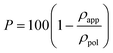Monolithic nanoporous–crystalline aerogels based on PPO
Christophe
Daniel
a,
Simona
Longo
a,
Stefano
Cardea
b,
Jenny G.
Vitillo
c and
Gaetano
Guerra
a
aDepartment of Chemistry and Biology, INSTM and NANOMATES Research Units, Università degli Studi di Salerno, via Ponte Don Melillo Fisciano, 84084, SA, Italy
bDepartment of Industrial Engineering, Università degli Studi di Salerno, via Ponte Don Melillo Fisciano, 84084, SA, Italy
cDipartimento di Chimica and NIS Centre of Excellence, Università di Torino, Via Giuria 7, Torino, 10125, Italy
First published on 1st October 2012
Abstract
Monolithic and robust aerogels exhibiting nanoporous–crystalline modifications of an industrially relevant polymer (poly(2,6-dimethyl-1,4-phenylene)oxide, generally known as polyphenyleneoxide, PPO), have been obtained from thermoreversible gels, by sudden solvent extraction with supercritical carbon dioxide. The aerogel formation occurs only in the presence of semicrystalline nanofibrils of syndiotactic polystyrene, a polymer presenting molecular miscibility with PPO in the amorphous phase. These mixed monolithic aerogels can present nanoporous–crystalline phases of both polymers and hence are very promising for water and air purification. For instance, the carbon tetrachloride uptake from 10 ppm aqueous solutions can be as high as 19 wt%.
Introduction
Aerogels are a unique class of materials characterized by a highly porous network, being attractive for many applications such as thermal and acoustic insulation, capacitors or catalysis.1 Although most aerogels are inorganic,1a–h many organic and polymeric aerogels1i,j have also been developed. The relevance of polymeric aerogels is mainly related to low cost, robustness, durability and easy processing typical of polymers.Polymeric aerogels are generally characterized, as the other aerogels, by chemically bonded three-dimensional networks. In fact, highly cross-linked aerogels are obtained by using thermosetting polymers, e.g. resorcinol–formaldehyde or melamine–formaldehyde.1i,j In more recent years, polymeric aerogels based on thermoplastic uncrosslinked polymers, where the knots of the three-dimensional networks are physical, i.e. not formed by covalent chemical bonds but by thin crystallites, have also been obtained.2–4 These physically cross-linked aerogels are generally prepared by supercritical CO2 (scCO2) extraction5 of thermoreversible hydrogels2 and organogels.3,4
Besides aerogels, another class of porous materials, which has received attention in the last decade by the scientific community, is constituted by semicrystalline thermoplastic polymers whose crystalline phase is nanoporous, i.e. presents a density lower than the corresponding amorphous phase.6 These nanoporous–crystalline polymers are able to absorb low molecular-mass molecules even when present in traces and have been proposed for molecular separation,7 sensor8 and catalysis9 applications.
The preparation of aerogels with thermoplastic materials exhibiting nanoporous–crystalline forms has resulted in a special class of physically cross-linked aerogels, where the crystallites that constitute the physical knots of the aerogel exhibit a nanoporous–crystalline form.4 These nanoporous–crystalline aerogels also exhibit, beside disordered amorphous micropores (typical of all aerogels), all identical nanopores of the crystalline phases. In particular, monolithic aerogels based on the nanoporous–crystalline δ4a–e and ε4f forms of syndiotactic polystyrene (s-PS), with their typical crystalline cavities and channels, have been obtained. These sPS nanoporous–crystalline aerogels, due to their fast kinetics and high capacity of sorption of volatile organic compounds (VOCs) as well as their good handling characteristics, are particularly suitable for removal of traces of pollutants from water and air.4
Very recently, the presence of nanoporous crystalline modifications has been disclosed for another commercial thermoplastic polymer: poly(2,6-dimethyl-1,4-phenylene)oxide (PPO).10 However, extraction from PPO gels of the solvent, independently of its chemical nature and of the extraction procedure, leads to powders rather than to aerogels.10a
In this paper, the preparation of monolithic aerogels, including PPO nanoporous–crystalline phases, is reported. The described preparation method is based on the formation in the gels (and in the derived aerogels) of fibrillar crystalline morphology as a consequence of blending PPO with s-PS. The method, by using gels with suitable PPO/s-PS compositions, also allows the formation of monolithic aerogels that include, beside amorphous nanopores, nanoporous–crystalline phases of both PPO and s-PS.
Experimental section
Materials and samples preparation
The PPO used in this study was purchased by Sigma Aldrich and presents weight-averaged and number-averaged molecular masses Mw = 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1 and Mn = 17
500 g mol−1 and Mn = 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, respectively. The syndiotactic polystyrene used in this study was manufactured by Dow Chemicals under the trademark Questra 101. 13C nuclear magnetic resonance characterization showed that the content of syndiotactic triads was over 98%. Weight-averaged and number-averaged molecular masses were found to be Mw = 320
000 g mol−1, respectively. The syndiotactic polystyrene used in this study was manufactured by Dow Chemicals under the trademark Questra 101. 13C nuclear magnetic resonance characterization showed that the content of syndiotactic triads was over 98%. Weight-averaged and number-averaged molecular masses were found to be Mw = 320![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and Mn = 82
000 g mol−1 and Mn = 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1. Solvents were purchased from Aldrich and used without further purification.
000 g mol−1. Solvents were purchased from Aldrich and used without further purification.
All gel samples were prepared in hermetically sealed test tubes by heating the mixtures until complete dissolution of the polymer and the appearance of a transparent and homogeneous solution had occurred. Then the hot solution was cooled down to room temperature where gelation occurred.
Gels were extracted with an SFX 200 supercritical carbon dioxide extractor (ISCO Inc.), generally using the following conditions: T = 40 °C, P = 250 bar, extraction time t = 300 min.
For monolithic aerogels with a regular cylindrical shape, the total porosity, including macroporosity, mesoporosity and microporosity, can be estimated from the volume/mass ratio of the aerogel.
Then, the percentage of porosity P of the aerogel samples can be expressed as:11
Techniques
The degree of crystallinity of the samples was evaluated from X-ray diffraction data applying the standard procedure of resolving the diffraction pattern into two areas corresponding to the contributions of the crystalline and amorphous fractions for the 2θ range 6–35°.
Results and discussion
Preparation and structural characterization of PPO/s-PS aerogels
X-ray diffraction patterns of physical PPO gels with a polymer content in the range 30–40 wt%, with four different solvents, are shown in Fig. 1 A–D. All these patterns show, beside broad amorphous halos associated with the solvent and the polymer amorphous phase, well-defined crystalline peaks associated with the three-dimensional physical networks holding up the gel. In particular, the gels including decalin and α-pinene (Fig. 1A and 1B, respectively) exhibit diffraction peaks of the corresponding co-crystalline phases with PPO. In particular, the 101, 110, 102, 200, 201, 211, 220 and 221 reflections of the PPO/α-pinene co-crystalline form, located at 2θCu-Kα ≈ 8.9°, 10.5°, 13.1°, 15.0°, 15.8°, 17.5°, 21.3° and 21.8°,9,14 are clearly apparent in Fig. 1B. As for the gel with decalin, peaks at 2θCu-Kα ≈ 9.1°, 10.6°, 12.7°, 17.4° and 21.8 are observed (Fig. 1A), which, from analogy with the X-ray diffraction pattern of the co-crystalline phase with α-pinene, can be indexed as 101, 110, 102, 211 and 220–221, respectively. The gels including CCl4 (Fig. 1C) and benzene (Fig. 1D) show diffraction patterns that are markedly different from those of the co-crystals with α-pinene and decalin gels but also definitely different from each other as well as from those of the known PPO crystalline modifications.9,15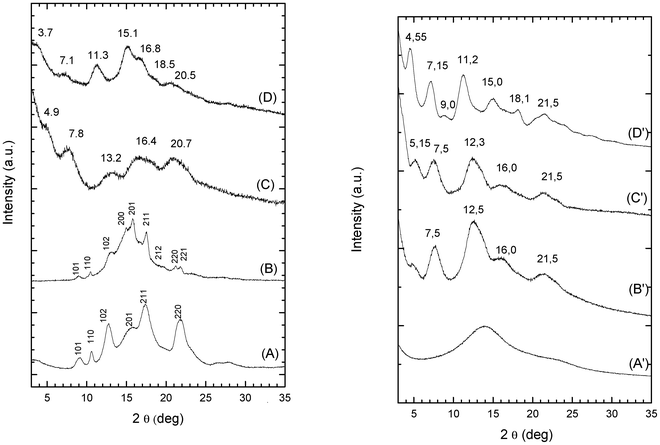 | ||
| Fig. 1 X-ray diffraction patterns (Cu-Kα radiation) of PPO gels (PPO content 30–40 wt%), prepared with different solvents (A–D), and of the corresponding powders, as obtained by complete solvent extraction by scCO2 (A′–D′): (A, A′) in decalin; (B, B′) in α-pinene; (C, C′) in benzene; (D, D′) in carbon tetrachloride. | ||
The solvent removal from all these physical gels by supercritical carbon dioxide does not lead to aerogels,10a as observed instead for many other thermoplastic polymers,4 but only leads to powders. These powders can be amorphous (Fig. 1A′) or can exhibit a PPO crystalline modification (Fig. 1B′–D′). In particular, as a consequence of complete benzene and CCl4 removal from their gels (Fig. 1C′ and 1D′), the limit nanoporous–crystalline modifications with highest and lowest diffraction angles (similar to those described in the first and last columns of Table 1 of ref. 10a) are obtained, respectively.
It is worth noting that the X-ray diffractions of the gels of Fig. 1C, D are similar to those of the corresponding nanoporous–crystalline phases of Fig. 1C′, D′. This similarity suggests the presence in these gels of co-crystalline phases, being structurally related to the corresponding nanoporous–crystalline phases. In particular, the shift to a higher 2θ value (from 3.7° up to 4.55° and from 4.9° up to 5.15°) observed for the lowest diffraction peak, as a consequence of CCl4 and benzene removal from their PPO gels, suggests unit cell reductions as a consequence of removal of guest molecules from the co-crystalline phases. This shift of the first reflection toward higher diffraction angle, as a consequence of guest removal, is analogous to those observed for the monoclinic δ-clathrate5a–c,16 and intercalate17 co-crystalline forms of s-PS.
Hence, the present results confirm that, independently of the nature of the solvent in the gels and of the nature of the crystalline phase holding up the gel, solvent removal procedures lead to powders or extremely brittle samples rather than to robust aerogels. This is shown for instance for the PPO gel in 1,2-dichloroethane with a polymer content of 20 wt%, in the lower part of Fig. 2.
 | ||
| Fig. 2 Photographs of pieces of gels prepared in 1,2-dichloroethane with a polymer concentration of 20 wt%, before and after complete solvent extraction via supercritical carbon dioxide (units on the ruler are cm). Lower part: PPO; upper part: PPO/s-PS, 90/10 w/w. | ||
Monolithic physically crosslinked aerogels can be instead easily prepared from mixed PPO/s-PS gels, by the usual scCO2 extraction procedure, provided that the s-PS fraction is higher than 0.05. This is shown, for instance, for a PPO/s-PS, 90/10 gel in 1,2-dichloroethane with a polymer content of 20 wt%, in the upper part of Fig. 2. The obtained aerogels exhibit a toughness comparable to those of s-PS aerogels and the monoliths do not break even after heavy handling.
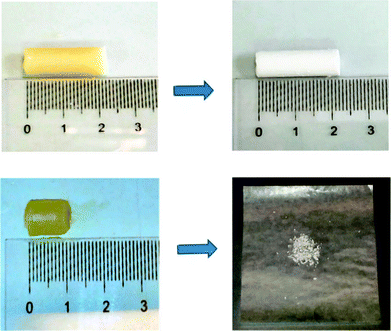
The X-ray diffraction patterns of aerogels obtained from gels in 1,2-dichloroethane18 (DCE) with a polymer content of 20 wt% are shown, for instance, in Fig. 3. The s-PS aerogel (Fig. 3A) shows strong reflections located at 2θ (Cu-Kα) ≈ 8.3° (010), 20.7° ((![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) 21) and (301)) and 23.5° (
21) and (301)) and 23.5° (![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 11), indicating, in agreement with a previous report,17d the presence of the nanoporous δ-form.5a–c
11), indicating, in agreement with a previous report,17d the presence of the nanoporous δ-form.5a–c
 | ||
| Fig. 3 X-ray diffraction patterns (Cu-Kα radiation) of PPO/s-PS gels in DCE, prepared with a polymer content of 20 wt%, after complete solvent extraction by scCO2 , for different PPO fractions: (A) s-PS aerogel; aerogels with (B) xPPO = 0.25, (C) xPPO = 0.50, (D) xPPO = 0.75, (E) xPPO = 0.9; (F) PPO powder. For some patterns (C and F), diffraction peaks of PPO and s-PS (δ form) are labeled by bold and italic numbers, respectively. | ||
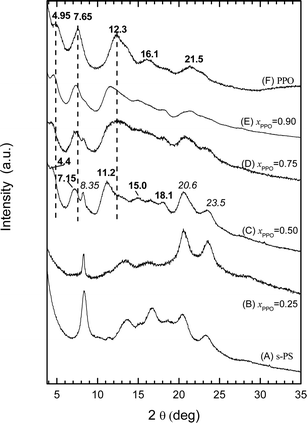
The PPO/s-PS aerogels, for low PPO contents (xPPO = 0.25, Fig. 3B), show only the crystallinity of the δ form of s-PS. For intermediate polymer composition (e.g. xPPO = 0.50, Fig. 3C) both s-PS and PPO crystallinities are clearly present. As for the PPO crystallinity, well apparent for xPPO > 0.5, diffraction peak positions change with xPPO. In fact, for the pure PPO powder, as obtained by DCE removal from the corresponding gel, diffraction peaks at 4.95°, 7.65°, 12.3°, 16.1°, 21.5° are observed (Fig. 3F), which are not far from those observed for powders obtained by desiccation of benzene gels (Fig. 1C′, indicated as high limit diffraction angles in the first column of Table 1 in ref. 10a). As the PPO fraction decreases, a progressive shift of the PPO crystalline peaks is observed and for xPPO = 0.50 (Fig. 3C) the diffraction peaks are observed at 4.4°, 7.15°, 11.2°, 15.0° and 18.1°, i.e. at 2θ values lower than those of powders obtained by desiccation of carbon tetrachloride gels (Fig. 1D′, indicated as low limit diffraction angles in the last column of Table 1 in ref. 10a).
The formation of PPO physical aerogels, already in the presence of small amount of s-PS, clearly indicates a strong influence of s-PS on PPO crystallization. This influence is confirmed by the remarkable shift between PPO crystalline modifications (approximately from the limit modification with lowest diffraction angles toward the limit modification with highest diffraction angles), which is induced by the increase of the s-PS content in the blend (Fig. 3).
The observed s-PS influence on PPO crystallization is not surprising, due to the well-known miscibility of the two polymers in their amorphous phases.19 In fact, it has been clearly established that PPO is able to alter the s-PS polymorphic behavior, favoring the densest and thermodynamically stable β crystalline form,20 both for melt crystallization19a and for solvent-induced crystallization from the amorphous state.19b The patterns of Fig. 3 show that, in turn, s-PS is able to alter the PPO polymorphic behavior favoring, for solution crystallization, the crystalline modifications exhibiting highest diffraction angles.
Scanning Electron Microscopy (SEM) images of some of the samples of Fig. 3 are shown in Fig. 4. The differences between the morphologies of the s-PS and PPO semicrystalline samples prepared in the same conditions are remarkable. In fact, the δ form s-PS aerogel (Fig. 4A) presents a fibrillar morphology with fibril diameters of 60–150 nm, while the PPO nanoporous–crystalline powder exhibits granules of micrometric size, possibly constituted by aggregates of spherulites (Fig. 4D). The SEM images of the aerogels obtained from PPO/s-PS blends (Fig. 4B, C) clearly show the maintenance of the fibrils typical of s-PS aerogels, together with a size reduction of the PPO granules (whose diameter becomes roughly lower than 1 μm). Hence, the miscibility between the amorphous phases of PPO and s-PS leads to finer PPO morphologies. It is reasonable to hypothesize that the maintenance of the fibrillar morphology of the s-PS crystalline phases in mixed gels and aerogels (Fig. 4B, C) makes for feasible PPO-rich monolithic aerogels.
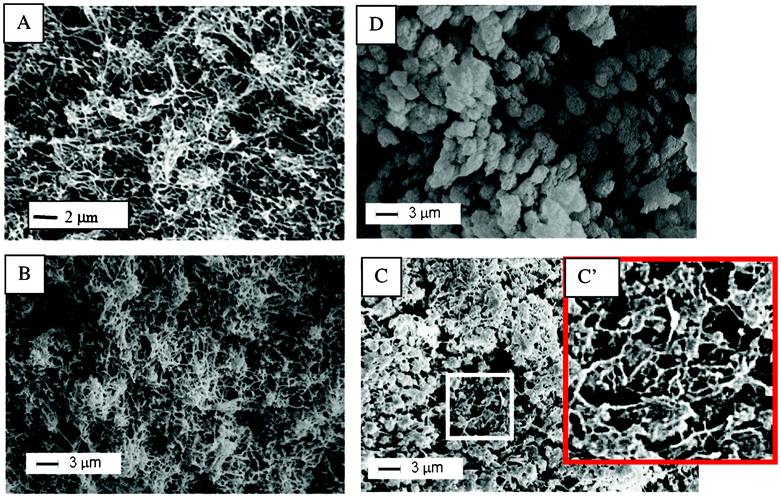 | ||
| Fig. 4 SEM images of samples, as prepared from DCE gels with polymer content of 20 wt%, after complete solvent extraction by scCO2: (A) s-PS δ form aerogel; (B) aerogel with xPPO = 0.5; (C, C′) aerogel with xPPO = 0.90; (D) PPO powder. | ||
Monolithic aerogels can be obtained from PPO/s-PS gels for a large range of polymer concentrations. The X-ray diffraction patterns of aerogels obtained from gels with 5, 10, 20 and 30 wt% of polymer, with xPPO = 0.50, are shown in Fig. 5. The obtained aerogels present a nearly constant s-PS crystallinity (roughly 50%) while showing a variable PPO crystallinity (in the range 15–48%), reaching a minimum for the aerogel prepared from the most diluted gel (for Cpol =5 wt%, χPPO = 15%).
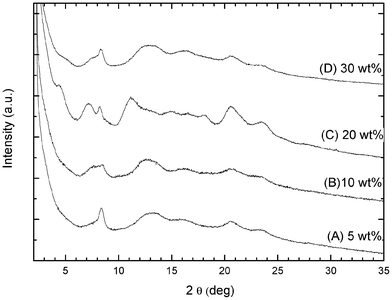 | ||
| Fig. 5 X-ray diffraction patterns (Cu-Kα radiation) of PPO/s-PS aerogels with xPPO = 0.50 , prepared from DCE gels after complete solvent extraction by scCO2, for different polymer contents: (A) 5 wt%; (B) 10 wt%; (C) 20 wt%; (D) 30 wt%. | ||
Properties of aerogels based on nanoporous-crystalline PPO
Total surface areas SBET and micropore areas Smicro, as obtained from N2 adsorption data at 77 K are compared in Table 1 and Fig. 6 for the mixed PPO/s-PS aerogels. It is noteworthy that SBET increases with the PPO content (Fig. 6A), but only for aerogels with crystalline PPO. In fact, the sample with xPPO = 0.25 exhibiting only the s-PS crystallinity (Fig. 3B) shows a surface area close to that of the pure s-PS aerogel. Accordingly, an almost perfect linear correlation between aerogels’ SBET values and their content of crystalline PPO (xPPO,crys) is instead observed, as clearly shown in Fig. 6B.| x PPO | C pol (wt%) | S BET a(m2 g−1) | S micro b (m2 g−1) | x PPO,crys c | χ PPO d(%) |
|---|---|---|---|---|---|
| a Total area evaluated following the BET model in the standard 0.05 < P/P0 < 0.25 pressure range. b Micropore area obtained from the t-plot. c Aerogel fraction constituted by crystalline PPO, as evaluated from the patterns of Fig. 3 and 5. d Degree of crystallinity relative to the PPO fraction, as evaluated by the X-ray diffraction patterns of Fig. 3 and 5. | |||||
| s-PS | 20 | 206 | 27 | 0 | 0 |
| 0.25 | 20 | 217 | 49 | 0 | 0 |
| 0.5 | 20 | 337 | 118 | 0.24 | 48 |
| 0.75 | 20 | 341 | 141 | 0.27 | 36 |
| 0.9 | 20 | 483 | 172 | 0.53 | 58 |
| PPO (powder) | 25 | 535 | 195 | 0.59 | 59 |
| 0.5 | 5 | 112 | 19 | 0.08 | 15 |
| 0.5 | 10 | 273 | 92 | 0.20 | 39 |
| 0.5 | 20 | 337 | 118 | 0.24 | 48 |
| 0.5 | 30 | 343 | 108 | 0.18 | 35 |
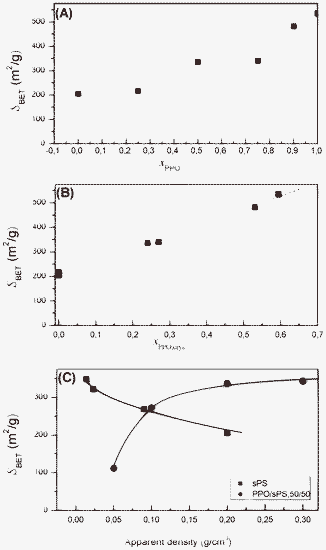 | ||
| Fig. 6 Surface area (SBET , m2 g−1) of PPO/s-PS aerogels (prepared from DCE gels and after complete solvent extraction by scCO2), as evaluated by the BET model in the standard 0.05 < P/P0 < 0.25 pressure range: (A, B) from gels with a polymer content of 20 wt% (aerogel apparent density of 0.2 g cm−3) versus the PPO fraction (xPPO; A) or versus the crystalline PPO fraction (xPPO,crys; B); (C) versus the aerogel apparent density, for xPPO = 0.50. | ||
The predominant contribution of PPO crystallinity to the aerogel surface area is also confirmed by comparing aerogels exhibiting equal PPO content. In fact, the high-porosity PPO/s-PS – 50/50 wt% aerogel prepared from diluted gels (Cpol = 5 wt%, 7th line in Table 1), which presents a low degree of crystallinity, exhibits a surface area nearly three times lower than for the denser highly crystalline aerogels, as obtained from more concentrated gels (Cpol = 10–30 wt%, last three lines in Table 1).
Particularly interesting is the comparison of the SBET dependence on the apparent density for s-PS and PPO/s-PS aerogels, which is shown in Fig. 6C. In fact, while for s-PS aerogels SBET increases as expected when the density decreases (black squares), the opposite unexpected behavior is observed for the PPO/s-PS aerogels (red circles). This behavior can be rationalized by the achievement of maximum content of nanoporous–crystalline phase of PPO only for denser PPO/s-PS aerogels (Cpol ≥ 10 wt%, Table 1).
For these monolithic PPO-based aerogels exhibiting high surface areas (up to 350–500 m2 g−1), also for densities higher than 0.2 g cm−3, the uptake of pollutant molecules as guests of the nanoporous-crystalline phases is expected to be large, mainly if expressed as content per volume rather than per weight.
The high potential of these nanoporous–crystalline PPO based aerogels for molecular separations is here shown, for instance, for the removal of traces of carbon tetrachloride from aqueous solutions. The reported results refer to CCl4 uptake from 10 ppm aqueous solutions.
The FTIR spectra in the 800–770 cm−1 range of the PPO/s-PS – 50/50 aerogel with crystalline PPO of Fig. 5C, before and after CCl4 equilibrium sorption from a 10 ppm aqueous solution, are compared in Fig. 7. The appearance of the peaks at 788 and 785 cm−1 clearly indicates the occurrence of a substantial sorption of CCl4, which has been quantified by thermogravimetric analysis (Fig. 8). In particular, the aerogel of lower porosity (P = 80%) and high PPO crystallinity (χPPO = 48%, whose WAXD pattern is shown in Fig. 5C) and the aerogel of higher porosity (P = 95%) and poor PPO crystallinity (χPPO =15%, whose WAXD pattern is shown in Fig. 5A) exhibit a CCl4 uptake of 13 wt% (Fig. 8c) and 7 wt% (Fig. 8b), respectively. This result clearly confirms that the uptake of carbon tetrachloride from diluted aqueous solutions is mainly due to the PPO nanoporous-crystalline phase of the aerogel.
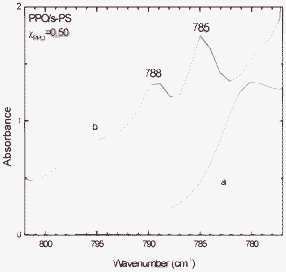 | ||
| Fig. 7 FTIR spectra in the 800–770 cm−1 range of the PPO/s-PS - 50/50 aerogel with crystalline PPO (WAXD of Fig. 4C), before (a) and after (b) CCl4 equilibrium sorption from a 10 ppm aqueous solution. | ||
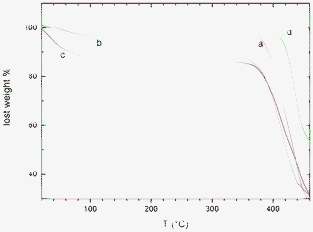 | ||
| Fig. 8 TGA scans at 10 °C min−1 of PPO/s-PS 50/50 aerogels. (a) untreated; (b, c) after CCl4 equilibrium sorption from a 10 ppm aqueous solution: (b) with P = 95% and χPPO = 15% ;(c) with P = 80% and χPPO = 48%. The TGA scan of a PPO amorphous film is shown, for comparison, as (d). | ||
In the same conditions, the CCl4 uptake from the PPO/s-PS – 90/10 wt% aerogel with crystalline PPO (of Fig. 3E), exhibiting surface area higher than for 50/50 aerogels (Table 1), is close to 19 wt%. For the sake of comparison, it is worth adding that after three days of sorption, nanoporous–crystalline aerogels based on the δ form of s-PS, show a CCl4 sorption lower than 0.5 wt% while PPO films exhibiting a nanoporous–crystalline form of PPO show a guest uptake lower than 1 wt%. The much higher guest uptake from aerogels with respect to PPO films, also exhibiting nanoporous–crystalline phases, are due to their much faster sorption kinetics associated with the aerogel morphology.
It is worth adding that the occurrence of higher molecular diffusivities in aerogels can also be responsible for the observed decrease of the temperature of onset of thermal degradation in TGA scans. In this respect, curve d in Fig. 8 shows the TGA of an amorphous PPO film obtained by compression moulding (being similar to scans of semicrystalline PPO films as obtained by different procedures like casting or solvent-induced crystallization, not shown). It is apparent that the aerogels (curves a, b and c in Fig. 8) present degradation phenomena shifted toward lower temperatures of nearly 40–50 °C.
Conclusions
X-ray diffraction characterizations of PPO gels with different solvents have shown that the gel physical cross-linking is constituted by co-crystalline phases between the polymer and solvent molecules. In particular, PPO gels with carbon tetrachloride and benzene exhibit co-crystalline phases, whose X-ray diffraction patterns are rather similar to those of the two nanoporous–crystalline limit modifications, obtained after complete solvent extraction by scCO2.All attempt to obtain monolithic aerogels from PPO gels were unsuccessful. PPO based monolithic aerogels were instead easily obtained starting from gels containing, beside PPO, a polymer miscible with PPO at molecular level and able to form aerogels (s-PS). SEM experiments suggest that the stability of PPO/s-PS aerogels is due to the s-PS fibrillar morphology, which is still apparent also for PPO/s-PS – 90/10 wt% aerogels.
X-ray diffraction experiments show that these mixed aerogels can exhibit nanoporous–crystalline phases of both polymers. In particular, aerogels with high degrees of PPO crystallinity (in the range 35–50%) can be easily obtained, at least for high PPO fractions (xPPO ≥ 0.5) and high densities (ρapp ≥ 0.1 g cm−3).
These nanoporous–crystalline PPO rich aerogels present high surface areas (up to 480 m2 g−1) and high guest solubilities and diffusivities, due to the presence of the crystalline cavities of both polymers, and hence are particularly suitable for removal of traces of organic pollutants from water and air. For instance, the carbon tetrachloride uptake from 10 ppm aqueous solutions for these aerogels can be as high as 19 wt%, while the corresponding uptake for s-PS aerogels and PPO films is lower than 1 wt%, after 3 days of exposure.
Acknowledgements
We thank Prof. Ernesto Reverchon, Prof. Vincenzo Venditto and Dr. Gianluca Fasano of the University of Salerno, Prof. Vittorio Petraccone and Dr. Oreste Tarallo of the University of Naples “Federico II”, and Prof. Giuseppe Spoto of the University of Turin for useful discussions. Financial support of the “Ministero dell'Istruzione, dell'Università e della Ricerca” and of Regione Campania is gratefully acknowledged.References
- (a) D. W. Schaefer and K. D. Keefer, Phys. Rev. Lett., 1986, 56, 2199 CrossRef CAS; (b) G. M. Pajonk, Appl. Catal., 1991, 72, 217 CrossRef CAS; (c) S. T. Mayer, J. L. Pekala and R. W. Kaschmitter, J. Electrochem. Soc., 1993, 140, 446 CrossRef CAS; (d) D. A. Ward and E. I. Ko, J. Catal., 1994, 150, 18 CrossRef CAS; (e) M. L. Anderson, R. M. Stroud and D. R. Rolison, Nano Lett., 2002, 2, 235 CrossRef CAS; (f) A. C. Pierre and G. M. Pajonk, Chem. Rev., 2002, 102, 4243 CrossRef CAS; (g) T. K. Nielsen, K. Manickam, M. Hirscher, F. Besenbacher and T. R. Jensen, ACS Nano, 2009, 3, 3521 CrossRef CAS; (h) T.-Y. Wei, C.-H. Chen, K.-H. Chang, S. Y. Lu and C. C. Hu, Chem. Mater., 2009, 21, 3228 CrossRef CAS; (i) R. W. Pekala, J. Mater. Sci., 1989, 24, 3221 CrossRef CAS; (j) R. W. Pekala, C. T. Alviso, F. M. Kong and S. S. Hulsey, J. Non-Cryst. Solids, 1992, 145, 90 CrossRef CAS.
- (a) H. Jin, Y. Nishiyama, M. Wada and S. Kuga, Colloids Surf., A, 2004, 240, 63 CrossRef CAS; (b) R. Gavillon and T. Budtova, Biomacromolecules, 2008, 9, 269 CrossRef CAS; (c) Z. J. Miao, K. L. Ding, T. B. Wu, Z. M. Liu, X. B. Han, G .M. An, S. D. Miao and G. Y. Yang, Microporous Mesoporous Mater., 2008, 111, 104 CrossRef CAS; (d) M. Robitzer, L David, C. Rochas, F. Di Renzo and F. Quignard, Langmuir, 2008, 24, 12547 CrossRef CAS; (e) F. Quignard, R. Valentin and F. Di Renzo, New J. Chem., 2008, 32, 1300 RSC.
- (a) G. Beaucage, J. H. Aubert, R. R. Lacasse, D. W. Schaefer, T. P. Rieker, P. R. Erlich, S. Stein, S. Kulkarni and P. D. Whaley, J. Polym. Sci., Part B: Polym. Phys., 1996, 34, 3063 CrossRef CAS; (b) D. Dasgupta and A. K. Nandi, Macromolecules, 2005, 36, 6504 CrossRef; (c) D. Dasgupta and A. K. Nandi, Macromolecules, 2007, 40, 2008 CrossRef CAS; (d) S. Cardea, A. Gugliuzza, M. Sessa, M. C. Aceto, E. Drioli and E. Reverchon, ACS Appl. Mater. Interfaces, 2009, 1, 171 CrossRef CAS; (e) C. Daniel, J. G. Vitillo, G. Fasano and G. Guerra, ACS Appl. Mater. Interfaces, 2011, 3, 969 CrossRef CAS.
- (a) C. Daniel, D. Alfano, V. Venditto, S. Cardea, E. Reverchon, D. Larobina, G. Mensitieri and G. Guerra, Adv. Mater., 2005, 17, 1515 CrossRef CAS; (b) G. Guerra, G. Mensitieri, V. Venditto, E. Reverchon and C. Daniel, PCT Int. Appl., 2005 Search PubMed WO 2005012402; (c) S. Malik, C. Rochas and J.-M. Guenet, Macromolecules, 2005, 38, 4888 CrossRef CAS; (d) S. Malik, D. Roizard and J.-M. Guenet, Macromolecules, 2006, 39, 5957 CrossRef CAS; (e) C. Daniel, D. Sannino and G. Guerra, Chem. Mater., 2008, 20, 577 CrossRef CAS; (f) C. Daniel, S. Giudice and G. Guerra, Chem. Mater., 2009, 21, 1028 CrossRef CAS.
- (a) E. Reverchon, G. Guerra and V. Venditto, J. Appl. Polym. Sci., 1999, 74, 2077 CrossRef CAS; (b) W. Ma, J. Yu and J. He, Macromolecules, 2005, 38, 4755 CrossRef CAS; (c) J. Fang and E. Kiran, Macromolecules, 2008, 41, 7525 CrossRef CAS.
- (a) C. De Rosa, G. Guerra, V. Petraccone and B. Pirozzi, Macromolecules, 1997, 30, 4147 CrossRef CAS; (b) G. Milano, V. Venditto, G. Guerra, L. Cavallo, P. Ciambelli and D. Sannino, Chem. Mater., 2001, 13, 1506 CrossRef CAS; (c) E. B. Gowd, N. Shibayama and K. Tashiro, Macromolecules, 2006, 39, 8412 CrossRef CAS; (d) P. Rizzo, C. Daniel, A De Girolamo Del Mauro and G. Guerra, Chem. Mater., 2007, 19, 3864–66 CrossRef CAS; (e) V. Petraccone, O. Ruiz de Ballesteros, O. Tarallo, P. Rizzo and G. Guerra, Chem. Mater., 2008, 20, 3663 CrossRef CAS; (f) A. R. Albunia, P. Rizzo and G. Guerra, Chem. Mater., 2009, 21, 3370–75 CrossRef CAS; (g) G. Guerra, C. Daniel, P. Rizzo and O. Tarallo, J. Polym. Sci., Part B: Polym. Phys., 2012, 50, 305 CrossRef CAS.
- (a) C. Manfredi, M. A. Del Nobile, G. Mensitieri, G. Guerra and M. Rapacciuolo, J. Polym. Sci., Part B: Polym. Phys., 1997, 35, 133 CrossRef CAS; (b) G. Guerra, C. Manfredi, P. Musto and S. Tavone, Macromolecules, 1998, 31, 1329 CrossRef CAS; (c) P. Musto, G. Mensitieri, S. Cotugno, G. Guerra and V. Venditto, Macromolecules, 2002, 35, 2296 CrossRef CAS; (d) K. P. O. Mahesh, M. Sivakumar, Y. Yamamoto, Y. Tsujita, H. Yoshimizu and S. Okamoto, J. Membr. Sci., 2005, 262, 11 CrossRef CAS; (e) G. Mensitieri, D. Larobina, G. Guerra, V. Venditto, M. Fermeglia and S. Pricl, J. Polym. Sci., Part B: Polym. Phys., 2008, 46, 8 CrossRef CAS; (f) S. Figueroa-Gerstenmaier, C. Daniel, G. Milano, J. G. Vitillo, O. Zavorotynska, G. Spoto and G. Guerra, Macromolecules, 2010, 43, 8594 CrossRef CAS.
- (a) G. Mensitieri, V. Venditto and G. Guerra, Sens. Actuators, B, 2003, 92, 255 CrossRef; (b) M. Giordano, M. Russo, A. Cusano, G. Mensitieri and G. Guerra, Sens. Actuators, B, 2005, 109, 177 CrossRef; (c) A. Cusano, A. Iadicicco, P. Pilla, L. Contessa, S. Campopiano, A. Cutolo, M. Giordano and G. Guerra, J. Lightwave Technol., 2006, 24, 1776 CrossRef CAS; (d) A. Buono, P. Rizzo, I. Immediata and G. Guerra, J. Am. Chem. Soc., 2007, 129, 10992 CrossRef CAS; (e) P. Pilla, A. Cusano, A. Cutolo, M. Giordano, G. Mensitieri, P. Rizzo, L. Sanguigno, V. Venditto and G. Guerra, Sensors, 2009, 9, 9816 CrossRef CAS; (f) M. Erdogan, Z. Ozbek, R. Capan and Y. Yagci, J. Appl. Polym. Sci., 2012, 123, 2414 CrossRef CAS.
- A. Buonerba, C. Cuomo, S. O. Sanchez, P. Canton and A. Grassi, Chem.–Eur. J., 2012, 18, 709 CrossRef CAS.
- (a) C. Daniel, S. Longo, J. G. Vitillo, G. Fasano and G. Guerra, Chem. Mater., 2011, 23, 3195 CrossRef CAS; (b) O. Tarallo, V. Petraccone, C. Daniel, G. Fasano, P. Rizzo and G. Guerra, J. Mater. Chem., 2012, 22, 11672 RSC; (c) M. Galizia, C. Daniel, G. Fasano, G. Guerra and G. Mensitieri, Macromolecules, 2012, 45, 3604 CrossRef CAS.
- E. I. Ko, in Kirk-Othmer Encyclopedia of Chemical Technology, 4th ed., John Wiley & Sons, 1998, Suppl. Vol.“Aerogels”, pp 1–22 Search PubMed.
- S. Brunauer, P. H. Emmett and E. Teller, Adsorption of Gases in Multimolecular Layers, 1938, 60, 309–319 CAS.
- S. J. Gregg and K. S. W. Sing, Adsorption, Surface Area and Porosity. In Academic Press, London, 1982, p. 113 Search PubMed.
- (a) M. Barrales-Rienda and J. M. G. Fatou, Kolloid. Z. Z. Polym., 1971, 244, 317 CrossRef; (b) S. Horikiri, J. Polym. Sci., Part A-2, 1972, 10, 1167 CrossRef CAS; (c) J. Hurek and E. Turska, Acta Polym., 1984, 35, 201 CrossRef CAS.
- (a) A. Factor, G. E. Heinsohn and L. H. Vogt Jr., J. Polym. Sci., Part B: Polym. Lett., 1969, 7, 205 CrossRef CAS; (b) O. M. Ilinitch, G. L. Semin, M. V. Chertova and K. I. Zamaraev, J. Membr. Sci., 1992, 66, 1 CrossRef; (c) M. Aguilar-Vega and D. R. Paul, J. Polym. Sci., Part B: Polym. Phys., 1993, 31, 1577 CrossRef CAS; (d) A. Alentiev, E. Drioli, M. Gokzhaev, G. Golemme, O. M. Ilinitch, A. A. Lapkin, V. Volkov and Yu Yampolskii, J. Membr. Sci., 1998, 138, 99 CrossRef CAS; (e) K.C. Khulbe, T. Matsuura, G. Lamarche and A.-M. Lamarche, J. Membr. Sci., 2000, 170, 81 CrossRef CAS; (f) D. M. Sterescu, D. F. Stamatialis, E. Mendes, J. Kruse, K. Rätzke, F. Faupel and M. Wessling, Macromolecules, 2007, 40, 5400 CrossRef CAS.
- (a) Y. Chatani, T. Inagaki, Y. Shimane, T. Ijitsu, T. Yukimori and H. Shikuma, Polymer, 1993, 34, 1620 CrossRef CAS; (b) Y. Chatani, Y. Shimane, T. Inagaki and H. Shikuma, Polymer, 1993, 34, 4841–45 CrossRef CAS; (c) C. De Rosa, P. Rizzo, O. Ruiz de Ballesteros, V. Petraccone and G. Guerra, Polymer, 1999, 40, 2103 CrossRef CAS; (d) O. Tarallo, V. Petraccone, A. R. Albunia, C. Daniel and G. Guerra, Macromolecules, 2010, 43, 8549 CrossRef CAS.
- (a) V. Petraccone, O. Tarallo, V. Venditto and G. Guerra, Macromolecules, 2005, 38, 6965 CrossRef CAS; (b) O. Tarallo, V. Petraccone, V. Venditto and G. Guerra, Polymer, 2006, 47, 2402 CrossRef CAS; (c) C. D'Aniello, D. Dondi, A. Faucitano and G. Guerra, Macromolecules, 2010, 43, 10560 CrossRef CAS.
- (a) C. Daniel, P. Musto and G. Guerra, Macromolecules, 2002, 35, 2243 CrossRef CAS; (b) C. Daniel, D. Alfano, G. Guerra and P. Musto, Macromolecules, 2003, 36, 5742 CrossRef CAS; (c) K Senoo, S. Matsuda and S. Kohjiya, Polymer, 2005, 46, 7819 CrossRef CAS; (d) C. Daniel, A. Avallone and G. Guerra, Macromolecules, 2006, 39, 7578 CrossRef CAS.
- (a) G. Guerra, C. De Rosa, V. M. Vitagliano, V. Petraccone and P. Corradini, J. Polym. Sci., Polym. Phys. Ed., 1991, 23, 265 Search PubMed; (b) G. Guerra, C. De Rosa, V. M. Vitagliano, V. Petraccone, P. Corradini and F. E. Karasz, Polym. Commun., 1991, 32, 30 CAS; (c) S. Cimmino, E. Di Pace, E. Martuscelli and C. Silvestre, Polymer, 1993, 34, 2799 CrossRef CAS; (d) E. M. Woo and F. S. Wu, Macromol. Chem. Phys., 1998, 199, 2041 CrossRef CAS; (e) B. K. Hong, W. H. Jo, S. C. Lee and J. Kim, Polymer, 1998, 39, 1793 CrossRef CAS.
- (a) C. De Rosa, M. Rapacciuolo, G. Guerra, V. Petraccone and P. Corradini, Polymer, 1992, 33, 1423 CrossRef CAS; (b) Y. Chatani, Y. Shimane, T. Ijitsu and T. Yukinari, Polymer, 1993, 34, 1625 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |