could provide information on a reference value for future device investigation.12 The SEM image in Fig. 4 illustrates the aggregation state of 3a (3a′) on a Pt-chip. The solid from the evaporation of CH2Cl2 and methanol (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) on the substrate shows a self-assembled morphology composed of nanobelts with a maximum width of up to 565 nm. The as-formed nanobelt films contribute to the electron transport, and can be readily transferred onto a desired solid substrate for device applications, which means 3a (3a′) is suitable for solution processing to fabricate thin-film devices.
9) on the substrate shows a self-assembled morphology composed of nanobelts with a maximum width of up to 565 nm. The as-formed nanobelt films contribute to the electron transport, and can be readily transferred onto a desired solid substrate for device applications, which means 3a (3a′) is suitable for solution processing to fabricate thin-film devices.
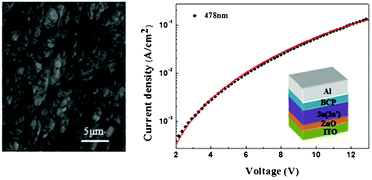 | ||
| Fig. 4 SEM image of bisaza CBI3a (3a′) aggregate suspension on a Pt-chip (left). Current density vs. voltage (J–V) characteristics of an electron-only device based on bisaza CBIs 3a (3a′) (right) at an electric field strength of 0.3 MV cm−1, inset: electron-only device schematic diagram of bisaza CBIs 3a (3a′). | ||
The charge-carrier mobility in bisaza CBIs 3 (3′) was investigated using the steady-state space-charge-limited current (SCLC) technique (Fig. 4),13 which has been widely used to investigate carrier mobility in organic materials. Using spin coating, we fabricated electron-only devices with the structure of ITO/ZnO (25 nm)/3a (3a′) (478 nm)/BCP (15 nm)/Al (100 nm) (ITO = indium tin oxide, BCP = 10-phenanthroline) (Fig. 4). ZnO was used as a hole blocking layer in order to hinder the injection of holes from ITO. BCP was used as a buffer layer between the 3a (3a′) layer and Al electrode, because effectively reducing the electron injection barrier from the Al electrode to the organic layer could significantly enhance the electron injection, and BCP played another role in preventing the reaction between 3a (3a′) and the Al electrode. The SCLC in this case of mobility depending on the field can be approximated by:14
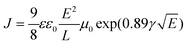 | (1) |
In summary, novel bisazacoronene diimides (bisaza CBIs) were developed through efficient photocyclization involving a Schiff base attached to the bay sites of a perylene core. One of the bisaza CBIs, 3a (3a′) showed interesting self-assembly into ordered nanobelts and reasonable electron mobility in an SCLC device. Further work on the devices is ongoing.
Acknowledgements
Yi Xiao thanks the National Natural Science Foundation of China (No. 21174022) and Chuanhui Cheng thanks the Fundamental Research Funds for the Central Universities (No. DUT11LK40) for financial support.Notes and references
- (a) G. Horowitz, F. Kouki, P. Spearman, D. Fichou, C. Nogues, X. Pan and F. Garnier, Adv. Mater., 1996, 8, 242 CrossRef CAS; (b) C. W. Struijk, A. B. Sieval, J. E. J. Dakhorst, M. van Dijk, P. Kimkes, R. B. M. Koehorst, H. Donker, T. J. Schaafsma, S. J. Picken, A. M. van de Craats, J. M. Warman, H. Zuilhof and E. J. R. Sudholter, J. Am. Chem. Soc., 2000, 122, 11057 CrossRef CAS; (c) L. Schmidt-Mende, A. Fechtenkötter, K. Müllen, E. Moons, R. H. Friend and J. D. MacKenzie, Science, 2001, 293, 1119 CrossRef CAS; (d) W. S. Shin, H. H. Jeong, M. K. Kim, S. H. Jin, M. R. Kim, J. K. Lee, J. W. Lee and Y. S. Gal, J. Mater. Chem., 2006, 16, 384 RSC; (e) X. Zhan, Z. A. Tan, B. Domercq, Z. An, X. Zhang, S. Barlow, Y. Li, D. Zhu, B. Kippelen and S. R. Marder, J. Am. Chem. Soc., 2007, 129, 7246 CrossRef CAS; (f) H. Z. Chen, M. M. Ling, X. Mo, M. M. Shi, M. Wang and Z. Bao, Chem. Mater., 2007, 19, 816 CrossRef CAS; (g) C. Huang, S. Barlow and S. R. Marder, J. Org. Chem., 2011, 76, 2386 CrossRef CAS.
- (a) C. Lütke Eversloh, C. Li and K. Müllen, Org. Lett., 2011, 13, 4148 CrossRef; (b) W. Yue, A. Lv, J. Gao, W. Jiang, L. Hao, C. Li, Y. Li, L. E. Polander, S. Barlow and W. Hu, J. Am. Chem. Soc., 2012, 134, 5770 CrossRef CAS.
- F. Würthner, Chem. Commun., 2004, 1564 RSC.
- W. G. Wang, X. Y. Liu, L. Liu and J. L. Pu, Key Eng. Mater., 2010, 428–429, 75 CAS.
- (a) U. Rohr, P. Schlichting, A. Böhm, M. Gross, K. Meerholz, C. Bräuchle and K. Müllen, Angew. Chem., Int. Ed., 1998, 37, 1434 CrossRef CAS; (b) U. Rohr, C. Kohl, K. Müllen, A. van de Craats and J. Warman, J. Mater. Chem., 2001, 11, 1789 RSC; (c) Z. An, J. Yu, B. Domercq, S. C. Jones, S. Barlow, B. Kippelen and S. R. Marder, J. Mater. Chem., 2009, 19, 6688 RSC.
- H. Choi, S. Paek, J. Song, C. Kim, N. Cho and J. Ko, Chem. Commun., 2011, 47, 5509 RSC.
- W. Jiang, Y. Li, W. Yue, Y. Zhen, J. Qu and Z. H. Wang, Org. Lett., 2010, 12, 228 CrossRef CAS.
- L. Hao, W. Jiang and Z. H. Wang, Tetrahedron, 2012, 68, 9234 CrossRef CAS.
- R. Nandha Kumar, T. Suresh and P. S. Mohan, Tetrahedron Lett., 2002, 43, 3327 CrossRef.
- Z. Yuan, Y. Xiao and X. Qian, Chem. Commun., 2010, 46, 2772 RSC.
- B. C. Thompson, Y. G. Kim, T. D. McCarley and J. R. Reynolds, J. Am. Chem. Soc., 2006, 128, 12714 CrossRef CAS.
- (a) Y. Che, A. Datar, K. Balakrishnan and L. Zang, J. Am. Chem. Soc., 2007, 129, 7234 CrossRef CAS; (b) X. Yang, X. Xu and H. F. Ji, J. Phys. Chem. B, 2008, 112, 7196 CrossRef CAS; (c) M. T. Usowicz, M. J. Kelley, K. D. Singer and V. V. Duzhko, J. Phys. Chem. B, 2011, 115, 9703 CrossRef CAS.
- (a) B. R. Kaafarani, T. Kondo, J. Yu, Q. Zhang, D. Dattilo, C. Risko, S. C. Jones, S. Barlow, B. Domercq and F. Amy, J. Am. Chem. Soc., 2005, 127, 16358 CrossRef CAS; (b) C. H. Woo, B. C. Thompson, B. J. Kim, M. F. Toney and J. M. J. Fréchet, J. Am. Chem. Soc., 2008, 130, 16324 CrossRef CAS; (c) Y. Zhang, D. Hanifi, S. Alvarez, F. Antonio, A. Pun, L. M. Klivansky, A. Hexemer, B. Ma and Y. Liu, Org. Lett., 2011, 13, 6528 CrossRef CAS.
- (a) P. N. Murgatroyd, J. Phys. D: Appl. Phys., 1970, 3, 151 Search PubMed; (b) G. G. Malliaras, J. R. Salem, P. J. Brock and J. C. Scott, J. Appl. Phys., 1998, 84, 1583 CrossRef CAS.
- K. Shum, Z. Chen, C. M. Xue and S. Jin, MRS Proceedings, Cambridge Univ. Press, 2009 Search PubMed.
- G. D. Sharma, P. Suresh, J. A. Mikroyannidis and M. M. Stylianakis, J. Mater. Chem., 2010, 20, 561 RSC.
- D. K. Mohamad, A. Fischereder, H. Yi, A. J. Cadby, D. G. Lidzey and A. Iraqi, J. Mater. Chem., 2011, 21, 851 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and compound characterization. See DOI: 10.1039/c2ra22488g |
| This journal is © The Royal Society of Chemistry 2012 |
