A facile synthesis method for Ni(OH)2 ultrathin nanosheets and their conversion to porous NiO nanosheets used for formaldehyde sensing†
Guanghui
Li‡
abc,
Xuewen
Wang‡
a,
Haiyan
Ding
a and
Ting
Zhang
*a
ai-LAB, Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences, Suzhou, 215123, China. E-mail: tzhang2009@sinano.ac.cn
bInstitute of Semiconductors, Chinese Academy of Sciences, Beijing, 100083, China
cGraduate University of Chinese Academy of Sciences, Beijing, 100049, China
First published on 23rd October 2012
Abstract
Single-crystalline β-Ni(OH)2 ultrathin nanosheets were synthesized via a simple electrochemical reaction of Ni electrodes with a mixed solution of NaCl, NaOH, and NH4Cl at room temperature. The average thickness of β-Ni(OH)2 nanosheets is in the range 1–15 nm, which can be readily tuned by changing the concentration of NaCl. The phase structure, composition, morphology, and thickness of Ni(OH)2 nanosheets were characterized by X-ray diffraction (XRD), transmission electron microscopy (TEM), and atomic force microscopy (AFM). The mechanism of the nanosheet formation is proposed as the selective adsorption of NH3 molecules on the (001) crystal face of β-Ni(OH)2 which suppresses growth in the [001] direction. Porous NiO ultrathin nanosheets were obtained by thermal decomposition of β-Ni(OH)2 nanosheets in air at 400 °C for 2 h. Gas sensing properties of NiO ultrathin nanosheets were investigated, and the sensors exhibited high sensitivity, low detection limit, and wide dynamic range for detection of formaldehyde.
Introduction
Recently, indoor air pollutant detection and environmental evaluation have attracted more and more attention. Formaldehyde (HCHO) is one of the most harmful gases among indoor air pollutants, which has been proven to be a human carcinogen and an allergen, and can cause dermatitis, respiratory irritation, asthma, and pulmonary edema.1,2 It is difficult to achieve selective and accurate detection of HCHO when it is coupled with other volatile organic compounds (VOCs) including benzene (C6H6), toluene (C7H8), etc. Up to now, several methods, such as spectroscopic, electrochemical, and chemiluminescent methods have been developed for the detection of HCHO.3–6 However, most of these methods require expensive and bulky instruments, which cannot be used for on-site detection. Colorimetric detection offers a cheap alternative method, but it is generally slow (typically >30 min) and inaccurate. Metal oxide based chemical sensors have been widely used for the detection of toxic gases including HCHO owing to their simple design, easy fabrication, good stability, high sensitivity, wide detection range, and fast response time. Gas sensors with nanostructured metal oxides have been proven to possess enhanced sensing capacity compared to bulk materials, and this research field remains active to be explored.Nickel oxide (NiO), as a p-type semiconductor with a wide band gap of 3.6 eV,7 has attracted considerable attention due to its unique properties and wide practical applications.8–11 Because of the high surface-to-volume ratio and high surface activity, NiO nanostructures exhibited superior performance for lithium ion batteries,12,13 fuel cells,14 supercapacitors,15 catalysts,16,17 and gas sensors.18–22 For these applications, the morphology and size of NiO nanostructures have great influence on their properties and performance, especially for gas sensing applications. For example, traditional film-like structured NiO showed good sensitivities for the detection of CO, H2, NH3, NO2 and HCHO.18–20 NiO nanowires exhibited strong response and high sensitivity towards toluene, ethanol, acetone, triethylamine and methanol.21 Mesoporous NiO nanosheet based sensors showed a reliable response to low-level (1–20 ppm) NO2 with good recovery behaviours.21 Among NiO nanostructures, 2-dimensional (2-D) NiO nanosheets are important basic building blocks for nanodevices due to their unique structural characteristics (i.e., their thickness is in the molecular range, but their lateral size ranges from nanoscale to microscale). However, synthesis of NiO ultrathin nanosheets, especially with a thickness of less than 10 nm, is still a challenging task. For example, Liang et al. synthesized β-Ni(OH)2 nanosheets with thickness in the range 12–20 nm by the hydrothermal method at 200 °C using nickel acetate as the nickel source and aqueous ammonia as a complexing reagent, and NiO nanosheets have been obtained by thermal decomposition of the as-prepared β-Ni(OH)2 precursor at 400 °C.23 Ida et al. prepared 1-nm-thick β-Ni(OH)2 nanosheets by exfoliation of layered Ni(OH)2 intercalated with dodecyl sulphate ions at 120 °C.24 Wang et al. synthesized β-Ni(OH)2 and NiO nanosheets by hydrothermal treatment of nickel oxalate source and methylamine at 120 °C.25 Recently, Hoa et al. reported the synthesis of crystalline mesoporous NiO nanosheets (~20 nm of thickness) by the same method.21 However, the hydrothermal method requires expensive autoclaves, high reaction pressure and temperature. To the best of our knowledge, controlled synthesis of Ni(OH)2 and NiO ultrathin (less than 10 nm) nanosheets at room temperature and atmospheric pressure has not been reported.
Herein, we demonstrate an efficient and facile electrochemical method for the synthesis of ultrathin single-crystalline β-Ni(OH)2 nanosheets, which can be thermally decomposed to polycrystalline NiO ultrathin nanosheets to make sensitive HCHO sensors. Single-crystalline β-Ni(OH)2 nanosheets were synthesized in solution by a simple electrochemical reaction of Ni electrodes with a mixture solution of NaCl, NaOH, and NH4Cl under ambient conditions. Control over the average thickness of single-crystalline β-Ni(OH)2 nanosheets was achieved by changing the concentration of NaCl. Meanwhile, the growth mechanism of ultrathin β-Ni(OH)2 nanosheets and the formaldehyde sensing properties of the polycrystalline NiO ultrathin nanosheets were systematically studied.
Experimental
Materials and synthesis conditions
All of the chemicals were of analytical grade and were used directly without further purification. Nickel wires (Ni, >99.5%), sodium chloride (NaCl, >99.5), ammonium chloride (NH4Cl, >99.5%) and sodium hydroxide (NaOH, >99.5%) were purchased from the Sinopharm Chemical Reagent Co. Ltd., China. Nickel wires were pre-treated in 1.0 M HCl solution for 30 s, ultrasonically washed in absolute ethanol for 15 min, and then dried naturally.In a typical synthesizing experiment, a mixture of 60 mmol NaCl, 10 mmol NH4Cl, and 7.5 mmol NaOH solution was prepared in 100 ml deionized water and magnetically stirred to form the electrolyte. Two pre-treated nickel wires were used as the cathode and anode electrodes, and the electrochemical reaction was performed under potentiostatic control (5 V) under ambient conditions for 90 min. During the process, the color of the electrolyte changed from transparent to light-blue, and then light-green precipitates were produced in the solution, as shown in Fig. S1.† The precipitates were separated from the solution by centrifugation at 8000 rpm, then washed thoroughly with deionized water and ethanol, and finally dried at 50 °C in air. The as-prepared Ni(OH)2 nanosheets were converted into NiO by calcining at 400 °C for 2 h in air.
Characterization and measurements
The as-prepared Ni(OH)2 and NiO nanosheets were characterized by X-Ray diffraction (XRD), atomic force microscopy (AFM) and transmission electron microscopy (TEM). The XRD analysis was performed using a BRUKER D8 Discover X-Ray diffractometer with Cu-Kα Radiation (λ = 1.5405 Å). The TEM images were obtained using FEI Tecnai G2 F20 S-Twin at 200 kV and the AFM images were obtained using Agilent 5500 with tapping mode. The electrical properties (I–V curves) were measured with a KEITHLEY 2602A System Source Meter (Keithley Instruments, Inc.) with potentials sweeping from −5 V to 5 V at different elevated temperatures.Sensor fabrication and sensing tests
The gas sensors were fabricated by the method described in the literature.22 First, NiO nanosheets were mixed with terpineol to form a paste, then the paste was coated onto ceramic tubes with a pair of pre-printed gold electrodes, which were connected by four platinum wires. A Ni–Cr heating wire was inserted into the tube to provide the working temperature for the gas sensors. In order to evaporate terpineol and improve the stability and repeatability of the sensors, the fabricated sensors were calcinated at 600 °C for 2 h and then aged at 350 °C for 5 days in air. The HCHO sensing tests were measured by the WS-30A system (Zhengzhou Winsen Electrics Technology Co. Ltd., China). A customized sealed testing chamber over the sensors was made with gas inlet and outlet ports for gas flow-through. During all the sensing tests, air was used as the reference gas and diluting gas for the different concentrations of HCHO.Results and discussion
The morphology and detailed structure of the as-synthesized β-Ni(OH)2 and NiO nanosheets were measured by TEM as shown in Fig. 1a–d. Fig. 1a and b show the typical TEM and corresponding high-resolution TEM (HRTEM) images of β-Ni(OH)2 with a hexagonal structure. The morphology of β-Ni(OH)2 is a 2-D shaped nanosheet with size ranging from 40 to 100 nm. The fringe spacing of the observed lattice planes is ~0.2707 nm in the HRTEM image, which agrees with the d spacing of the (100) crystal planes in hexagonal β-Ni(OH)2. The insert of Fig. 1b is the corresponding fast Fourier transform (FFT) pattern of the HRTEM images, and it can be indexed as the [001] zone axis, which is perpendicular to the nanosheet surface of single crystalline β-Ni(OH)2 with a hexagonal structure. The HRTEM and corresponding FFT reveal that the β-Ni(OH)2 nanosheets are well-crystallized single crystalline and grow along the a–b plane. Fig. 1c and d show the typical TEM and HRTEM images and corresponding selected-area electron diffraction (SAED) pattern of NiO nanosheets. Fig. 1c gives a general morphology of the NiO nanosheets and indicates that NiO nanosheets are in aggregated shapes and are assembled with a porous structure consisting of interconnected nano-crystallites, which are supported by the HRTEM image shown in Fig. 1d.26 The corresponding SAED pattern inset in Fig. 1d displays several sharp rings, which are indexed to the (111), (200) and (220) planes of the cubic phase of NiO (JCPDS card no. 65-2901). It further confirms that the nanosheets are polycrystalline cubic NiO. | ||
| Fig. 1 (a) TEM and (b) HRTEM images of as-obtained β-Ni(OH)2 nanosheets. The inset of (b) is the corresponding FFT pattern of the HRTEM image. (c) TEM and (d) HRTEM images of porous NiO ultrathin nanosheets prepared by thermal decomposition of β-Ni(OH)2 nanosheets. The inset of (d) is the SAED pattern of NiO nanosheets. (e) XRD patterns of Ni(OH)2 and porous NiO nanosheets. | ||
Fig. 1e shows the XRD patterns of Ni(OH)2 and porous NiO nanosheets. As can be seen in Fig. 1e-I, all the diffraction peaks can be indexed to hexagonal β-Ni(OH)2 with lattice parameters a = 3.12 Å and c = 4.605 Å, which are consistent with the values in standard data files (JCPDS no. 14-0117). No other peaks have been found, suggesting high purity of the as-synthesized single crystalline Ni(OH)2 nanosheets. From Fig. 1e-II, three peaks at 2θ = 37.1°, 43.1° and 62.6° are observed. According to reported values (JCPDS card no. 65-2901), these peaks are assigned to (111), (200) and (220) diffraction lines of the cubic NiO phase, respectively. From the XRD results we can see that β-Ni(OH)2 is totally transformed to NiO after calcination at 400 °C for 2 h.
During the electrochemical reactions, NaCl concentration is found to be the key factor in controlling the thickness of single-crystalline β-Ni(OH)2 ultrathin nanosheets. To compare the thicknesses, β-Ni(OH)2 nanosheets were synthesized with 0.2 M, 0.6 M, and 1.0 M of NaCl, respectively, and ultrasonically dispersed into deionized water, then drop-deposited onto silicon substrates for AFM analysis. Fig. 2a shows a typical AFM image of a single β-Ni(OH)2 nanosheet, and indicates that the thickness is about 1 nm. Fig. 2b–d show the histograms of thickness distribution of β-Ni(OH)2 nanosheets by counting more than 60 sheets for each sample. At 0.2 M NaCl, the thickness distribution is in the range 1–9 nm (Fig. 2b). When the concentrations of NaCl are increased to 0.6 M and 1.0 M, the typical thicknesses of the nanosheets are in the range 3–12 nm and 4–15 nm, respectively (Fig. 2c,d). The standard deviations of the thicknesses were calculated to be 3 nm, 6 nm, and 10 nm. The average thickness of the nanosheets increases with increasing concentration of NaCl. The above result suggests that controllable synthesis with different thicknesses of β-Ni(OH)2 ultrathin nanosheets can be achieved by tuning the concentration of NaCl.
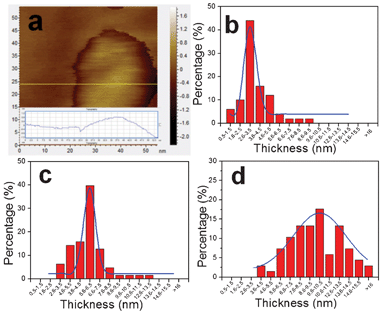 | ||
| Fig. 2 (a) AFM image of a typical Ni(OH)2 nanosheet. (b, c, d) Histograms of the thickness distribution of β-Ni(OH)2 nanosheets prepared with three different concentrations of NaCl as 0.2 M, 0.6 M, and 1.0 M, respectively. | ||
The growth mechanism of ultrathin β-Ni(OH)2 nanosheets is depicted in Fig. 3a. We believe that NH3 molecules produced by the reaction of NaOH and NH4Cl play a vital role. In a typical crystal unit of hexagonal β-Ni(OH)2, each Ni atom is surrounded by six O atoms, whereas each O atom is associated with three Ni atoms and a H atom.27 The H atoms form as a parallel plane for the (001) lattice plane of hexagonal β-Ni(OH)2. NH3 molecules produced by the reaction of NaOH and NH4Cl are linked with H atoms of the (001) plane by hydrogen bonding,28 and suppress growth in the [001] direction. In order to further illuminate the function of NH3 and understand the growth mechanism, the products obtained via the electrochemical reaction with only 1 M NaCl (without NaOH and NH4Cl) were characterized by SEM and XRD, as shown in Fig. S2a and b.† The obtained powders are irregularly shaped α-Ni(OH)2 without 2-D sheet-like structures, which further indicates that an important role in the formation of β-Ni(OH)2 ultrathin nanosheets is not NaCl, but rather NH3.
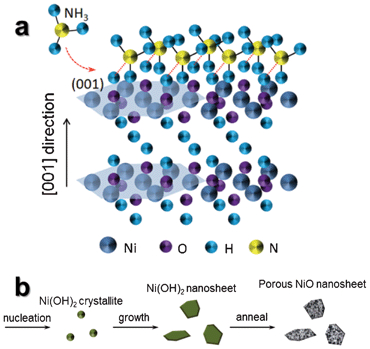 | ||
| Fig. 3 (a) Schematic of NH3 molecules attached to (001) faces of the β-Ni(OH)2 nanosheet. (b) Schematic of formation steps of porous NiO ultrathin nanosheets. | ||
Tuning the concentrations of NaCl and NaOH is an effective way to form the electrolyte with different cation concentrations and pH values, and regulates the reaction and nucleation kinetics for the controllable synthesis of β-Ni(OH)2 ultrathin nanosheets. In the electrochemical reaction process, the reactions to form Ni(OH)2 nanosheets are formulated as follows:
Anode reactions:
| Ni − 2e− → Ni2+ | (1) |
| 4OH− − 4e− → O2(gas) + 2H2O | (2) |
Cathode reactions:
| 2H2O + 2e− → H2(gas) + 2OH− | (3) |
| Ni2+ + 2e− → Ni | (4) |
Solution reactions:
| NH4+ + OH− ↔ NH3 + H2O | (5) |
| Ni2+ + 6NH3 ↔ [Ni(NH3)6]2+ | (6) |
| Ni2+ + 2OH− ↔ Ni(OH)2 | (7) |
As described in the previous report,29 [Ni(NH3)6]2+ coordination compound is formed by Ni2+ reacting with NH3. When the evaporation of NH3 and reaction (7) take place, reaction (6) will shift to the left-hand side in order to keep reaction (7) going continuously. We think reaction (6) is the important step to slowly release Ni2+ into the solution, so that most of the Ni(OH)2 nanosheets are formed in the solution instead of on the cathode electrode. Fig. 3b shows the formation of ultrathin NiO nanosheets in three steps. In the first step, reaction (7) takes place to form Ni(OH)2 nanoclusters in the presence of NH3 molecules. The Ni(OH)2 nanoclusters continue to grow by sustaining hydrolyzation of [Ni(NH3)6]2+. In the second step, NH3 molecules adsorb on the (001) plane of the Ni(OH)2 crystal nucleus, and passivate the (001) plane for the formation of ultrathin 2-D nanosheets growing along the a–b plane. The thickness of obtained nanosheets can be controlled by changing the NaCl concentrations. In the last step, polycrystalline NiO nanosheets can be obtained by the thermal decomposition of the corresponding β-Ni(OH)2 in air. The color change of the electrolyte further supports those reactions during the synthesis process (Fig. S1†).
2-D nanosheet structures are promising sensing materials for gas sensors due to several advantages, such as large specific surface area, large number of lattice defects, and high surface activity.22,30 In this work, the specific surface area and pore-size distribution of the as-obtained NiO nanosheets were measured by a typical N2 adsorption–desorption experiment, and the results are shown in Fig. S3.† The BET surface area calculated from N2 adsorption is 65.6 m2 g−1, which is higher than the values reported in the previous work for NiO with meso- and nano-porous structures.31–33 The BJH calculations for the pore-size distribution show that NiO nanosheets have nanopores in the main range 2–5 nm. The sensors based on ultrathin NiO nanosheets were prepared, and the electrical properties and gas sensing performance towards HCHO were investigated at temperatures ranging from 160 °C to 400 °C (Fig. 4). Fig. 4a shows the I–V curves of a typical sensor at different temperatures. Linear behaviour is normally observed, which indicates the ohmic contact between the NiO nanosheet and the Au electrodes. The resistance of the sensor markedly decreases with the increase in temperature, which can be explained by the semiconducting properties of the obtained NiO nanosheets. Fig. 4b displays the sensor's typical response to 50 ppm HCHO at different temperatures. The sensitivity is defined as Rg/Ra, where Rg and Ra are the resistance of the sensors in target gas and in air, respectively. The sensitivity reaches its maximum at 240 °C, and then decreases when the temperature is further increased (Fig. 4c). Therefore, the sensing tests towards HCHO with different concentrations ranging from 1 to 1000 ppm were carried out at 240 °C. The real-time dynamic response and corresponding sensitivity are displayed in Fig. 4c and d, respectively. After exposure to HCHO, the resistance of the sensor increases abruptly and gradually approaches steady state and then decreases immediately to recover its initial value when purged with dry air.
 | ||
| Fig. 4 (a) I–V curves of NiO nanosheet based sensor at different temperatures (ranging from 160 to 400 °C). (b) Sensitivity (Rg/Ra) of the sensor toward 50 ppm HCHO at different temperatures. (c) Real-time response to HCHO from 1 ppm to 1000 ppm at 240 °C. (d) Sensitivity of NiO nanosheet based sensor vs. the square root of different HCHO concentrations. (e) Sensor's response to 100 ppb HCHO at 240 °C. (f) Selectivity of NiO nanosheet based sensor to different gases with different concentrations (50 ppm HCHO, 1000 ppm C6H6, 500 ppm C7H8, 500 ppm NH3, 500 ppm CH4, 500 ppm H2, and 500 ppm H2O). | ||
The response time and recovery time, defined as the time required to reach 90% of the steady state value, are generally less than 1 min. Fig. 4d shows the linear relationship between the sensitivity and the square root of HCHO concentration, which can be explained with the Langmuir adsorption isotherm model at low analyte concentrations.34 As shown in Fig. 4e, the detection limit (defined as the concentration providing a signal-to-noise ratio of at least 335) of this specific sensor for HCHO is about 100 ppb. By employing noise filtering methods, lower detection limits could be possible with reduced electrical noise of the sensing system. The sensor was also exposed to different typical indoor air pollutants for selectivity evaluation, such as 1000 ppm C6H6, 500 ppm C7H8, 500 ppm NH3, 500 ppm CH4, 500 ppm H2, and 500 ppm H2O. The results are shown in Fig. 4f, which demonstrate that the sensor displays higher selectivity for detection of HCHO from these interfering gases.
NiO is a p-type semiconductor, in which vacancies occur in cation sites. For each cation vacancy, two Ni2+ at lattice sites are oxidized to Ni3+ to keep the electrical neutrality, hence there must be two electron holes formed from each metal vacancy.22 Cation vacancies act as electron donors and oxygen molecules act as acceptors. According to the gas sensing mechanism of metal oxide semiconductors, when a NiO based sensor is working at high temperature (at 240 °C) in air, atmospheric oxygen will adsorb onto the surface of the porous NiO nanosheets, and forms a great number of O2−, O− and O2− ions on the NiO surface.18,22 The electrical conductivity of the sensor significantly depends on the oxygen partial pressure, adsorption of gas molecules, and electron density on the surface of NiO.36 When the sensor is exposed to HCHO gas, the HCHO molecules will adsorb and react with oxygen ions (e.g. O2−, O− and O2−) on the surface of porous NiO nanosheets. The possible reactions of oxygen ions with HCHO molecules are described as follows:
| HCHO(gas) + 2O− → H2O(gas) + CO2(gas) + 2e− | (8) |
| HCHO(gas) + 2O2− → H2O(gas) + CO2(gas) + 4e− | (9) |
When reactions (8) and (9) take place, the electrical holes and oxygen partial pressure on the surface of NiO decrease, and hence decrease the electrical conductivity of the NiO nanosheets. According to the previous report,18 NiO is one of the most promising materials used for the oxidation of HCHO. The primary reason is that NiO is the most active catalytic oxide with a Ni electro-negativity of 1.9, which is higher than those values of all the other oxide metals with high catalytic activities (between 1.2 and 1.9). This is probably one of the reasons that the porous NiO nanosheet based sensor has a high sensitivity and great selectivity towards HCHO.
Conclusions
In summary, single-crystalline β-Ni(OH)2 ultrathin nanosheets were obtained by a facile and novel electrochemical method at room temperature. The thickness of β-Ni(OH)2 nanosheets can be controlled by tuning the compositions of the electrolyte solution. It is anticipated that this facile electrochemical method under ambient conditions can be easily scaled up to synthesize 2-D sheet-like nanostructures of other metal hydroxides, such as iron hydroxide and zinc hydroxide (Fig. S4†). Porous NiO ultrathin nanosheets with a polycrystalline cubic structure were obtained by thermal decomposition of single-crystalline β-Ni(OH)2. NiO nanosheet based chemiresistive sensors showed high sensitivity, great selectivity, and a large detection range (1–1000 ppm) towards HCHO, which will have great potential in applications for practical indoor air quality monitoring.Acknowledgements
We acknowledge the funding support from the National Natural Science Foundation of China (91123034, 21107132), the Hong Kong, Macao and Taiwan Science & Technology Cooperation Program of China (2012DFH50120), the Key Industrial Supporting Project of Jiangsu Province (SIP1104PT051), and the Science and Technology Program of Suzhou (SH201010, SYG201144). We thank the Platforms of Characterization & Test and Nanofabrication of Suzhou Institute of Nanotech and Nanobionics, Chinese Academy of Sciences.References
- M. Agathos and H. A. Bernecker, Derm. Beruf. Umwelt., 1982, 30, 43 CAS.
- A. J. Hempel, K. S. Kjaergaard, L. Molhave and H. K. Hundnell, Arch. Environ. Health, 1999, 54, 416 CrossRef.
- G. J. Mohr, U. E. Spichiger, W. Jona and H. Langhals, Anal. Chem., 2000, 72, 1084 CrossRef CAS.
- Y. Suzuki, N. Nakano and K. Suzuki, Environ. Sci. Technol., 2003, 37, 5695 CrossRef CAS.
- J. R. Nicole and V. D. Serger, Sensors, 2008, 8, 2569 CrossRef.
- L. Feng, C. J. Musto and K. S. Suslick, J. Am. Chem. Soc., 2010, 132, 4046 CrossRef CAS.
- R. A. Bari, D. Adler and R. V. Lange, Phys. Rev. B: Solid State, 1970, 2, 2898 CrossRef.
- K. Oka, T. Yanagida, K. Nagashima, H. Tanaka and T. Kawai, J. Am. Chem. Soc., 2009, 131, 3434 CrossRef CAS.
- Z. Jiao, M. Wu, Z. Qin and H. Xu, Nanotechnology, 2003, 14, 458 CrossRef CAS.
- X. Song and L. Gao, J. Am. Ceram. Soc., 2008, 91, 4105 CrossRef CAS.
- H. Yang, G. Guai, C. Guo, Q. Song, S. Jiang, Y. Wang, W. Zhang and C. Li, J. Phys. Chem. C, 2011, 115, 12209 CAS.
- B. Varghese, M. Reddy, Z. Yanwu, C. Lit, T. Hoong, G. Rao, B. Chowdari, A. Wee, C. Lim and C. Sow, Chem. Mater., 2008, 20, 3360 CrossRef CAS.
- L. Liu, Y. Li, S. Yuan, M. Ge, M. Ren, C. Sun and Z. Zhou, J. Phys. Chem. C, 2010, 114, 251 CAS.
- B. Park and E. J. Cairns, Electrochem. Commun., 2011, 13, 75 CrossRef CAS.
- S. Ding, T. Zhu, J. Chen, Z. Wang, C. Yuan and X. Lou, J. Mater. Chem., 2011, 21, 6602 RSC.
- B. Zhao, X. Ke, J. Bao, C. Wang, L. Dong, Y. Chen and H. Chen, J. Phys. Chem. C, 2009, 113, 14440 CAS.
- J. Park, E. Kang, S. Son, H. Park, M. Lee, J. Kim, K. Kim, H. Noh, J. Park, C. Bae, J. Park and T. Hyeon, Adv. Mater., 2005, 17, 429 CrossRef CAS.
- J. A. Dirksen, K. Duval and T. A. Ring, Sens. Actuators, B, 2001, 80, 106 CrossRef.
- I. Hotovy, V. Rehacek, P. Siciliano, S. Capone and L. Spiess, Thin Solid Films, 2002, 418, 9 CrossRef CAS.
- I. Hotovy, J. Huran, P. Siciliano, S. Capone, L. Spiess and V. Rehacek, Sens. Actuators, B, 2001, 78, 126 CrossRef.
- N. D. Hoa and S. A. El-Safty, Chem.–Eur. J., 2011, 17, 12896 CrossRef CAS.
- B. Liu, H. Yang, H. Zhao, L. An, L. Zhang, R. Shi, L. Wang, L. Bao and Y. Chen, Sens. Actuators, B, 2011, 156, 251 CrossRef.
- Z. Liang, Y. Zhu and X. Hu, J. Phys. Chem. B, 2004, 108, 3488 CrossRef CAS.
- S. Ida, D. Shisuke, M. Koinuma and Y. Matsumoto, J. Am. Chem. Soc., 2008, 130, 14038 CrossRef CAS.
- X. Wang, L. Li, Y. Zhang, S. Wang, Z. Zhang, L. Fei and Y. Qian, Cryst. Growth Des., 2006, 6, 2163 CAS.
- W. Zhou, M. Yao, L. Guo, Y. Li and J. Li, J. Am. Chem. Soc., 2009, 131, 2959 CrossRef CAS.
- Y. Ichiyanagi, H. Kondoh, T. Yokoyama, K. Okamoto, K. Nagai and T. Ohta, Chem. Phys. Lett., 2003, 379, 345 CrossRef CAS.
- S. Sarkar, M. Pradhan, A. K. Sinha, M. Basu, Y. Negishi and T. Pal, Inorg. Chem., 2010, 49, 8813 CrossRef CAS.
- Y. Li, B. Tan and Y. Wu, Chem. Mater., 2008, 20, 567 CrossRef CAS.
- K. Li, Y. Li, M. Lu, C. Kuo and L. Chen, Adv. Funct. Mater., 2009, 19, 2453 CrossRef CAS.
- Y. Wang and Y. Xia, Electrochim. Acta, 2006, 51, 3223 CrossRef CAS.
- T. Sreethawong, S. Chavadej, S. Ngamsinlapasathian and S. Yoshikawa, Colloids Surf., A, 2007, 296, 222 CrossRef CAS.
- S. Sumikura, S. Mori, S. Shimizu, H. Usami and E. Suzuki, J. Photochem. Photobiol., A, 2008, 199, 1 CrossRef CAS.
- X. Wang, G. Li, R. Liu, H. Ding and T. Zhang, J. Mater. Chem., 2012, 22, 21824 RSC.
- L. A. Currie, Pure Appl. Chem., 1995, 67, 1699 CrossRef CAS.
- C. Lee, C. Chiang, Y. Wang and R. Ma, Sens. Actuators, B, 2007, 122, 50 CrossRef.
Footnotes |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra22049k |
| ‡ X. Wang and G. Li contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2012 |
