Dicationic AC regioisomer cyclodextrins: mono-6A-ammonium-6C-alkylimidazolium-β-cyclodextrin chlorides as chiral selectors for enantioseparation†
Yun
Dai
,
Shuye
Wang
,
Jianhua
Wu
,
Jian
Tang
* and
Weihua
Tang
*
Key Laboratory of Soft Chemistry and Functional Materials, Ministry of Education, Nanjing University of Science and Technology, Nanjing, P. R. China. E-mail: tangj2005@hotmail.com; whtang@mail.njust.edu.cn; Fax: (86) 25 8431 7311; Tel: (86) 25 8431 7311
First published on 16th October 2012
Abstract
Dicationic cyclodextrins with AC regioisomer structure were prepared by monosubstitution of 6A-azido-β-cyclodextrin with 2-mesitylenesulfonyl chloride and the Staudinger reaction. One family of the desired cyclodextrins, the 6A-ammonium-6C-butylimidazolium-β-cyclodextrin chlorides, demonstrated a good chiral recognition ability towards both acidic and even neutral racemates even at concentrations as low as 0.5 mM.
The Staudinger reaction is known as the reaction of tertiary phosphine with an organic azide to produce an iminophosphorane after nitrogen evolution, which has proved to be very useful in organic synthetic chemistry.1 When an electrophilic trap is placed on the tertiary phosphine, the phosphine-activated reagents can react with azide-labeled target molecules to form aza-ylide intermediates that quickly rearrange in aqueous conditions to form stable amide bonds between reactant molecules, which find many applications in the field of chemical biology.1,2 In the absence of an electrophilic trap, an amine can be formed by the hydrolysis of the aza-ylide intermediate. Immobilization of highly functionalized molecules onto solid (or biomacromolecules) support surfaces by the Staudinger reaction affords a facile approach to achieve a variety of functional materials. For instance, a series of structurally well-defined chiral stationary phases (CSPs) have been successfully developed by immobilizing azido-functionalized cyclodextrin (CD) derivatives onto aminised silica gel.3–5 These CSPs have presented excellent enantioselectivities for a large pool of racemic drugs in chiral chromatography.
CDs and their derivatives have also been widely used as chiral mobile phases for the enantioseparation in capillary electrophoresis (CE).6 Especially, charged CDs have been developed for the efficient analysis of both neutral and oppositely charged enantiomers in a certain separation window, where CDs and enantiomers have different charges. The enatioselectivities can thus be enhanced by taking advantage of both inclusion complexation and electrostatic interactions as extra driving-forces for chiral recognition.7,8 Most of these charged CDs were complex mixtures of isomers which differ in their degrees and loci of substituents, which exert a great impact on their chiral selectivities and reproductivities in practical applications.9 Hence the use of structurally well-defined (single-isomer) charged CDs was recommended to eliminate both the batch-to-batch composition variations and the undesirable analytical result variations,7,8 whose critical role in molecular level studies of the chiral recognition process and chiral CE assay validation has been emphasized in several excellent reviews.8,9 A library of single-isomer CDs has been developed as either monosubstituted or persubstituted CD derivatives.8,9 Comparably, fewer explorations have been conducted in the development of AC, AB or AD disubstituted regioisomers of CDs, mainly due to difficulties in synthesis. Capping of CD is the typical strategy to achieve the desired regioisomers.10–12
Under this context, we initiated a long-term research programme to develop single-isomer cationic CDs, among which a series of both mono-ammonium and mono-imidazolium substituted CDs were developed.7a,7f,13 These CDs have demonstrated outstanding enantioselectivities towards a variety of acidic racemates. In review of the successful applications diamino-β-cyclodextrins (AB, AC, AD) in the enantioseparation of acids, where hydroxy acids were best separated by the AB regioisomer and carboxylic acids were best separated by the AC regioisomer,14 it is intriguing to us whether we can develop efficient regioisomers with both ammonium and imidazolium functionality as chiral selectors for the enantioseparation of acids.
In this paper, we describe the synthesis of a family of disubstituted, dicationic AC regioisomer CDs, 6A-ammonium-6C-alkylimidazolium-β-cyclodextrin chlorides. This leads to novel chiral mobile phases which have better enantioselectivities for acidic enantiomers in CE than their mono-imidazolium15 and mono-ammonium counterpart CDs7f in the literature.
As depicted in Scheme 1, the synthetic route for dicationic CDs was established as follows: an azide group was first introduced onto the C(6A) position of the CD;7c mono-azido-β-CD 1 was further nucleophilic substituted with 2-mesitylenesulfonyl chloride to give a mixture of three regioisomers, 6A-azido-6X-mesitylene-sulfonyl-β-cyclodextrin (X = B, C or D). The mixture was then subjected to a reversed phase column liquid chromatography12 to afford the desired AC regioisomer, 6A-azido-6C-mesitylene-sulfonyl-β-cyclodextrin (Mess-N3-CD) 2 with a yield of 42%. The AC regiochemistry was supported by comparison with the known AC, AB and AD regioisomers synthesized by capping of the CD.10–12 The AC regioisomer structure 2 is confirmed by its characteristic absorptions [101.68 (C1), 81.53 (C4)] in its 13C NMR, where the strong peak centered at 71.82 is assigned to C2, C3 and C5, while the peak at 59.67 is assigned to C6 adjacent to the hydroxyl group on the primary rim of the CD.
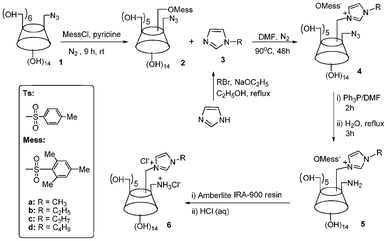 | ||
| Scheme 1 Synthetic approach to 6A-ammonium-6C-alkylimidazolium-β-cyclodextrin chlorides. | ||
The as-prepared Mess-N3-CD 2 was then reacted with 3-alkylimidazoles via nucleophilic addition to introduce an imidazolium cation onto the C(6C) position of the CD. The Staudinger reaction was further employed to achieve 6A-amino-6C-alkylimidazolium-β-cyclodextrin mesitylene sulfonate 5. The final dicationic CDs were synthesized via protonation with dilute hydrochloric acid and anionic exchange with Amberlite (Cl) resin.
In the synthesis of 2, a more sterically hindered 2-mesitylenesulfonyl chloride was used, since the commonly used nucleophile, p-toluenesulfonyl chloride,7 did not work with 1. The nucleophilic substitution of 2 with alkylimidazole 3 worked smoothly by refluxing in DMF, although the yield (75%–81%) is slightly different for 3 with different alkyl chain length. The Staudinger reduction of azido-containing CD 4 proceeded as well as that for N3-CD in our previous report,7c where a yield between 75 and 86% was achieved for 5.
By varying the alkyl chain length of the alkylimidazole, a series of 6A-ammonium-6C-alkylimidazolium-β-cyclodextrin chlorides was synthesized. All of them exhibit good enantioselectivities towards negatively charged racemates. Herein, we take 6A-ammonium-6C-butylimidazolium-β-CD chloride (6d, BAMIMCD) as an example to illustrate their enantioseparation capability in chiral CE. The buffer pH was first optimized to be in the range of 5.5 to 7.0 with 1.0 mM CD by considering the pKa (∼3.5–4) of acidic analytes or pI values (∼5.5–6.5) of amino acids (structure in Fig. S1†).16 The separation selectivity (α) and chiral resolution (Rs) values of 11 model analytes are summarized in Table 1.
| Analytes | pH 5.5 | pH 6.0 | pH 6.5 | pH 7.0 | ||||
|---|---|---|---|---|---|---|---|---|
| α | R s | α | R s | α | R s | α | R s | |
| a Conditions: 50 mM acetate buffer (pH 5.5, 6.0) or phosphate buffer (pH 6.5, 7.0), 25 °C. Dns-Aca: dansyl DL-α-aminocaprylic acid, Dns-Nleu: dansyl DL-norleucine, Dns-Phe: dansyl DL-phenylalanine, PLA: 3-phenyllactic acid, HPLA: DL-p-hydroxylphenyllactic acid, MoPPA: 2-methoxy-3-phenylpropanoic acid, PCPA: α-phenylcyclopentaneacetic acid, 2-PPA: 2-phenylpropionic acid, 2-TPA: 2-(p-tolyl)propionic acid, 2-POPA: 2-phenoxypropionic acid, 3-Cl POPA: 2-(3-chlorophenoxy)propionic acid. | ||||||||
| Dns-Aca | 1.07 | 1.8 | 1.07 | 2.4 | 1.05 | 1.9 | 1.06 | 1.6 |
| Dns-Nleu | 1.01 | 1.6 | 1.02 | 1.9 | 1.01 | 1.7 | 1.01 | 1.7 |
| Dns-Phe | 1.01 | 1.6 | 1.02 | 1.7 | 1.01 | 1.6 | 1.01 | 1.6 |
| PLA | 1.05 | 2.9 | 1.08 | 3.2 | 1.05 | 2.7 | 1.07 | 3.4 |
| HPLA | 1.08 | 2.2 | 1.10 | 3.5 | 1.05 | 3.3 | 1.07 | 3.1 |
| MoPPA | 1.09 | 3.6 | 1.11 | 5.6 | 1.06 | 3.9 | 1.02 | 1.7 |
| PCPA | 1.01 | 1.6 | 1.02 | 1.8 | 1.02 | 1.9 | 1.02 | 1.7 |
| 2-PPA | 1.01 | 1.4 | 1.01 | 1.6 | 1.03 | 1.9 | 1.01 | 1.6 |
| 2-TPA | 1.01 | 1.9 | 1.03 | 2.4 | 1.02 | 2.3 | 1.02 | 2.1 |
| 2-POPA | 1.01 | 1.3 | 1.03 | 1.6 | 1.04 | 2.5 | 1.03 | 2.0 |
| 3-Cl POPA | 1.06 | 1.9 | 1.05 | 1.8 | 1.05 | 2.1 | 1.05 | 1.9 |
As shown in Table 1, BAMIMCD exhibited good chiral recognition towards all analytes, with Rs over 1 in the studied pH range. A close look at the α and Rs values of all analytes reveals a local maximum was achieved at pH 6.0. The versatile chiral resolution ability of BAMIMCD towards both amino acids and acidic racemates validates the applicability of our design principle, in which alkylimidazolium substituted CDs target for resolution of amino acids and ammonium group substituted CDs for acidic enantiomers.7,8,16,17 As known to us, the high enantioselectivity of cationic CDs is ascribed to inclusion phenomena and the additional electrostatic interactions between the protonated CD and the anionic analyte.7,9,14 And the enantioselectivities can be further enhanced if extra hydrogen bonding can be formed between CD and analyte.17 In our case, BAMIMCD is positively charged in the pH range below 8 used in CE experiments, since the side rim has an imidazolium and a C6 protonated amino group (pKa ∼ 8.5),18 which is particularly suitable for the separation of acidic and amino acids in the pH range where they are deprotonated. For hydroxy acids, the extra hydrogen bonding formed between CD and analytes can contribute to even better enantioselectivities.
A typical electropherogram of such a hydroxy acid, HPLA, with 1.0 mM CD at varied pH values is depicted in Fig. 1 to demonstrate the powerful enantioseparation capability of BAMIMCD. The elution profile of HPLA changed at pH 6.5, probably attributable to the different buffer utilized at this pH (phosphate instead of acetate). On the basis of these results we chose to perform further experiments at pH 6.0 with BAMIMCD.
 | ||
| Fig. 1 Enantioseparation of HPLA with 1.0 mM CD at varied pH buffer. | ||
The enantioseparation capability was further explored by varying its concentration from 0.5 mM to 1.5 mM, with the separation selectivity (α) and chiral resolution (Rs) listed in Table 2. In general, chiral resolutions of most enantiomers were enhanced with CD concentration in the range of 0.5 mM to 1.5 mM. Seven out of eighteen studied analytes were baseline separated with a CD concentration even as low as 0.5 mM. Especially for acidic racemates such as PLA, HPLA and 3-ClPOPA, significantly improved resolutions (ca. 7.6) were obtained with 1.5 mM CD. The –OH group of analytes may form hydrogen bonding with the N atom of the CD, contributing as an extra driving-force for the enantioseparation. However, Dns-Ser was best resolved with 0.5 mM CD. To the best of our knowledge, few cationic chiral selectors can demonstrate such versatile and good enantioseparation towards acidic racemates even at CD concentrations as low as 0.5 mM.8
| Analytesa | 0.5 mM | 1.0 mM | 1.5 mM | |||
|---|---|---|---|---|---|---|
| α | R s | α | R s | α | R s | |
| a Dns-Ser: dansyl DL-serine. | ||||||
| Dns-Aca | 1.04 | 1.4 | 1.07 | 2.4 | 1.07 | 3.0 |
| Dns-Ser | 1.09 | 3.7 | 1.02 | 1.9 | 1.01 | 1.2 |
| Dns-Nleu | 1.01 | 0.5 | 1.02 | 0.9 | 1.02 | 1.2 |
| PLA | 1.03 | 1.9 | 1.08 | 3.1 | 1.12 | 6.5 |
| HPLA | 1.04 | 1.5 | 1.11 | 3.5 | 1.11 | 7.2 |
| MoPPA | 1.01 | 2.7 | 1.11 | 5.6 | 1.11 | 5.2 |
| 2-POPA | 1.02 | 1.2 | 1.03 | 1.6 | 1.01 | 1.5 |
| 3-Cl POPA | 1.05 | 1.8 | 1.05 | 1.8 | 1.11 | 7.6 |
Typical electropherograms of enantioseparations of Dns-Aca at different CD concentrations are shown in Fig. 2. Dns-Aca experiences increased chiral resolution with increasing CD concentration. Impressively, the highest Rs of 4.6 can be readily achieved for Dns-Aca with 4.0 mM CD. The different elution profile of Dns-Aca observed at 1.5 mM and 2.5 mM CD may be attributed to the viscosity change of buffer.
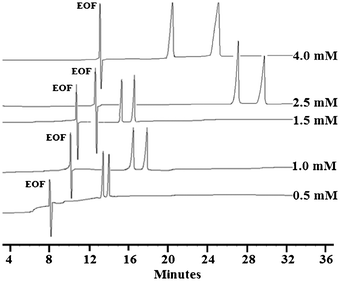 | ||
| Fig. 2 Enantioseparation of Dns-Aca at varied CD concentrations. | ||
The chiral recognition ability of dually cationic CDs was further demonstrated with the good resolution of 8 model analytes including dansyl amino acids and acidic racemates with BAMIMCD (Fig. 3). Even at CD concentrations as low as 0.5 mM, chiral resolutions over 2 can be easily achieved. At the same separation conditions, these dicationic CDs displayed improved enantioseparations over their mono-imidazolium11 and mono-ammonium counterpart CDs.7,8 It should be noted that neutral compounds like benzoin and anisoin can also be well resolved (Rs over 2) with 2 mM CD.
 | ||
| Fig. 3 Capillary electrochromatography of racemates using 1.0 mM BAMIMCD in 50 mM pH 6.0 acetate buffer. | ||
In summary, a new series of ammonium and alkylimidazolium disubstituted AC regioisomer CDs have been successfully developed. The good enantioselectivities towards acidic racemates was demonstrated by 6A-ammonium-6C-butylimidazolium-β-cyclodextrin chlorides in CE. Under optimum buffer pH 6.0 conditions, BAMIMCD exhibited good chiral resolutions even at concentrations as low as 0.5 mM.
Experimental section
All reagents were of analytical grade quality as obtained from commercial suppliers and used without further purification. Ethanol and DMF were freshly dried prior to use using standard procedures. The synthesis of 6A-azido-β-cyclodextrin (N3-CD) 1 was carried out as previously reported.7c6 A -Azido-6C-mesitylenesulfonyl-β-cyclodextrin (Mess-N3-CD, 222): To a solution of 1 (5 g, 4.3 mmol) in freshly distilled pyridine (25 mL) was added 2-mesitylenesulfonyl chloride (4.3 g, 19.4 mmol). The reaction mixture was stirred at 25 °C for 9 h. The crude product was obtained by precipitating the reaction mixture out of vigorously stirred acetone (200 mL). The precipitate was collected by filtration and washed with acetone to give a mixture of three regioisomers, 6A-azido-6X-mesitylene-sulfonyl-β-cyclodextrin (X = B, C or D) as a white solid (5.32 g, yield 82%). The mixture was further subjected to a reversed phase column chromatography to afford the regioisomer, 6A-azido-6C-mesitylene-sulfonyl-β-cyclodextrin 2 with a yield of 42%. Most characteristic for Mess-N3-CD in 13C NMR are: 101.68 (C1), 81.53 (C4), strong peak centered at 71.82 (C2, C3, C5), and 59.67 (C6 adjacent to hydroxyl). The AC regioisomer structure of 2 is supported by comparison with known regioisomers in the literature.11,12
1H NMR (500 MHz, DMSO-d6) δ: 7.11 (m, 2H), 5.70–5.83 (m, 14H), 4.82–4.88 (m, 7H), 4.45–4.51 (m, 5H), 4.21 (br, 1H), 4.09 (s, 1H), 3.55–3.64 (m, 26H), 3.26–3.36 (m, 14H), 2.29 (s, 6H), 2.17 (s, 3H). 13C NMR (125 MHz, DMSO-d6) δ: 142.99, 138.92, 131.25, 129.53, 101.68, 81.53, 71.92–70.27, 68.79, 59.67, 59.29, 21.75, 20.24. Decomposition temperature (dp): 203.5 °C. FT-IR (cm−1, KBr): 3387, 2928, 2104, 1644, 1637, 1157, 1080, 1030. Anal. Calcd. for C44H79N3O36S: C, 42.00; H, 6.33. Found: C, 41.84; H, 6.72.
Alkylimidazole 3b3b–3d3d: To a degassed and N2-filled flask was added imidazole (4 g, 58.8 mmol), sodium ethoxide (4.4 g, 64.6 mmol) and anhydrous ethanol (20 mL). Afterwards, excessive 1-bromoethane (7.04 g, 64.6 mmol) or 1-bromopropane (7.95 g, 64.6 mmol) or 1-bromobutane (8.85 g, 64.6 mmol) was added dropwise. The reaction mixture was refluxed at 50 °C for 4 h before cooling down to room temperature. The insoluble solid was filtered off and washed with dichloromethane. The collected filtrate was concentrated and further distilled under reduced pressure to afford the title products as yellowish liquids.
1-Ethylimidazole 3b3b (4.98 g, 88%). 1H NMR (500 MHz, CDCl3) δ: 7.41 (s, 1H), 6.97 (s, 1H), 6.86 (s, 1H), 3.88–3.93 (m, 2H), 1.36 (t, 3H).
1-Propylimidazole 3c3c (5.37 g, 83%). 1H NMR (500 MHz, CDCl3) δ: 7.31 (s, 1H), 6.94 (s, 1H), 6.76 (s, 1H), 3.74 (t, 2H), 1.60–1.68 (m, 2H), 0.78 (t, 3H).
1-Butylimidazole 3d3d (5.84 g, 80%). 1H NMR (500 MHz, CDCl3) δ: 7.38 (s, 1H), 7.01 (s, 1H), 6.83 (s, 1H), 3.85 (t, 2H), 1.64–1.70 (m, 2H), 1.22–1.26 (m, 2H), 0.86 (t, 3H).
6 A -Azido-6C-alkylimidazolium-β-cyclodextrin mesitylene sulfonate44: To a degassed and N2-filled two-necked, round-bottomed flask, was added 2 (3.0 mmol, 4.02 g), alkylimidazole (9.0 mmol, 3a, 0.74 g; 3b, 0.86 g; 3c, 1.0 g; 3d, 1.12 g) and freshly distilled N,N-dimethylformamide (DMF, 15 mL). The reaction mixture was stirred at 90 °C for 48 h under N2 atmosphere. Cooling down to room temperature, the reaction solution was precipitated using acetone (100 mL). The precipitate was collected by filtration and washed with acetone (50 mL × 2) before drying in vacuo at 60 °C overnight. The crude product was recrystallized from water to obtain the title product 4 as a white solid.
6 A -Azido-6C-methylimidazolium-β-cyclodextrin mesitylene sulfonate4a4a (3.63 g, 75%). 1H NMR (500 MHz, DMSO-d6) δ: 8.75 (s,1H), 7.58 (s, 1H), 7.47 (s, 1H), 7.10 (m, 2H), 5.59–5.93 (m, 14H), 4.76–4.94 (m, 7H), 4.45–4.51 (m, 5H), 3.94 (br, 1H), 3.88 (s, 1H), 3.82 (s, 3H), 3.50–3.71 (m, 26H), 3.21–3.49 (m, 14H), 2.32 (s, 6H), 2.27 (s, 3H). 13C NMR (125 MHz, DMSO-d6) δ: 142.04, 136.23, 136.08, 129.70, 129.51, 122.96, 126.61, 101.54, 81.17, 72.70–71.64, 69.82, 59.58, 33.79, 22.30, 20.41. dp: 201.4 °C. FT-IR (cm−1, KBr): 3383, 2928, 2106, 1649, 1637, 1564, 1157, 1084, 1032. Anal. Calcd. for C48H85N5O36S: C, 43.01; H, 6.39. Found: C, 42.79; H, 6.52.
6 A -Azido-6C-ethylimidazolium-β-cyclodextrin mesitylene sulfonate4b4b (3.49 g, 81%). 1H NMR (500 MHz, DMSO-d6) δ: 9.01 (s, 1H), 8.15 (s, 1H), 8.13 (s, 1H), 7.10 (m, 2H), 6.08–5.54 (m, 14H), 5.01–4.62 (m, 7H), 4.28–4.50 (m, 5H), 4.09 (m, 2H), 3.90 (m, 1H), 3.84 (s, 1H), 3.50–3.80 (m, 26H), 3.50–3.19 (m, 14H), 2.38 (s, 6H), 2.27 (s, 3H), 1.55 (t, 3H). 13C NMR (125 MHz, DMSO-d6) δ: 145.52, 142.91, 136.41, 130.39, 128.66, 124.08, 120.51, 102.53, 83.40, 82.36, 72.95–70.88, 69.47, 62.01, 59.22, 45.65, 34.55, 23.18, 20.76. dp: 203.8 °C. FT-IR (cm−1, KBr): 3394, 2928, 2106, 1643, 1636, 1157, 1072, 1047. Anal. Calcd. for C49H87N5O36S: C, 43.46; H, 6.48. Found: C, 43.14; H, 6.63.
6 A -Azido-6C-propylimidazolium-β-cyclodextrin mesitylene sulfonate4c4c (3.48 g, 80%). 1H NMR (500 MHz, DMSO-d6) δ: 9.19 (s, 1H), 8.22 (s, 1H), 8.13 (s, 1H), 7.45 (m, 2H), 5.62–5.97 (m, 14H), 4.87 (s, 1H), 4.82 (s, 6H), 4.37–4.63 (m, 5H), 4.15 (t, 2H), 3.93 (m, 1H), 3.88 (m, 1H), 3.48–3.72 (m, 26H, H-5CD), 3.16–3.48 (m, 14H), 2.39 (s, 6H), 2.18 (s, 3H), 1.78–1.82 (m, 2H), 0.84 (t, 3H). 13C NMR (125 MHz, DMSO-d6) δ: 150.03, 145.96, 142.12, 136.64, 130.34, 128.45, 124.38, 102.73, 83.33, 82.02, 73.54–70.73, 60.42, 45.66, 36.30, 31.29, 23.19, 21.85. dp: 207.1 °C. FT-IR (cm−1, KBr): 3393, 2928, 2106, 1647, 1635, 1157, 1078, 1032. Anal. Calcd. for C50H89N5O36S: C, 43.89; H, 6.56. Found: C, 43.41; H, 6.84.
6 A -Azido-6C-butylimidazolium-β-cyclodextrin mesitylene sulfonate4d4d (3.34 g, 76%). 1H NMR (500 MHz, DMSO-d6) δ: 9.03 (s, 1H), 8.32 (s, 1H), 8.13 (m, 1H), 7.54 (m, 2H), 5.60–5.92 (m, 14H), 4.86 (s, 1H), 4.84 (s, 6H), 4.27–4.63 (m, 5H), 4.11 (t, 2H), 3.90 (m, 1H), 3.83 (m, 1H), 3.49–3.74 (m, 26H), 3.20–3.48 (m, 14H), 2.32(s, 6H), 2.16 (s, 3H), 1.71–1.78 (m, 2H), 1.20–1.25 (m, 2H), 0.88 (t, 3H). 13C NMR (125 MHz, D2O): 149.10, 144.53, 143.03, 135.53, 132.32, 128.41, 126.24, 102.39, 83.41, 82.30, 73.52–72.47, 70.67, 60.41, 51.54, 45.66, 36.28, 31.27, 24.13, 19.94; ESI-MS (m/z): 1267.25 (calcd.), 1268.69 (found) for [M+]. dp: 221.8 °C. FT-IR (cm−1, KBr): 3396, 2928, 2106, 1644, 1636, 1155, 1084, 1030. Anal. Calcd. for C51H91N5O36S: C, 44.31; H, 6.64. Found: C, 43.98; H, 6.92.
6 A -Amino-6C-alkylimidazolium-β-cyclodextrin mesitylene sulfonate5555: A mixture of 4 (2.0 mmol, 4a, 2.85 g; 4b, 2.88 g; 4c, 2.90 g; 4d, 2.93 g), triphenylphosphine (2.2 mmol, 0.52 g) and DMF (10 mL) was stirred at room temperature for 2 h before the addition of deionized water (1.0 mL). The reaction mixture was then heated at 90 °C for 3 h. The reaction solution was cooled down and the crude product was precipitated using acetone (60 mL). The crude product was filtered off, washed with acetone (50 mL × 2), and dried in vacuo at 60 °C overnight. Further recrystallization from water yielded the title product 5 as a white solid.
6 A -Amino-6C-methylimidazolium-β-cyclodextrin mesitylene sulfonate5a5a (2.29 g, 82%). 1H NMR (500 MHz, DMSO-d6) δ: 9.05 (s, 1H), 7.98 (s, 1H), 7.57 (s, 1H), 7.10 (m, 2H), 5.59–5.98 (m, 14H), 4.72–5.06 (m, 7H), 4.38–4.61 (m, 5H), 3.92 (m, 1H), 3.82 (s, 1H), 3.64 (s, 3H), 3.72–3.48 (m, 26H), 3.15–3.47 (m, 14H), 2.89 (s, 6H), 2.72 (s, 3H). 13C NMR (125 MHz, D2O): δ 142.93, 138.33, 136.40, 134.04, 130.35, 128.67, 121.08, 102.41, 83.31, 82.08, 73.34–72.07, 70.78, 60.47, 33.26, 23.14, 20.73. dp: 191.8 °C. FT-IR (cm−1, KBr): 3383, 2926, 1649, 1635, 1155, 1078, 1041. Anal. Calcd. for C48H87N3O36S: C, 43.87; H, 6.67. Found: C, 43.12; H, 6.95.
6 A -Amino-6C-ethylimidazolium-β-cyclodextrin mesitylene sulfonate5b5b (2.21g, 78%). 1H NMR (500 MHz, DMSO-d6) δ: 9.04 (s, 1H), 8.29 (s, 1H), 8.14 (s, 1H), 7.74 (m, 2H), 5.67–5.81 (m, 14H), 4.49–4.56 (m, 7H), 4.28–4.50 (m, 5H), 3.92 (m, 2H), 3.88 (m, 1H), 3.49–3.72 (m, 27H), 3.25–3.49 (m, 14H), 2.92 (s, 6H), 2.74 (s, 3H), 1.45 (t, 3H). 13C NMR (125 MHz, D2O): 149.13, 142.78, 136.99, 134.05, 130.37, 129.31, 122.29, 102.40, 83.16, 81.99, 72.85–71.73, 70.67, 60.48, 44.77, 31.14, 23.34, 20.74. dp: 196.4 °C; FT-IR (cm−1, KBr): 3385, 2926, 1653, 1637, 1157, 1078, 1034. Anal. Calcd. for C49H89N3O36S: C, 44.31; H, 6.75. Found: C, 43.86; H, 7.10.
6 A -Amino-6C-propylimidazolium-β-cyclodextrin mesitylene sulfonate5c5c (2.45 g, 86%). 1H NMR (500 MHz, DMSO-d6) δ: 9.09 (s, 1H), 8.52 (s, 1H), 8.33 (s, 1H), 7.60 (m, 2H), 5.87–5.63 (m, 14H), 4.86 (s, 1H), 4.82 (s, 6H), 4.3–4.45 (m, 5H), 3.89 (t, 2H), 3.83 (m, 1H), 3.52–3.73 (m, 27H), 3.25–3.46 (m, 14H), 3.22 (m, 1H), 2.79 (s, 6H), 2.61 (s, 3H3), 1.82–1.78 (m, 2H), 1.08 (t, 3H). 13C NMR (125 MHz, D2O): 145.37, 138.56, 134.05, 131.97, 129.22, 128.64, 125.92, 102.38, 83.24, 81.98, 73.51–72.48, 70.87, 60.40, 45.17, 36.32, 31.28, 23.34, 21.27. dp: 103.7 °C. FT-IR (cm−1, KBr): 3423, 2928, 1655, 1630, 1157, 1078, 1030. Anal. Calcd. for C50H91N3O36S: C, 44.74; H, 6.83. Found: C, 44.56; H, 7.06.
6 A -Amino-6C-butylimidazolium-β-cyclodextrin mesitylene sulfonate5d5d (2.19 g, 76%). 1H NMR (500 MHz, DMSO-d6) δ: 9.03 (s, 1H), 8.62 (s, 1H), 8.33 (m, 1H), 7.85 (m, 2H), 5.52–6.10 (m, 14H), 4.96 (s, 1H), 4.84 (s, 6H), 4.27–4.63 (m, 5H), 3.91 (t, 2H), 3.82 (m, 1H), 3.53–3.79 (m, 27H), 3.18–3.53 (m, 14H), 3.05 (m, 1H), 2.88 (s, 6H), 2.73 (s, 3H), 1.59 (m, 2H2), 1.22 (m, 2H2), 0.85 (t, 3H). 13C NMR (125 MHz, DMSO-d6) δ: 145.95, 139.29, 137.83, 133.48, 131.98, 129.20, 128.44, 102.42, 83.38, 82.02, 73.50–72.55, 71.29, 60.43, 36.27, 35.02, 31.30, 29.57, 21.83, 19.30. dp: 214.7 °C. FT-IR (cm−1, KBr): 3383, 2928, 1667, 1658, 1155, 1078, 1032. Anal. Calcd. for C51H93N3O36S: C, 45.16; H, 6.91. Found: C, 44.92; H, 7.23.
6 A -Ammonium-6C-alkylimidazolium-β-cyclodextrin chlorides66: A solution of 5 (1.4 mmol, 5a, 1.96 g; 5b, 1.98 g; 5c, 2.00 g; 5d, 2.02 g) in deionized water (50 mL) was added into a column packed with Amberlite IRA-900 ion-exchange resin and left to stand for 5 h. The eluent was then collected. To the collected solution was added dropwise 1.5 equiv. hydrochloric acid solution (0.1 M, 21 mL) over 1 h. The solution was concentrated to ∼10 mL before precipitating out the product with acetone (100 mL). The solid was obtained by filtration and washed with acetone (50 mL × 2). After drying in vacuo at 60 °C overnight, the final product 6 was obtained by further recrystallization from deionized water as a white solid. The complete conversion of chloride anion was confirmed by ESI-MS measurement.
6 A -Ammonium-6C-methylimidazolium-β-cyclodextrin chloride6a6a (1.72 g, 83%). 1H NMR (500 MHz, DMSO-d6) δ: 8.98 (s, 1H), 7.78 (s, 1H), 7.57 (s, 1H), 5.88–5.59 (m, 14H), 4.96–4.62 (m,7H), 4.56–4.32 (m, 5H), 3.90 (m, 1H), 3.84 (s, 1H), 3.66 (s, 3H), 3.48–3.62 (m, 26H), 3.25–3.48 (m, 14H). 13C NMR (125 MHz, DMSO-d6) δ: 135.36, 134.03, 130.34, 102.38, 83.27, 82.06, 73.54–72.50, 70.74, 60.43, 31.18. IR (cm−1, KBr): 3383, 2928, 1083, 1030. Anal. Calcd. for C39H77Cl2N3O33: C, 39.46; H, 6.54. Found: C, 39.08; H, 6.86. ESI-MS (m/z): 1115.44 (calcd. for C39H77N3O33) and 1115.50 found for [M+], 35.45 (calcd.) and 35.58 found for [Cl−].
6 A -Ammonium-6C-ethylimidazolium-β-cyclodextrin chloride6b6b (1.64 g, 74%). 1H NMR (500 MHz, DMSO-d6) δ: 8.84 (s, 1H), 8.19 (s, 1H), 8.08 (s, 1H), 5.76–5.57 (m, 14H), 4.52–4.47 (m, 7H), 4.32–4.42 (m, 5H), 3.88 (m, 2H), 3.86 (m, 1H), 3.48–3.76 (m, 27H), 3.32–3.48 (m, 14H), 1.42 (t, 3H). 13C NMR (125 MHz, DMSO-d6) δ: 135.36, 134.03, 130.34, 102.38, 83.27, 82.06, 73.54, 72.88, 72.50, 70.74, 60.43, 31.18. FT-IR (cm−1, KBr): 3415, 2928, 1078, 1030. Anal. Calcd. for C40H79Cl2N3O33: C, 40.00; H, 6.63. Found: C, 39.68; H, 6.88. ESI-MS (m/z): 1129.46 (calcd. for C40H79N3O33) and 1129.61 found for [M+], 35.45 (calcd.) and 35.58 found for [Cl−].
6 A -Ammonium-6C-propylimidazolium-β-cyclodextrin chloride6c6c (1.76 g, 69%). 1H NMR (500 MHz, DMSO-d6) δ: 9.01 (s, 1H), 8.32 (s, 1H), 8.13 (s, 1H), 5.77–5.60 (m, 14H), 4.80 (s, 1H), 4.76 (s, 6H), 4.26–4.40 (m, 5H), 3.89 (t, 2H), 3.82 (m, 1H), 3.54–3.72 (m, 27H), 3.32–3.54 (m, 14H), 3.27 (m, 1H), 1.76–1.57 (m, 2H), 1.28 (t, 3H). 13C NMR (125 MHz, DMSO-d6) δ: 135.33, 134.00, 130.30, 101.81, 83.22, 81.95, 73.51–72.47, 70.33, 61.21, 45.70, 34.57; FT-IR (cm−1, KBr): 3387, 2928, 1078, 1030. Anal. Calcd. for C41H81Cl2N3O33: C, 40.53; H, 6.72. Found: C, 40.26; H, 6.93. ESI-MS (m/z): 1143.47 (calcd. for C41H81N3O33) and 1143.68 found for [M+], 35.45 (calcd.) and 35.58 found for [Cl−].
6 A -Ammonium-6C-butylimidazolium-β-cyclodextrin chloride6d6d (1.65 g, 66%). 1H NMR (500 MHz, DMSO-d6) δ: 8.93 (s, 1H), 8.22 (s, 1H), 8.13 (m, 1H), 5.50–6.14 (m,14H), 4.96 (s, 1H), 4.88 (s, 6H), 4.53–4.27 (m, 5H), 4.13 (m, 2H), 3.90 (t, 2H), 3.84 (m, 1H), 3.50–3.72 (m, 27H), 3.28–3.50 (m, 14H), 3.15 (m, 1H), 1.58 (m, 2H), 1.32 (m, 2H), 0.91 (t, 3H). 13C NMR (125 MHz, DMSO-d6) δ: 132.81, 130.52, 128.45, 102.92, 83.98, 73.22, 72.57–71.92, 70.12, 60.54, 34.67, 30.42, 20.71, 19.46. FT-IR (cm−1, KBr): 3387, 2928, 1084, 1030. Anal. Calcd. for C42H83Cl2N3O33: C, 41.05; H, 6.81. Found: C, 40.92; H, 6.83. ESI-MS (m/z): 1157.49 (calcd. for C42H83N3O33) and 1157.35 found for [M+], 35.45 (calcd.) and 35.58 found for [Cl−].
Acknowledgements
This work was kindly supported by the Natural Science Foundation of Jiangsu Province (Grant No. BK2010486) and Research Fund for the Doctoral Program of Higher Education of China (Grant No. 20113219110037).References
- M. Köhn and R Breinbauer, Angew. Chem., Int. Ed., 2004, 43, 3106 CrossRef.
- E. Saxon and C. R. Bertozzi, Science, 2000, 287, 2007 CrossRef CAS.
- C. Xu, S. C. Ng and H. S. O. Chan, Langmuir, 2008, 24, 9118 CrossRef CAS.
- L. S. Li, Y. Wang, D. J. Young, S. C. Ng and T. T. Y. Tan, Electrophoresis, 2010, 31, 378 CrossRef CAS.
- R.-Q. Wang, T. T. Ong, W. H. Tang and S. C. Ng, TrAC, Trends Anal. Chem., 2012, 37, 83 CrossRef.
- T. J. Ward and K. D. Ward, Anal. Chem., 2010, 82, 4712 CrossRef CAS.
- (a) W. H. Tang and S. C. Ng, Nat. Protoc., 2007, 2, 3195 CrossRef CAS; (b) W. H. Tang and S. C. Ng, J. Sep. Sci., 2008, 31, 3246 CrossRef CAS; (c) W. H. Tang and S. C. Ng, Nat. Protoc., 2008, 3, 691 CrossRef CAS; (d) W. H. Tang, I. W. Muderawan, T. T. Ong and S. C. Ng, Electrophoresis, 2005, 26, 3125 CrossRef CAS; (e) W. H. Tang, I. W. Muderawan, T. T. Ong and S. C. Ng, J. Chromatogr., A, 2005, 1091, 152 CrossRef CAS; (f) W. H. Tang, I. W. Muderawan, S. C. Ng and H. S. O. Chan, J. Chromatogr., A, 2005, 1094, 187 CrossRef CAS; (g) W. H. Tang, I. W. Muderawan, T. T. Ong and S. C. Ng, Anal. Chim. Acta, 2007, 585, 227 CrossRef CAS.
- (a) T. de Boer, R. A. de Zeeuw, G. J. de Jong and K. Ensing, Electrophoresis, 2000, 21, 3220 CrossRef CAS; (b) K. Nzeadibe and G. Vigh, Electrophoresis, 2007, 28, 2589 CrossRef CAS; (c) E. Tutu and G. Vigh, Electrophoresis, 2011, 32, 2655 CrossRef CAS.
- V. Cucinotta, A. Contino, A. Giuffrida, G. Maccarrone and M. Messina, J. Chromatogr., A, 2010, 1217, 953 CrossRef CAS.
- K. Fujita, A. Matsunaga and T. Imoto, Tetrahedron Lett., 1984, 25, 5533 CrossRef CAS.
- L. Tabushi, Y. Kuroda, K. Yokota and L. C. Yuan, J. Am. Chem. Soc., 1981, 103, 711 CrossRef.
- (a) I. Tabushi, K. Yamamura and T. Nabeshima, J. Am. Chem. Soc., 1984, 106, 5267 CrossRef CAS; (b) R. Bredow, J. W. Canary, M. Varney, S. T. Waddell and D. Yang, J. Am. Chem. Soc., 1990, 112, 5212 CrossRef.
- W. H. Tang, I. W. Muderawan, T. T. Ong and S. C. Ng, Tetrahedron: Asymmetry, 2007, 18, 1548 CrossRef CAS.
- G. Galaverna, M. C. Paganuzzi, R. Corradini, A. Dossena and R. Marchelli, Electrophoresis, 2001, 22, 3171 CrossRef CAS.
- T.-T. Ong, W. H. Tang, I. W. Muderawan, S.C. Ng and H. S. O. Chan, Electrophoresis, 2005, 26, 3839 CrossRef CAS.
- (a) S. Fanali, C. Desiderio and Z. Aturki, J. Chromatogr., A, 1997, 772, 185 CrossRef CAS; (b) W. H. Tang, I. W. Muderawan, S. C. Ng and H. S. O. Chan, Anal. Chim. Acta, 2005, 554, 156 CrossRef CAS.
- Y. Dai, S. Wang, J. Zhou, Y. Liu, D. Sun, J. Tang and W. H. Tang, J. Chromatogr., A, 2012, 1246, 98 CrossRef CAS.
- S. E. Brown, J. H. Coates, P. A. Duckworth, S. F. Lincoln, C. J. Easton and B. L. May, J. Chem. Soc., Faraday Trans., 1993, 89, 1035 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Instrumentation, 1H and 13C NMR spectra of 2, 4, 5 and 7. See DOI: 10.1039/c2ra21940a |
| This journal is © The Royal Society of Chemistry 2012 |
