From isoxazolidines to tetrahydro-1,3-oxazines for the synthesis of chiral pyrrolidines†‡
MariFe
Flores
a,
Pilar
García-García
b,
Narciso M.
Garrido
a,
Isidro S.
Marcos
a,
Francisca
Sanz
c and
David
Díez
*a
aDepartamento de Química Orgánica, Facultad de Ciencias Químicas, Universidad de Salamanca, Plaza de los Caídos 1-5, 37008, Salamanca, Spain. E-mail: ddm@usal.es; Fax: +34 923294574; Tel: +34 923294474
bInstituto de Tecnología Química (UPV-CSIC), Universidad Politécnica de Valencia-Consejo Superior de Investigaciones Científicas, Av. De los Naranjos s/n, 46022, Valencia, Spain
cServicio de Difracción de Rayos X, Universidad de Salamanca, Salamanca, Spain. E-mail: sdrayosx@usal.es; Fax: +34 923294574; Tel: +34 923294500
First published on 17th September 2012
Abstract
A novel approach for the synthesis of chiral tetrasubstituted pyrrolidines has been developed. The rearrangement of isoxazolidines into tetrahydro-1,3-oxazines using reactive organic bromides is herein described for the first time. The subsequent opening reaction of these tetrahydro-1,3-oxazines with nucleophiles probes the usefulness of the method for the synthesis of biologically active compounds.
Introduction
Nitrones and sulfones are two of the most important functional groups in organic chemistry due to their versatility.1 Nitrones undergo several synthetically useful reactions such as 1,3-dipolar cycloadditions and nucleophilic additions, which make them ideal tools for the construction of highly functionalized nitrogen heterocycles.2 Especially important are the cyclic nitrones, as they have been applied to the synthesis of many biologically active natural products.3 The excellent properties of the sulfone group as well as its being easily removable, have made it increasingly important in synthetic chemistry, for example in the synthesis of demanding and sophisticated complex molecules such as peptide-based inhibitors.4 Although there is very extensive literature dedicated to the study of cyclic nitrones5 and vinyl sulfones,6 studies of the reactivity of both together are scarce.7 Recently, we studied the reactivity of several cyclic nitrones with phenylvinylsulfone (Scheme 1).8 | ||
| Scheme 1 Reaction of cyclic nitrones with phenylvinylsulfone. | ||
This study started with the aim of obtaining pyrrolidine-based organocatalysts with a phenylsulfone group. In order to synthesize the required organocatalysts it was necessary to open the isoxazolidine ring. This step has usually been achieved by cleavage of the N–O bond, mainly by reduction,9 oxidation10 with m-CPBA or alkylation of the nitrogen atom followed by treatment with base.11
Results and discussion
We initially focused our attention on the ring opening reaction of compounds 2a–c and 3a–c (Scheme 2), as they are the straightforward precursors for the organocatalysts. | ||
| Scheme 2 Ring opening reaction of isoxazolidines 2a–c and 3a–c (details of reaction conditions A or B are given in Table 1). | ||
Treatment of isoxazolidines 2a,2b and 3a,3b with Mo(CO)6, for the reductive cleavage of the N–O bond9c (Table 1, entries 1, 3, 5 and 7) gave the expected products, 4a,4b and 5a,5b, respectively. Surprisingly, when compounds 2c and 3c were submitted to these conditions, not only were the corresponding pyrrolidines 4c and 5c obtained, but two other rearranged products, identified as the corresponding bicyclic tetrahydro-1,3-oxazines 10 and 11 respectively (entries 9 and 11), were obtained.
| Entry | Compound | Conditionsa | Product (% yield)b |
|---|---|---|---|
| a Conditions A: Mo(CO)6, H2O–MeCN, reflux, 24 h; conditions B: BnBr, CHCl3, reflux, 20 h. b Yield of isolated product. | |||
| 1 | 2a | A | 4a(35) |
| 2 | 2a | B | 6a (24) |
| 3 | 3a | A | 5a (35) |
| 4 | 3a | B | 7a (55) |
| 5 | 2b | A | 4b (20) |
| 6 | 2b | B | 8b (10) |
| 7 | 3b | A | 5b (—) |
| 8 | 3b | B | 9b (10) |
| 9 | 2c | A | 4c (50), 10 (50) |
| 10 | 2c | B | 12a (46) |
| 11 | 3c | A | 5c (20), 11 (53) |
| 12 | 3c | B | 13a (67) |
As we were aware of the importance of this rearrangement, we focused our attention on the alkylation conditions, in order to see if this rearrangement takes place with alkylating agents (Table 1). When isoxazolidines 2a–c and 3a–c were submitted to treatment with benzyl bromide it was observed that pyrrolidines with no substituents in the 3 or 4 positions (entries 2 and 4) undergo alkylation of the nitrogen atom with neither ring opening nor rearrangement. Pyrrolidines 2b and 3b, protected with benzyl groups (entries 6 and 8), gave products resulting from alkylation, followed by ring opening (8b and 9b respectively) in low yield by transformation in the work up or chromatography. To our delight, for compounds with an acetonide group (entries 10 and 12) the rearrangement was the only reaction, producing tetrahydro-1,3-oxazines 12a and 13a in moderate and good yields respectively. The stereochemistry of the rearranged compounds was easily established by the observation of no coupling between the H-5 and H-4 hydrogens in the 1H NMR spectra and corroborated by X-ray analysis for compound 13a (Fig. 1).12
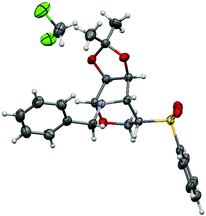 | ||
| Fig. 1 X-ray crystal structure of compound 13a. Displacement ellipsoids are drawn at the 30% probability level. Hydrogen atoms are shown as spheres of arbitrary radius. | ||
This rearrangement could be understood as a 1,3-hydride shift from the C–H in the α-position with regard to the tertiary amine (tert-amino effect) to the oxygen of the isoxazolidine (Pathway A in Scheme 3). The structural rigidity of the tricyclic derivatives 2c and 3c could be responsible for their unique reactivity, forcing the topology required in the transition state. Therefore, this could represent a nice example of a 1,3-hydride shift triggered reaction cascade involving ring opening–ring closure sequences which, to the best of our knowledge, are quite uncommon. Related intramolecular hydride shift–ring closure transformations are known, including some recent advances through catalytic approaches.13 It could also be considered a deprotonation–ring opening followed by the cyclization step (Pathway B in Scheme 3). More synthetic studies to better understand the mechanism are being conducted.
 | ||
| Scheme 3 Suggested mechanisms for the synthesis of tetrahydro-1,3-oxazines. | ||
This kind of behaviour of the isoxazolidine moiety for these kinds of compounds has rarely been observed. The related formation of oxazines from isoxazolidines has been observed by Uccella and co-workers through alkylation of the isoxazolidines to give isoxazolidinium salts, followed by treatment with a base.14
The novelty of the reactions reported herein rests in their occurrence under thermal conditions with no reagent added, which would further support a 1,3-hydride shift mechanism. Formation of tetrahydro-1,3-oxazines from tertiary amines bearing an OH group able to trap the intermediate iminium ion is a well established process occurring usually by oxidation or under photochemical conditions.15 The particular structure of adducts 2c and 3c seems to suggest kinetic lability of the C–H α to N for stereoelectronic reasons, according to similar observations reported for the oxidation of the parent hydroxylamines to the corresponding nitrones.16
Having obtained bicyclic aminals 12 and 13, and taking into account the importance of nitrones such as 1c, which has been used in the synthesis of many natural and biologically active compounds,3b,3c we decided to explore the scope of the reaction using different alkylating agents (Table 2). The rearrangement of isoxazolidines 2c and 3c to the bicyclic system only works with very effective alkylating agents such as allylic or benzylic species, and in higher yields with bromides than with chlorides (entries 5–8 and 9, 10). When geranyl bromide was employed, no reaction with 2c occurred due to the steric hinderence of the sulfonyl group; the yield was low with 3c (entry 11). Moreover, no reaction occurred in any case with either saturated alkyl bromides or acyl compounds.
| Entry | S. M. | RX | Product | Yield (%) | |
|---|---|---|---|---|---|
| a Isoxazolidine (1 mmol) in CHCl3 (0.06 M); RX (1 mmol), 60 °C for 20 h. b In this case, only the alkylation product was isolated in 30% yield. | |||||
| 1 | 2c |

|
12a | 46 | |
| 2 | 2c |

|
12b | 60 | |
| 3 | 2c |

|
b | 30 | |
| 4 | 2c |
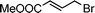
|
12d | 25 | |
| 5 | 3c |

|
13a | 67 | |
| 6 | 3c |

|
13a | 20 | |
| 7 | 3c |

|
13b | 74 | |
| 8 | 3c |

|
13b | — | |
| 9 | 3c |

|
13c | 25 | |
| 10 | 3c |

|
13c | — | |
| 11 | 3c |
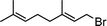
|
13d | 30 | |
| 12 | 3c |

|
13e | 80 | |
| 13 | 3c |
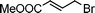
|
13f | 35 | |
| 14 | 3c |
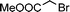
|
13g | 20 | |
To demonstrate the importance of this reaction, we decided to open the bicyclic system with different nucleophiles as has been done with other aminals, in particular by Bosch and Amat.17 Compounds 12a and 13a were chosen as starting materials. First of all, 12a and 13a were treated with methylmagnesium bromide. When the reaction was performed using 1, 3 or even 5 equivalents of the Grignard reagent, ring opening either did not occur or only occurred in poor yield. The desired products (14a and 15a) were formed in high yield only when using at least 10 equivalents of the nucleophile (Table 3, entries 1 and 2). Moreover, lithium nucleophiles resulted in quite poor yields or no reaction, irrespective of the number of equivalents used (entries 3 and 4). Other Grignard reagents led to the opening of the aminal in high yields when 10 equivalents were used (entries 5–10). In this manner, pyrrolidines with four chiral centers were obtained in high yield in a simple way.
| Entry | S. M. | Nucleophile | Product | Yield (%) |
|---|---|---|---|---|
| a 12a or 13a (1 mmol) , Et2O (0.08 M); NuMgBr (10 mmol) or NuLi (10 mmol), −60 °C for 2 h. | ||||
| 1 | 12a | MeMgBr | 14a Nu = Me | 85 |
| 2 | 13a | MeMgBr | 15a Nu = Me | 98 |
| 3 | 13a | MeLi | 15a Nu = Me | 40 |
| 4 | 13a | n-BuLi | 15b Nu = Bu | — |
| 5 | 13a | EtMgBr | 15c Nu = Et | 98 |
| 6 | 13a | PhMgBr | 15d Nu = Ph | 70 |
| 7 | 13a | AllylMgBr | 15e Nu = Allyl | 85 |
| 8 | 13a | 2-NaphCH2MgBr | 15f Nu = 2-NaphCH2- | 80 |
| 9 | 13a | c-hexCH2MgBr | 15g Nu = c-hexCH2- | 90 |
| 10 | 13a | VinylMgBr | 15h Nu = Vinyl | 54 |
It can be observed that the configuration of the sulfone group does not influence the stereochemistry of the incoming nucleophile; in all cases, the same α-Nu were obtained independently of the starting material 12a or 13a. The stereochemistry of the ring opened product was established by analysis of the NMR experiments (bidimensional and NOE, see ESI†) and confirmed by transformation of 14a and 15a into pyrrolidine 16 by treatment with Na(Hg) amalgam as shown in Scheme 4. Since the stereochemistry of 16 is known,18 it could be established that the nucleophile had entered through the α side. The stereochemistry was also corroborated by transformation of compound 15h in the same manner to form meso diolefin 17.
 | ||
| Scheme 4 a. Na(Hg) 5%, MeOH, r.t., 2 h, 100%; b. 9-BBN, THF, NaBO3, r.t., 30%; c. Na(Hg) 5%, MeOH, r.t., 2 h, 100%. | ||
In order to further extend the applicability of this methodology, compound 16, which had previously been transformed by Palmer and Jäger into biologically active pyrrolidines,18 was submitted to hydroboration and oxidation affording pyrrolidine 18, the C-5 epimer of which has been previously transformed into a fucosidase inhibitor by Defoin et al..19
As depicted in Scheme 4, both compounds 14a and 15a led to the same olefin 16, which increased the yield of the final product (18).
Conclusions
A new method for the synthesis of chiral pyrrolidines is described. This approach is based on the rearrangement of chiral isoxazolidines into tetrahydro-1,3-oxazines by treatment with reactive organic bromides. Opening of the obtained tetrahydro-1,3-oxazines with different nucleophiles affords the corresponding chiral pyrrolidines in a diastereoselective manner. These compounds can be used for diversity oriented synthesis of biologically active compounds.Experimental section
N–O cleavage of isoxazolidines using Mo(CO)6: standard procedure
To a stirred solution of isoxazolidine (1 mmol) in 1 mL of H2O and 15 mL of MeCN was added 0.7 mmol of Mo(CO)6 and the mixture was heated at reflux. The solution was stirred for 24 h then concentrated in vacuo. The resulting crude residue was purified by flash chromatography (silica gel, hexane–EtOAc 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain rearranged compound.
1) to obtain rearranged compound.
Alkylation of heterocycles: standard procedure
To a stirred solution of isoxazolidine (1 mmol) in CHCl3 (0.06 M) was added dropwise RBr (1 mmol) and the solution heated at 60 °C. The solution was stirred at 60 °C for 20 h. Then it was quenched with saturated aqueous solution of NH4Cl and the product was extracted with DCM (3×15 mL). The combined organic layers were washed with brine, dried (Na2SO4), filtered, and concentrated in vacuo. The resulting crude residue was purified by flash chromatography (silica gel, hexane–EtOAc 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to obtain the rearranged compound.
2) to obtain the rearranged compound.
Addition of organometallic reagents: standard procedure
To a stirred solution of rearranged compound (1 mmol) in Et2O (0.08 M) was added dropwise RMgBr or RLi (10 mmol) at −60 °C. The solution was stirred at −60 °C for 2 h. Then the mixture was allowed to warm slowly to room temperature. It was quenched with a saturated aqueous solution of NH4Cl and the product was extracted with DCM (3×15 mL). The combined organic layers were washed with brine, dried (Na2SO4), filtered, and concentrated in vacuo. The resulting crude residue was purified by flash chromatography (silica gel, hexane–EtOAc 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain the pyrrolidine product.
1) to obtain the pyrrolidine product.
(2S,3S,4R,5R)-1-Benzyl-3,4-isopropylidenedioxy-5-methyl-2-vinylpyrrolidine 16
a) To a solution of pyrrolidine 15a (40 mg, 0.09 mmol) in MeOH (1.5 mL) was added 128 mg (0.28 mmol) of 5% Na (Hg) amalgam at r.t. The mixture was stirred for 2 h at this temperature under an argon atmosphere. Next, it was filtered to remove the Hg residue and diluted with DCM (30 mL). The mixture was washed with brine, dried (Na2SO4), filtered, and concentrated. The resulting crude residue was purified by flash chromatography (silica gel, n-hexane–EtOAc 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) to obtain 16 (25 mg, 100%). [α]20D −5.0 (c 0.5, CH2Cl2); IR (film): 2980, 2965, 2930, 1449, 1246, 1148, 1070, 866 cm−1; 1H NMR (200 MHz, CDCl3) δ 7.31–7.21 (5H, m, HAr), 5.84–5.66 (1H, m, H-1′), 5.39–5.20 (2H, m, H-2′), 4.29 (1H, dd, J = 5.0 and 6.8 Hz, H-3), 4.16 (1H, dd, J = 4.8 and 6.8 Hz, H-4), 3.84 (1H, d, J = 14.6 Hz, HA–CH2Bn), 3.49 (1H, d, J = 14.6 Hz, HB–CH2Bn), 3.09 (1H, dd, J = 5.0 and 8.4 Hz, H-2), 2.70–2.64 (1H, m, H-5), 1.43 (3H, s, Me-acetonide), 1.29 (3H, s, Me-acetonide), 1.22 (3H, d, J = 5.6 Hz, Me–C-5); 13C NMR (50 MHz, CDCl3) δ 138.8, 137.6, 129.5, 128.2, 127.1, 118.9, 113.5, 85.4, 83.5, 72.9, 63.9, 53.4, 27.5, 25.6, 18.5; HRMS (EI) calcd for C17H24NO2 (M + H)+ 274.1801; found 274.1800.
4) to obtain 16 (25 mg, 100%). [α]20D −5.0 (c 0.5, CH2Cl2); IR (film): 2980, 2965, 2930, 1449, 1246, 1148, 1070, 866 cm−1; 1H NMR (200 MHz, CDCl3) δ 7.31–7.21 (5H, m, HAr), 5.84–5.66 (1H, m, H-1′), 5.39–5.20 (2H, m, H-2′), 4.29 (1H, dd, J = 5.0 and 6.8 Hz, H-3), 4.16 (1H, dd, J = 4.8 and 6.8 Hz, H-4), 3.84 (1H, d, J = 14.6 Hz, HA–CH2Bn), 3.49 (1H, d, J = 14.6 Hz, HB–CH2Bn), 3.09 (1H, dd, J = 5.0 and 8.4 Hz, H-2), 2.70–2.64 (1H, m, H-5), 1.43 (3H, s, Me-acetonide), 1.29 (3H, s, Me-acetonide), 1.22 (3H, d, J = 5.6 Hz, Me–C-5); 13C NMR (50 MHz, CDCl3) δ 138.8, 137.6, 129.5, 128.2, 127.1, 118.9, 113.5, 85.4, 83.5, 72.9, 63.9, 53.4, 27.5, 25.6, 18.5; HRMS (EI) calcd for C17H24NO2 (M + H)+ 274.1801; found 274.1800.
b) To a solution of pyrrolidine 14a (10 mg, 0.02 mmol) in MeOH (1 mL) was added 48 mg (0.06 mmol) of 5% Na (Hg) amalgam at r.t. The mixture was stirred for 2 h at this temperature under an argon atmosphere. Next, it was filtered to eliminate the Hg residue and diluted with DCM (30 mL). The mixture was washed with brine, dried (Na2SO4), filtered, and concentrated. The resulting crude residue was purified by flash chromatography (silica gel, n-hexane–EtOAc 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) to obtain 16 (6 mg, 100%).
4) to obtain 16 (6 mg, 100%).
(2S,3S,4R,5R)-1-Benzyl-2,5-divinyl-3,4-isopropylidenedioxypyrrolidine 17
To a solution of pyrrolidine 15h (24 mg, 0.06 mmol) in MeOH (1 mL) was added 75 mg (0.16 mmol) of 5% Na (Hg) amalgam at r.t. The mixture was stirred for 2 h at this temperature under an argon atmosphere. Next, it was filtered to remove the Hg residue and diluted with DCM (30 mL). The mixture was washed with brine, dried (Na2SO4), filtered, and concentrated. The resulting crude residue was purified by flash chromatography (silica gel, n-hexane–EtOAc 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) to obtain 17 (17 mg, 100%). IR (film): 2982, 2924, 1375, 1267, 1072, 922, 866, 704 cm−1; 1H NMR (200 MHz, CDCl3) δ 7.26–7.21 (5H, m, HAr), 5.82–5.84 (2H, m, H-1′), 5.38 (2H, d, J = 1.8 Hz, HA-2′), 5.20 (2H, dd, J = 5.0 and 6.8 Hz, HB-2′), 4.30 (2H, dd, J = 1.2 and 3.0 Hz, H-3 and H-4), 3.70 (2H, bs, CH2Bn), 3.12 (2H, dd, J = 1.8 and 7.8 Hz, H-2 and H-5), 1.42 (3H, s, Me-acetonide), 1.27 (3H, s, Me-acetonide); 13C NMR (50 MHz, CDCl3) δ 138.5, 136.5, 130.1, 128.1, 127.1, 118.9, 113.7, 83.7, 77.3, 71.7, 52.6, 27.4, 25.6; HRMS (EI) calcd for C18H24NO2 (M + H)+ 286.1803; found 286.1801.
4) to obtain 17 (17 mg, 100%). IR (film): 2982, 2924, 1375, 1267, 1072, 922, 866, 704 cm−1; 1H NMR (200 MHz, CDCl3) δ 7.26–7.21 (5H, m, HAr), 5.82–5.84 (2H, m, H-1′), 5.38 (2H, d, J = 1.8 Hz, HA-2′), 5.20 (2H, dd, J = 5.0 and 6.8 Hz, HB-2′), 4.30 (2H, dd, J = 1.2 and 3.0 Hz, H-3 and H-4), 3.70 (2H, bs, CH2Bn), 3.12 (2H, dd, J = 1.8 and 7.8 Hz, H-2 and H-5), 1.42 (3H, s, Me-acetonide), 1.27 (3H, s, Me-acetonide); 13C NMR (50 MHz, CDCl3) δ 138.5, 136.5, 130.1, 128.1, 127.1, 118.9, 113.7, 83.7, 77.3, 71.7, 52.6, 27.4, 25.6; HRMS (EI) calcd for C18H24NO2 (M + H)+ 286.1803; found 286.1801.
(2S,3S,4R,5R)-1-Benzyl-3,4-isopropylidenedioxy-5-methylpyrrolidine-2-ethanol 18
9-BBN (1.8 ml, 0.9 mmol) was added to a solution of vinylpyrrolidine 16 (50 mg, 0.18 mmol) in THF (1.50 mL) at 0 °C. The reaction mixture was stirred at r.t. for 4 h. A saturated aqueous solution of NaBO3 was added and the resulting mixture was stirred at r.t. for 18 h. The reaction product was then extracted with DCM (3×15 mL). The combined organic layers were washed with brine, dried (Na2SO4), filtered, and concentrated. The resulting crude residue was purified by flash chromatography (silica gel, n-hexane–EtOAc 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) to obtain 18 (15 mg, 30%). [α]20D −12.8 (c 0.8, CH3Cl); IR (film): 3397, 2980, 2932, 2866, 1452, 1341, 1028, 733, 702 cm−1; 1H NMR (200 MHz, CDCl3) δ 7.33–7.17 (5H, m, HAr), 4.29 (1H, dd, J = 5.0 and 6.8 Hz, H-3), 4.16 (1H, dd, J = 4.6 and 6.8 Hz, H-4), 4.05–3.99 (1H, m, HA-2′), 3.84 (1H, d, J = 14.3 Hz, HA–CH2Bn), 3.54–3.40 (1H, m, HB-2′), 3.49 (1H, d, J = 14.3 Hz, HB–CH2Bn), 3.12–3.05 (1H, m, H-2), 2.70–2.64 (1H, m, H-5), 1.95–1.85 (1H, m, HA-1′), 1.83–1.75 (1H, m, HB-1′), 1.44 (3H, s, Me-acetonide), 1.29 (3H, s, Me-acetonide), 1.17 (3H, d, J = 5.6 Hz, Me–C-5); 13C NMR (50 MHz, CDCl3) δ 135.2, 132.3, 130.4, 128.6, 113.2, 83.5, 81.2, 72.7, 68.663.5, 54.4, 27.6, 24.3, 17.6; HRMS (EI) calcd for C17H26NO3 (M + H)+ 292.1907; found 292.1911.
4) to obtain 18 (15 mg, 30%). [α]20D −12.8 (c 0.8, CH3Cl); IR (film): 3397, 2980, 2932, 2866, 1452, 1341, 1028, 733, 702 cm−1; 1H NMR (200 MHz, CDCl3) δ 7.33–7.17 (5H, m, HAr), 4.29 (1H, dd, J = 5.0 and 6.8 Hz, H-3), 4.16 (1H, dd, J = 4.6 and 6.8 Hz, H-4), 4.05–3.99 (1H, m, HA-2′), 3.84 (1H, d, J = 14.3 Hz, HA–CH2Bn), 3.54–3.40 (1H, m, HB-2′), 3.49 (1H, d, J = 14.3 Hz, HB–CH2Bn), 3.12–3.05 (1H, m, H-2), 2.70–2.64 (1H, m, H-5), 1.95–1.85 (1H, m, HA-1′), 1.83–1.75 (1H, m, HB-1′), 1.44 (3H, s, Me-acetonide), 1.29 (3H, s, Me-acetonide), 1.17 (3H, d, J = 5.6 Hz, Me–C-5); 13C NMR (50 MHz, CDCl3) δ 135.2, 132.3, 130.4, 128.6, 113.2, 83.5, 81.2, 72.7, 68.663.5, 54.4, 27.6, 24.3, 17.6; HRMS (EI) calcd for C17H26NO3 (M + H)+ 292.1907; found 292.1911.
Acknowledgements
The authors gratefully acknowledge the help of A. Lithgow (NMR) and C. Raposo (MS) of Universidad de Salamanca; FSE, MICINN CTQ2009-11172BQU, Junta de Castilla and León for financial support. MFF is grateful to the JCyL for her fellowship.References
- (a) S. Patai, Z. Rappoport and C. Stirling, The Chemistry of Sulphones and Sulphoxides; John Wiley and Sons, Chichester, 1988 Search PubMed; (b) N. S. Simpkins, Sulphones in Organic Synthesis; Pergamon Press, Oxford, 1993 Search PubMed; (c) L. A. Paquette, Synlett, 2001, 1–12; (d) C. Nájera and J. M. Sansano, Recent Res. Devel. Org. Chem., 1998, 2, 637 Search PubMed; (e) R. Chinchilla and C. Najera, Recent Res. Devel. Org. Chem., 1997, 1, 437 Search PubMed; (f) H. Feuer, Nitrile Oxides, Nitrones and Nitronates in Organic Synthesis: Novel Strategies in Synthesis; John Wiley and Sons, New York, 2008 Search PubMed.
- (a) J. N. Martin and R. C. Jons, in Nitrones in Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Towards Heterocycles and Natural Products, ed. A. Padwa and W. H. Pearson, John Wiley & Sons, Hoboken NJ, 2003, ch. 1 Search PubMed; (b) T. Hashimoto and K. Maruoka, 1,3-Dipolar Cycloadditions, in Handbook of Cyclization Reactions; ed. S. Ma, Wiley-VCH, New York, 2009, ch. 3 Search PubMed; (c) K. V. Gothelf and K. A. Jørgensen, Chem. Commun., 2000, 1449 RSC; (d) P. Merino, in Science of Synthesis Knowledge Updates, ed. E. Schaumann, Thieme, Stuttgart, 2011,vol. 2010/4, pp. 325–403 Search PubMed.
- (a) F. Cardona, G. Moreno, F. Guarna, P. Vogel, C. Schuetz, P. Merino and A. Goti, J. Org. Chem., 2005, 70, 6552 CrossRef CAS; (b) A. Brandi, F. Cardona, S. Cicchi, F. Cordero and A. Goti, Chem.–Eur. J., 2009, 15, 7808 CrossRef CAS; (c) S. Cicchi, M. Marradi, P. Vogel and A. Goti, J. Org. Chem., 2006, 71, 1614 CrossRef CAS; (d) I. Delso, T. Tejero, A. Goti and P. Merino, Tetrahedron, 2010, 66, 1220 CrossRef CAS; (e) M. Closa and R. H. Wightman, Synth. Commun., 1998, 28, 3443 Search PubMed; (f) S. Desvergnes, Y. Vallee and S. Py, Org. Lett., 2008, 10, 2967 Search PubMed; (g) C. S. McKay, J. A. Blake, J. Cheng, D. C. Danielson and J. P. Pezacki, Chem. Commun., 2011, 47, 10040 RSC; (h) Y. Li, M. Huang, Y. Yamashita, A. Kato, Y. Jia, W. Wang, G.W. J. Fleet, R. J. Nash and C. Yu, Org. Biomol. Chem., 2011, 9, 3405 RSC; (i) J. Revuelta, S. Cicchi, A. Goti and A. Brandi, Synthesis, 2007, 485 CAS.
- B. Lovejoy, A. R. Welch, S. Carr, C. Luong, C. Broka, R. T. Hendriks, J. A. Campbell, K. A. M. Walker, R. Martin, H. Van Wart and M. F. Browner, Nat. Struct. Biol., 1999, 6, 217 CrossRef CAS.
- (a) S. A. Ali and M. I. M. Wazeer, J. Chem. Soc. Perkin Trans., 1986, 2, 1789 Search PubMed; (b) G. Giambastiani, S. Cicchi, A. Giannasi, L. Luconi, A. Rossin, F. Mercuri, C. Bianchini, A. Brandi, M. Melucci and G. Ghini, Chem. Mater., 2011, 23, 1923 CrossRef CAS; (c) Z. Zhang, S. Nakagawa, A. Kato, Y. Jia, X. Hua and C. Yu, Org. Biomol. Chem., 2011, 9, 7713 RSC; (d) M. Buchlovic, S. Man and M. Potacek, Synthesis, 2012, 44, 973 Search PubMed; (e) C. S. Mckay, M. Chigrinova, J. A. Blake and J. P. Pezacki, Org. Biomol. Chem., 2012, 10, 3066 RSC; (f) G. Gosset, J. L. Clement, M. Culcasi, A. Rockenbauer and S. Pietri, Org. Biomol. Chem., 2011, 19, 2218 Search PubMed; (g) S. Stecko, M. Jurczak, Z. Urbanczyk-Lipkowska, J. Solecka and M. Chmielewski, Carbohydr. Res., 2008, 343, 2215 CrossRef CAS; (h) E. Coutouli-Argyropoulou, C. Xatzis and N. Argyropoulos, Nucleosides, Nucleotides Nucleic Acids, 2008, 27, 84 CrossRef CAS; (i) A. Marwaha, R. K. Goel and M. P. Mahajan, Bioorg. Med. Chem. Lett., 2007, 17, 5251 Search PubMed.
- (a) M. F. Flores, P. Garcia, N. M. Garrido, I. S. Marcos, F. Sanz and D. Diez, Tetrahedron: Asymmetry, 2011, 22, 1467 Search PubMed; (b) D. Diez, M. T. Beneitez, I. S. Marcos, N. M. Garrido, P. Basabe, F. Sanz, H. B. Broughton and J. G. Urones, Org. Lett., 2003, 5, 4361 Search PubMed.
- (a) P. D. Croce, C. Rosa, R. Stradi and M. Ballabio, J. Heterocycl. Chem., 1983, 20, 819 Search PubMed; (b) A. El-Din and A. M. Nour, Bull. Chem. Soc. Jpn., 1986, 59, 1239 Search PubMed; (c) J. Sinkkonen, O. Martiskainen and K. Pihlaja, J. Heterocycl. Chem., 2006, 43, 1267 CrossRef CAS. For other interesting examples of 1,3-dipolar reaction of vinylsulfones: (d) J. K. Laha, Chem. Nat. Compd., 2010, 46, 254 Search PubMed; (e) T. Llamas, R. G. Arrayás and J. C. Carretero, Org. Lett., 2006, 8, 1795 CrossRef and references cited therein; (f) V. Padmavathi, K. V. Reddy, A. Valaiah, T. V. R. Reddy and D. B. Reddy, Heteroat. Chem., 2002, 13, 677 CrossRef CAS; (g) M. Burdisso, R. Gandolfi, P. Grunanger and A. Rastelli, J. Org. Chem., 1990, 55, 3427 CrossRef CAS; (h) J. L. García Ruano, A. Fraile, A. M. Martín Castro and M. R. Martín, J. Org. Chem., 2005, 70, 8825 CrossRef CAS. See also the 1,3-dipolar cycloaddition of a vinylsulfonates: (i) S. Caddick and H. D. Bush, Org. Lett., 2003, 5, 2489 CrossRef CAS.
- M. F. Flores, P. García, N. M. Garrido, C. T. Nieto, P. Basabe, I. S. Marcos, F. Sanz-González, J. M. Goodman and D. Díez, Tetrahedron: Asymmetry, 2012, 23, 76 Search PubMed.
- (a) J. Revuelta, S. Cicchi and A. Brandi, Tetrahedron Lett., 2004, 45, 8375 CrossRef CAS; (b) J. Revuelta, S. Cicchi and A. Brandi, J. Org. Chem., 2005, 70, 5636 CrossRef CAS; (c) S. Cicchi, A. Goti, A. Brandi, A. Guarna and F. De Sarlo, Tetrahedron Lett., 1990, 31, 3351 CrossRef CAS; (d) P. Cid, M. Closa, P. de March, M. Figueredo, J. Font, E. Sanfeliu and A. Soria, Eur. J. Org. Chem., 2004,(20), 4215 Search PubMed; (e) A. K. Lewis, J. B. Mok, D. A. Tocher, J. D. Wilden and S. Caddick, Org. Lett., 2006, 8, 5513 CrossRef; (f) F. Wierschem and K. Rueck-Braun, Eur. J. Org. Chem., 2004, 2321 CrossRef CAS; (g) M. Szostak, M. Spain, D. Parmar and D. J. Procter, Chem. Commun., 2012, 48, 330 RSC; (h) S. Cicchi, M. Bonanni, F. Cardona, J. Revuelta and A. Goti, Org. Lett., 2003, 5, 1773 CrossRef CAS and references therein.
- (a) N. A. Lebel, M. E. Post and D. Hwang, J. Org. Chem., 1979, 44, 1819 CrossRef CAS; (b) J. J. Tufariello, G. B. Mullen, J. J. Tegeler, E. J. Trybulski, S. C. Wong and S. A. Ali, J. Am. Chem. Soc., 1979, 101, 2435 CrossRef CAS.
- (a) M. P. Van Boggelen, A. G. F. B. Van Dommelen, S. Jiang and G. Singh, Tetrahedron, 1997, 53, 16897 CrossRef CAS; (b) P. Bayón, P. de March, M. Figueredo and J. Font, Tetrahedron, 1998, 54, 15691 Search PubMed; (c) C. Chevrier, D. LeNouen, M. Neuburger, A. Defoin and C. Tarnus, Tetrahedron Lett., 2004, 45, 5363 CrossRef; (d) W. Carruthers, P. Coggins and J. B. Weston, J. Chem. Soc., Chem. Commun., 1991, 117 RSC; (e) S. I. Murahashi, Y. Kodera and T. Hosomi, Tetrahedron Lett., 1988, 29, 5949 CrossRef CAS; (f) J. J. Tufariello and S. A. Ali, J. Am. Chem. Soc., 1979, 101, 7114 CrossRef CAS.
- Crystal data for 13a: C22H25NO5S, CH2Cl2, M = 500.42, monoclinic, space group P21, a = 6.0659(2) Å, b = 15.5656(6) Å, c = 12.9608(5) Å, α = γ = 90°, β = 99.791(3)°, V = 1205.93(8) Å3, Z = 2, Dc = 1.378 Mg m−3, μ(Cu-Kα) = 3.521 mm−1, F(000) = 524. 6938 reflections were collected at 4.48 ≤ 2θ ≤ 67.01 and merged to give 3318 unique reflections (Rint = 0.0254), of which 3132 with I > 2σ(I) were considered to be observed. Final values are R1 = 0.0379, wR2 = 0.1017, GOF = 1.039, max/min residual electron density 0.355 and −0.352 e. Å−3. A suitable single crystal of the 13a compound was mounted on a glass fibre for data collection on a Bruker Kappa APEX II CCD (charge coupled device) diffractometer. Data were collected at 298 K using Cu-Kα radiation (λ = 1.54178 Å) and ω scan technique, and were corrected for Lorentz and polarization effects. Structure solution, refinement and data output were carried out with the SHELXTLTM program package. The structure was solved by direct methods combined with difference Fourier synthesis and refined by full-matrix least-squares procedures, with anisotropic thermal parameters in the last cycles of refinement for all non-hydrogen atoms. Hydrogen atom positions were calculated by geometrical methods and refined as a riding model. CCDC 888605.
- K. R. Campos, Chem. Soc. Rev., 2007, 36, 1069 RSC.
- F. Casuscelli, U. Chiacchio, A. Rescifina, R. Romeo, G. Romeo, S. Tommasini and N. Uccella, Tetrahedron, 1995, 51, 2979 CrossRef CAS.
- (a) G. Pandey and S. R. Gadre, Arkivoc, 2003, iii, 45; (b) N. J. Leonard and W. K. Musker, J. Am. Chem. Soc., 1960, 82, 5148 CrossRef CAS; (c) G. Pandey, G. Kumaraswamy and P.Y. Reddy, Tetrahedron, 1992, 48, 8295 CrossRef CAS; (d) S. M. A. Hashmi, S. A. Ali and M. I. M. Wazeer, Tetrahedron, 1998, 54, 12959 CrossRef CAS; (e) S. A. Ali and H. A. AI-Muallem, Tetrahedron, 1993, 49, 7373 Search PubMed See too: N. Tokitoh and R. Okazaki, Tetrahedron Lett.,1984, 25, 4677.
- A. Goti, S. Cicchi, V. Fedi, L. Nannelli and A. Brandi, J. Org. Chem., 1997, 62, 3119 CrossRef CAS.
- (a) L. Guerrier, J. Royer, D. S. Grierson and H. P. Husson, J. Am. Chem. Soc., 1983, 105, 7754 CrossRef; (b) J. Royer and H. P. Husson, J. Org. Chem., 1985, 50, 670 CrossRef CAS; (c) P. Q. Huang, S. Arseniyadis and H.-P Husson, Tetrahedron Lett., 1987, 28 Search PubMed; (d) for an account: H. P. Husson and J. Royer, Chem. Soc. Rev., 1999, 28, 383 Search PubMed; (e) M. Amat, F. Subrizi, V. Elias, N. Llor, E. Molins and J. Bosch, Eur. J. Org. Chem., 2012, 1835 Search PubMed; (f) M. Amat, C. Arróniz, E. Molins, C. Escolano and J. Bosch, Org. Biomol. Chem., 2011, 9, 2175 RSC; (g) M. Amat, M. Pérez and J. Bosch, Chem.–Eur. J., 2011, 17, 7724 CrossRef CAS and references cited therein (h) G. Arena, N. Zill, J. Salvadori, N. Girard, A. Mann and M. Taddei, Org. Lett., 2011, 13, 2294 Search PubMed; (i) J. J. Zhuang, J. L. Ye, H. K. Zhang and P. Q. Huang, Tetrahedron, 2012, 68, 1750 Search PubMed; (j) L-H. Yan, F. Dagorn, E. Gravel, B. Séon-Méniel and E. Poupon, Tetrahedron, 2012, 68, 6276 Search PubMed; (k) J. Alladoum, S. Roland, E. Vrancken, P. Mangeney and C. Kadouri-Puchot, J. Org. Chem., 2008, 73, 9771 CrossRef CAS; (l) C. Berini, F. Minassian, N. Pelloux-Léon, J. Denis, Y. Vallée and C. Philouze, Org. Biomol. Chem., 2008, 6, 2574 RSC; (m) N. Kielland, F. Catti, D. Bello, N. Isambert, I. Soteras, F. J. Luque and R. Lavilla, Chem.–Eur. J., 2010, 16, 7904 CrossRef CAS; (n) N. Yamazaki, H. Suzuki and C. Kibayashi, J. Org. Chem., 1997, 62, 8280 CrossRef CAS.
- A. M. Palmer and V. Jäger, Eur. J. Org. Chem., 2001, 2547 CrossRef CAS.
- C. Chevrier, D. Le Nouën, A. Defoin and C. Tarnus, Carbohydr. Res., 2011, 346, 1202 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, optimization studies, complete characterization of products, NMR spectra, IR spectra, CCDC 888605. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ra22110a |
| ‡ This manuscript is dedicated to Prof. Arturo San Feliciano on the occasion of his 65th birthday. |
| This journal is © The Royal Society of Chemistry 2012 |


