One-pot efficient synthesis of pyrrolylBODIPY dyes from pyrrole and acyl chloride†
Min
Zhang
,
Erhong
Hao
*,
Yajun
Xu
,
Shengzhou
Zhang
,
Hongnian
Zhu
,
Qi
Wang
,
Changjiang
Yu
and
Lijuan
Jiao
*
Laboratory of Functional Molecular Solids; Anhui Laboratory of Molecule-Based Materials; College of Chemistry and Materials Science, and School of Life Science, Anhui Normal University, China 241000. E-mail: haoehong@mail.ahnu.edu.cn; jiao421@mail.ahnu.edu.cn
First published on 20th September 2012
Abstract
A facile one-pot synthesis of pyrrolylBODIPY dyes from acid chloride and excess pyrrole was developed through oxidative nucleophilic substitution of in situ formed dipyrromethene with pyrrole. Fluorescence imaging of living cells by using selected dyes has been successfully demonstrated.
Pyrrolyldipyrromethenes, in which each of the pyrrole units are covalently linked at the 2,2′-positions or via a methylene bridge, as in the core structure of natural red pigment prodigiosins (Fig. 1), have received special attention due to their wide range of interesting biological activities.1 Pyrrolyldipyrromethenes are also potentially interesting ligands as they can coordinate in the same fashion as a dipyrromethene ligand and have a pyrrolic substituent in the 9-position.2 Boron complexes of pyrrolyldipyrromethene have been used to achieve long wavelength absorbing and emitting fluorescent dyes. B and C marketed by Introvigen as BODIPY3 576/589 and BODIPY-650/665 have been commonly used as long wavelength biological labels due to their excellent photophysical properties and biocompatibility.4
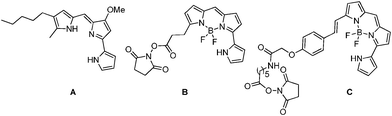 | ||
| Fig. 1 Chemical structures of Prodigiosin A, BODIPY 576/589 succinimidyl ester B and BODIPY 650/665-X succinimidyl ester C. | ||
The key step for the synthesis of pyrrolyldipyrromethene is the construction of the 2,2′-bipyrrole unit. These available synthetic methods5 involve multiple steps, and often require the use of expensive catalysts. For example, BODIPYs B and C were available in small quantities due to the complicated synthesis and the use of expensive precursors as described in a patent.5a Previously, we developed facile syntheses of 3-pyrrolyl isoindole-BODIPY dyes based on a nucleophilic substitution (SNAr) reaction of in situ formed chlorinated dipyrromethene by pyrrole.4c Herein, we report a facile one-pot synthesis of boron pyrrolyldipyrromethene dyes by reacting acyl chloride with an excess amount of pyrrole under oxygen atmosphere.
A small amount of a highly red fluorescent material (2a) was isolated from condensation between pyrrole and acetyl chloride (Scheme 1 and S1 in ESI†). 1a and 2a were fully characterized, and their structures were confirmed by X-ray analysis (Fig. 2). This interesting result prompted us to study the possible mechanism for the formation of 2a and to improve its yield.
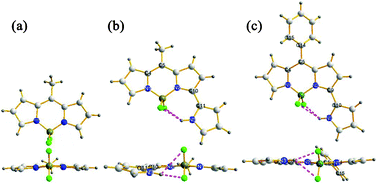 | ||
| Fig. 2 X-Ray structures: (a) 1a. Selected bond distance (Å): N1–B, 1.54; N2–B, 1.54; (b) 2a. Selected bond distance (Å): N1–B, 1.52; N2–B, 1.54; N3H⋯F, 1.97 and 2.85; Selected torsional angles (deg): N2–C10–C11–N3, −11.6°; (c) 2i. Selected bond distance (Å): N1–B, 1.52; N2–B, 1.54; N3H⋯F, 2.10 and 2.58; Selected torsional angles (deg): C4–C5–C14–C15, 50.5°; N2–C9–C10–N3, 5.5°. C grey, N blue, B brown, F green. | ||
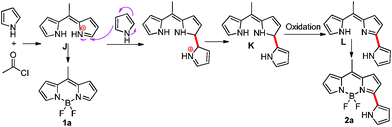 | ||
| Scheme 1 Possible mechanism for the formation of 3-pyrrolylBODIPY 2a. | ||
The α-position of BODIPY or dipyrromethene is subjective to nucleophilic attack. For example, direct (oxidative) nucleophilic substitution of the 3-hydrogen of the BODIPY core by various nucleophiles has been reported recently.6,7 Very few reactions of dipyrromethenes have been reported due to their instability. Among these, Cohen and coworkers8a disclosed a direct oxidative nucleophilic substitution of dipyrromethene (in situ formed from dipyrromethanes) by methanol with the assistance of an oxidant (hydroquinone). Recently, Pandy and co-workers8b greatly improved this type of reaction and reported Fe(III) assisted formation of α-alkoxy substituted 5-ferrocenyldipyrromethenes. Ravikanth and coworkers8c have reported several pyrrole substituted meso-aryldipyrromethenes from the oxidation of their corresponding dipyrromethanes with the assistance of an oxidant (2,3-dichloro-5,6-dicyano-1,4-benzoquinone) in the presence of pyrrole. Inspired by these reports, we proposed a mechanism for the formation of BODIPY 2a as shown in Scheme 1. Initially, dipyrromethene hydrochloride J intermediate was formed, which was attacked by pyrrole at the α-position to form intermediate K. The auto oxidation of intermediate K generated pyrrolyldipyrromethene, which was then complexed with BF2 to give 3-pyrrolylBODIPY 2a.
Both the concentration of acetyl chloride and the amount of pyrrole were essential for this reaction. When diluted acetyl chloride (1 mmol acetyl chloride in 100 mL dichloromethane) was used for this reaction, only a trace amount of 2a was obtained in the presence of 2 mmol pyrrole at room temperature and under air. At the same conditions, increasing the amount of pyrrole to 10 mmol improved the yield of 2a to 3%. The yield of 2a was further improved to 8% by increasing the concentration of acetyl chloride (1 mmol in 2 mL dichloromethane) in the presence of 10 mmol pyrrole. To further optimize this reaction, oxygen was used to substitute air for this reaction and 1,2-dichloroethane was used instead of dichloromethane to increase the reaction temperature, from which 2a was obtained in acceptable yield (19%) (Scheme 2). Thus, the optimized reaction conditions were set to be 1 mmol acetyl chloride in 2 mL 1,2-dichloroethane in the presence of 10 mmol pyrrole under oxygen atmosphere. It is worth mentioning that only a black mixture, and no target BODIPYs, was obtained in the absence of solvent.
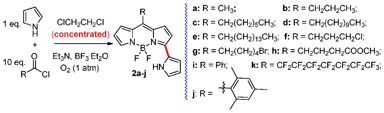 | ||
| Scheme 2 One-pot syntheses of BODIPYs 1a–i and 3-pyrrolylBODIPYs 2a–j from pyrrole and acyl chlorides. | ||
This optimized reaction conditions were further applied to the reactions of pyrroles and a variety of acyl chlorides (Scheme 2). Not only the aliphatic acyl chloride but also aromatic benzoyl chloride smoothly reacted with pyrroles to generate a series of 3-pyrrolylBODIPYs 2a–2j in 16–27% yields. Fluoro-BODIPY 2k was also synthesized in 19% yield. The yields for these reactions are acceptable considering the multiple steps involved in this reaction.
The single crystals of 1a, 2a and 2i, suitable for X-ray analysis, were obtained from the slow evaporation of their dichloromethane solutions. These BODIPYs all showed an almost planar structure for the BODIPY core and the B–N distance is within 1.52–1.54 Å, indicating the usual delocalization of the positive charge. The dihedral-angle between the meso-aryl substituent and the BODIPY core is 50.5° for 2i, which is similar to that of 1i reported in the literature9a and indicates the free rotation of the meso-aryl group. The 3-pyrrole substituents in 2a and 2i lie almost in the same plane of the BODIPY core, with deviations of 11.6° and 5.5°, respectively. Intramolecular hydrogen bonds between the hydrogen attached to the uncoordinated pyrrolic nitrogen and the fluorine atoms in 2a and 2i were observed (Fig. 2b/c). The observed NH⋯F distances were 1.97 and 2.85 Å for 2a and 2.10 and 2.58 Å for 2i. The intramolecular hydrogen bonds and the observed short NH⋯F distances may reduce the degree of rotation of the 3-substituted pyrrole and enhance the rigidity of the dyes.
The optical properties of 2a–k and 1a are summarized in Table 1. In comparison to that of 1a (Fig. 3), significant spectral red-shifts (71 and 82 nm in absorption and emission, respectively) were observed for 2a in dichloromethane with bright fluorescence emission (ϕ = 0.57) at 594 nm, indicating the enhancement in the π-electron delocalization due to the presence of the 3-substituted pyrrole group. These decent fluorescence quantum yields were also observed in other solvents studied (ϕhexane = 0.52, ϕtoluene = 0.45, ϕTHF = 0.45, ϕMeCN = 0.45, and ϕMeOH = 0.45, ESI†). The absorption and fluorescence properties of 2a–k were also studied in six different solvents of varying polarity/polarizability (ESI†). 2b–j all show similar strong absorption bands at around 570 ± 10 nm and shoulder peaks at around 540 ± 10 nm. These BODIPYs also show strong fluorescence emissions at wavelength over 580 nm with fluorescence quantum yields in the range 0.3–0.6 in various solvents studied (ESI†). These results are consistent with the general behavior of BODIPY chromophores.4,9
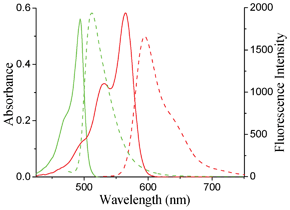 | ||
| Fig. 3 Overlapped absorption (solid line) and emission (dash line) spectra of BODIPYs 1a (green line) and 2a (red line) in dichloromethane. | ||
| λ abs; λem (nm), | ϕ a | Stokes-shift (cm−1) | τ b (ns) | k f (108 s−1) | k nr (108 s−1) | |
|---|---|---|---|---|---|---|
| a The fluorescence quantum yields (ϕ) were calculated using fluorescein (ϕ = 0.90 in 0.1 N NaOH aqueous solution) as standard for 1a and 3; Rhodamine B in anhydrous ethanol (ϕ = 0.49) as standard for 2a–k and 4. b The fluorescence lifetime. | ||||||
| 1a | 494, 512 | 0.87 | 711 | 11.77 | 0.7 | 0.1 |
| 2a | 565, 594 | 0.57 | 864 | 7.08 | 0.8 | 0.6 |
| 2b | 567, 593 | 0.35 | 773 | 6.56 | 0.5 | 1.0 |
| 2c | 567, 588 | 0.39 | 630 | 7.55 | 0.5 | 0.8 |
| 2d | 567, 588 | 0.45 | 630 | 8.00 | 0.6 | 0.7 |
| 2e | 567, 594 | 0.48 | 802 | 6.09 | 1.3 | 0.9 |
| 2f | 570, 597 | 0.36 | 793 | 5.89 | 0.6 | 1.1 |
| 2g | 568, 594 | 0.55 | 771 | 5.77 | 1.0 | 0.8 |
| 2h | 568, 594 | 0.49 | 771 | 5.72 | 0.9 | 0.9 |
| 2i | 576, 609 | 0.24 | 951 | 3.90 | 0.6 | 1.9 |
| 2j | 573, 600 | 0.60 | 785 | 5.84 | 1.0 | 0.7 |
| 2k | 602, 632 | 0.27 | 789 | 4.06 | 0.7 | 1.8 |
| 3 | 497, 515 | 0.90 | 703 | 7.12 | 1.3 | 0.1 |
| 4 | 571, 597 | 0.71 | 763 | 5.70 | 1.2 | 0.5 |
The time-resolved emissions of these BODIPYs were studied and the fluorescence lifetimes (τ) were determined in dichloromethane (Table 1). In contrast to 1a that shows higher fluorescence quantum yield (0.87) and longer fluorescence lifetime in dichloromethane, the installation of pyrrole substituents at the 3-position extends the conjugation of the resultant dyes 2a–j but reduces the fluorescence quantum yields (0.34–0.60) and the fluorescence lifetimes. The similar radiate rate constants (kf) were observed for 1 and 2. By contrast, dramatic decreases of the nonradiate rate constants (knr) for 1a and 3 were observed in comparison with those of 2. This difference may be attributed to the rotations of the 3-pyrrole substituents that increase the S1 excited state nonradiative decay. 2i with a phenyl group at the meso position, which can freely rotate further, decreased its fluorescence quantum yield9a and increased the nonradiate rate constant (knr). 2k shows 30–40 nm red-shifts of absorption and emission maximum compared to those of 2a–j due to the strong electron-withdrawn effect of those fluorine atoms.
2g and 2h generated from the corresponding 6-bromohexanoyl chloride and methyl adipoyl chloride provide good reaction sites for further functionalization and reaction with some biomolecules of interest. To test the biological applicability of these dyes, we further synthesized 3 and 4 bearing the lipophilic and cationic triphenylphosphonium (TPP) ions. TPP ion is a very useful tool to deliver molecules inside cells since it allows the cytoplasm uptake of non-permeable drugs and it is non-toxic for cells and not expensive.103 and 4 were obtained in high yields by direct nucleophilic substitution of 1g and 2g by triphenylphosphine in refluxing toluene as shown in Scheme 3.
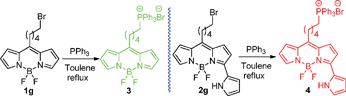 | ||
| Scheme 3 Syntheses of BODIPYs 4 and 5. | ||
For the fluorescent image in living cells, human gastric cancer SGC7901 cells were treated with 3 and 4 in Dulbecco's Modified Eagle Medium (DMEM) containing 0.1% dimethyl sulfoxide. After incubation with 10 μM 3 for 30 min, the cells showed strong green fluorescence (Fig. 4a) when imaged with a fluorescence microscope using a FITC (green channel) filter set. In contrast, after cells were incubated with 10 μM 4 for 30 min, strong red fluorescence (Fig. 4c) was observed when imaged using a TRITC (red channel) filter set. Bright-field transmission images of cells confirmed that the cells were viable throughout the imaging experiments (Fig. 4b and 4d). These results suggest these dyes can be easily taken up by cells and exhibit nontoxicity to the cells. The photostability of representative dye 4 in ethanol solution was measured by continuous irradiation with a Xe lamp (500 W) for 1 h. 4 shows excellent photostability which is comparable to a well-known dye Cresyl violet perchlorate (Fig. S15 and 16 in ESI†).
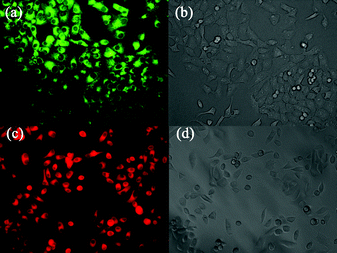 | ||
| Fig. 4 Fluorescence (a, c) and brightfield (b, d) images of living SGC7901 cells incubated with 10 μM 3 or 4 for 30 min. | ||
In conclusion, we have developed a facial one-pot synthesis of boron pyrrolyldipyrromethene dyes from excess pyrrole and acyl chlorides. These resultant BODIPYs as analogs to widely used BODIPY 576/589, have decent fluorescence quantum yields, longer absorptions/emissions, insensitivity to the solvent polarity, and high biocompatibility since they are structurally closely related to the natural red pigment prodigiosin.
Acknowledgements
This work is supported by the National Nature Science Foundation of China (Grants 20902004, 21072005 and 21272007) and Ministry of Education of China (Grant 20093424120001).References
- (a) A. Fűrstner, Angew. Chem., Int. Ed., 2003, 42, 3582 CrossRef; (b) J. L. Sessler, L. R. Eller, W. S. Cho, S. Nicolaou, A. Aguilar, J. T. Lee, V. M. Lynch and D. J. Magda, Angew. Chem., Int. Ed., 2005, 44, 5989 CrossRef CAS; (c) C. Yu, L. Jiao, X. Tan, J. Wang, Y. Xu, Y. Wu, G. Yang, Z. Wang and E. Hao, Angew. Chem., Int. Ed., 2012, 51, 7688 CrossRef CAS; (d) S. R. Chawrai, N. R. Williamson, T. Mahandiran, G. P. C. Salmond and F. J. Leeper, Chem. Sci., 2012, 3, 447 RSC.
- (a) T. E. Wood and A. Thompson, Chem. Rev., 2007, 107, 1831 CrossRef CAS; (b) D. T. Gryko, D. Gryko and C. Lee, Chem. Soc. Rev., 2012, 41, 3780 RSC; (c) V. S. Thoi, J. R. Stork, E. T. Niles, E. C. Depperman, D. L. Tierney and S. M. Cohen, Inorg. Chem., 2008, 47, 10533 CrossRef CAS.
- (a) A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891 CrossRef CAS; (b) R. Ziessel, G. Ulrich and A. Harriman, New J. Chem., 2007, 31, 496 RSC; (c) G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184 CrossRef CAS; (d) N. Boens, V. Leen and W. Dehaen, Chem. Soc. Rev., 2012, 41, 1130 RSC; (e) T. Uppal, X. Hu, F. R. Fronczek, S. Maschek, P. Bobadova-Parvanova and M. G. H. Vicente, Chem.–Eur. J., 2012, 18, 3893 CrossRef CAS; (f) A. Martin, C. Long, R. J. Forster and T. E. Keyes, Chem. Commun., 2012, 48, 5617 RSC; (g) S. L. Niu, C. Massif, G. Ulrich, P.-Y. Renard, A. Romieu and R. Ziessel, Chem.–Eur. J., 2012, 18, 7229 CAS; (h) A. B. Descalzo, H.-J. Xu, Z.-L. Xue, K. Hoffmann, Z. Shen, M. G. Weller, X.-Z. You and K. Rurack, Org. Lett., 2008, 10, 1581 CrossRef CAS; (i) J. Lee, S. Lee, D. Zhai, Y. Ahn, H. Y. Yeo, Y. L. Tan and Y. Chang, Chem. Commun., 2011, 47, 4508 RSC; (j) M. Zhang, Y. Wu, S. Zhang, H. Zhu, Q. Wu, L. Jiao and E. Hao, Chem. Commun., 2012, 48, 8925 RSC.
- (a) C. L. Dale, S. J. Hill and B. Kellam, Med. Chem. Commun., 2012, 3, 333 RSC; (b) K. Mizusawa, Y. Takaoka and I. Hamachi, J. Am. Chem. Soc., 2012, 134, 13386 CrossRef CAS; (c) C. Yu, Y. Xu, L. Jiao, J. Zhou, Z. Wang and E. Hao, Chem.–Eur. J., 2012, 18, 6437 CrossRef CAS; (d) S. M. Crawford, A. A. Ali, T. S. Cameron and A. Thompson, Inorg. Chem., 2011, 50, 8207 CrossRef CAS.
- (a) R. P. Haugland and H. C. Kang, U. S. Patent 5,248, 782, 1993 Search PubMed; (b) H. Rapoport and K. G. Holden, J. Am. Chem. Soc., 1962, 84, 635 CrossRef; (c) D. L. Boger and M. Patel, J. Org. Chem., 1988, 53, 1405 CrossRef CAS; (d) H. H. Wasserman, A. K. Peterson, M. Xia and J. Wang, Tetrahedron Lett., 1999, 40, 7587 CrossRef CAS; (e) A. Fürstner and H. Krause, J. Org. Chem., 1999, 64, 8281 CrossRef; (f) R. D'Alessio and A. Rossi, Synlett, 1996, 513 CrossRef CAS; (g) K. Dairi, S. Tripathy, G. Attardo and J. Lavalle, Tetrahedron Lett., 2006, 47, 2605 CrossRef CAS; (h) M. I. Uddin, S. Thirumalairajan, S. M. Crawford, T. S. Cameron and A. Thompson, Synlett, 2010, 2561 CAS.
- (a) V. Leen, V. Zaragozí Gonzalvo, W. M. Deborggraeve, N. Boens and W. Dehaen, Chem. Commun., 2010, 46, 4908 RSC; (b) V. Leen, M. Van der Auweraer, N. Boens and W. Dehaen, Org. Lett., 2011, 13, 1470 CrossRef CAS; (c) B. Verbelen, V. Leen, L. Wang, N. Boens and W. Dehaen, Chem. Commun., 2012, 48, 9129 RSC.
- M. Zhang, E. Hao, J. Zhou, C. Yu, G. Bai, F. Wang and L. Jiao, Org. Biomol. Chem., 2012, 10, 2139 CAS.
- (a) S. R. Halper, J. R. Stork and S. M. Cohen, Dalton Trans., 2007, 1067 RSC; (b) R. K. Gupta, R. Pandey, S. Sharma and D. S. Pandey, Dalton Trans, 2012, 41, 8556 RSC; (c) M. R. Rao, M. D. Tiwari, J. R. Bellare and M. Ravikanth, J. Org. Chem., 2011, 76, 7263 CrossRef CAS.
- (a) H. L. Kee, C. Kirmaier, L. Yu, P. Thamyongkit, W. J. Youngblood, M. E. Calder, L. Ramos, B. C. Noll, D. F. Bocian, R. Scheidt, R. R. Birge, J. S. Lindsey and D. Holten, J. Phys. Chem. B, 2005, 109, 20433 CrossRef CAS; (b) W. Qin, M. Baruah, M. Van der Auweraer, F. C. De Schryver and N. Boens, J. Phys. Chem. A, 2005, 109, 7371 CrossRef CAS.
- M. Duca, B. Dozza, E. Lucarelli, S. Santi, A. D. Giorgio and G. Barbarella, Chem. Comm., 2010, 46, 7948 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC reference numbers 881436–881438. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ra22203e |
| This journal is © The Royal Society of Chemistry 2012 |
