Effects of an ionic liquid solvent on the synthesis of γ-butyrolactones by conjugate addition using NHC organocatalysts†
Michelle H.
Dunn
,
Marcus L.
Cole
* and
Jason B.
Harper
*
School of Chemistry, University of New South Wales, Sydney, NSW 2052, Australia. E-mail: m.cole@unsw.edu.au; j.harper@unsw.edu.au; Fax: +61 2 9385 6141; Tel: +61 2 9385 4692
First published on 28th August 2012
Abstract
The potential of imidazolium ionic liquids as solvents and precatalysts for the N-heterocyclic carbene (NHC) catalysed preparation of γ-butyrolactones has been investigated. The effect of changing from a molecular solvent to an ionic liquid solvent is examined, as is the organocatalytic efficacy of an ionic liquid derived NHC.
One of the challenges facing the widespread application of imidazolium ionic liquids is that differences in the outcomes of reactions are often observed on changing from a molecular to an ionic liquid solvent. It has been shown that these differences can be highly beneficial, leading to increased rates of reaction and superior selectivities.1 It is imperative that the origin of these enhancements be understood if ionic liquids are to rival or even supersede molecular solvents as reaction media.
Organocatalysis is one field in which ionic liquids have substantial potential.2 As well as being attractive on the basis of cost and toxicity,3 1,3-dialkyl-2-hydroimidazolium ionic liquids represent ready-made precursors for the in situ preparation of imidazol-2-ylidene N-heterocyclic carbenes by deprotonation.4 This offers the enticing prospect of using an ionic liquid as the source of an organocatalyst.
Despite the widespread application of imidazol-2-ylidenes to organocatalysis in molecular solvents, reports pertaining to NHC based processes in ionic liquids are limited to a recent study of the stoichiometric electrochemical generation of NHCs and one example of their reactivity.5 Even in that case, neither the organocatalytic efficacy of ionic liquid derived imidazol-2-ylidenes, nor the utility of imidazolium based ionic liquids as organocatalysis reaction media, have been investigated. In the context of this study, it is noteworthy that heterocycle forming reactions have exhibited improved rates and selectivities in imidazolium ionic liquids.6,7
Our research focuses on the fundamental properties of NHCs, their application in catalysis,8,9 and the effect of imidazolium ionic liquids like 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([bmim][NTf2], 1), on reaction outcomes.6,7,10–16 It was therefore of interest to (i) study the organocatalytic activity of an NHC derived from the imidazolium cation of an ionic liquid (IBuMe, 2, vide infra) in molecular and ionic liquid solvents, and (ii) examine the effects of moving a well-known imidazol-2-ylidene catalysed process to an imidazolium cation ionic liquid solvent, [bmim][NTf2] 1. For this purpose, we chose a high yielding reaction with good diastereoselectivity, the homoenolate equivalent coupling of cinnamaldehyde 3 and benzaldehyde 4 to afford lactones 5 (Scheme 1).17,18 The typical catalyst for this reaction is 1,3-bis(2,4,6-trimethylphenyl)imidazol-2-ylidene, IMes 6.
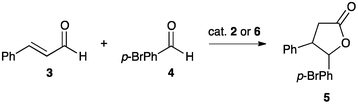 | ||
| Scheme 1 The NHC catalysed coupling of the aldehydes 3 and 4, and subsequent cyclisation to afford the γ-butyrolactones 5. | ||
The ionic liquid derived NHC, 1-butyl-3-methylimidazol-2-ylidene (IBuMe, 2), was prepared by deprotonation of its hydrochloride salt ([bmim][Cl]), followed by purification and isolation of the free NHC (cf. [bmim]+ = IBuMe·H+). Initial reactions were carried out in tetrahydrofuran (THF), the standard solvent for this process,17,18 with reaction progress determined using 1H NMR spectroscopy, cf. aldehyde resonance of the starting material 3 and 5-position resonances of the diastereoisomers of lactones 5.
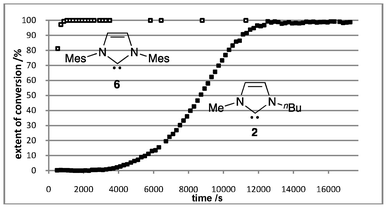 | ||
| Fig. 1 Extent of conversion of the reaction outlined in Scheme 1 carried out in THF at 24.9 °C using either carbene 2 (lower curve) or 6 (upper curve) as the catalyst (Mes = 2,4,6-Me3C6H2). | ||
The extent of conversion using 2 as catalyst with time when the reactions were carried out in THF is illustrated in Fig. 1 with equivalent data for IMes 6.‡ Reactions using catalyst 6 are very rapid, reaching completion in under one hour. This contrasts the prolonged reaction times reported for this process.17,19 At lower temperatures, the conversion of the starting materials to lactone 5 approaches linearity up to approximately 30% conversion (see ESI†). When IMes 6 is replaced with IBuMe 2, a sigmoidal reaction profile is observed, indicating that the reaction may be autocatalytic. Separate experiments demonstrated that the lactones 5 in the absence of IBuMe 2 did not catalyse the reaction, however addition of the lactones 5 to IBuMe 2 catalysed reaction media enhances the catalytic activity of the system (see ESI†). The origin of this enhancement is unknown at present, however it is clear that IBuMe 2 is a less effective catalyst for the preparation of lactones 5 relative to IMes 6.
To investigate the effect of an ionic liquid reaction medium (from which a catalyst might be generated) on the preparation of the lactones 5, the aforementioned reactions were repeated in the ionic liquid [bmim][NTf2] 1. In the specific case of IBuMe 2, no products were observed over a 14 h period. Separate experiments confirmed that IBuMe 2 was present at the conclusion of these reactions (see ESI†). This demonstrates that the ionic liquid 1 markedly retards formation of the lactones 5. This retardation may be the result of interaction of the cation of the ionic liquid 1 with the carbene 2; similar interactions have been observed previously.13,16
As illustrated in Fig. 2, the use of IMes 6 as a catalyst in [bmim][NTf2] 1 leads to a decrease in reaction rate relative to analogous reactions in THF. These reactions are most likely complicated by proton-transfer equilibria between IMes 6 and [bmim]+ to afford [IMes·H]+ and IBuMe 2 (eqn (1)), which, based on the non-catalytic nature of the carbene 2 in the ionic liquid 1, leads to a catalyst system with significantly reduced activity. An alternative explanation comes from so-called solvent effects, which impede the reaction by destabilising important intermediates during the formation of the lactones 5. Herein, the presence of an equilibrium, as per eqn (1), was confirmed by deuterium exchange experiments (see ESI†).20,21 According to a recent report by O'Donoghue and co-workers, the pKa value of [bmim]+ in water is 2.5 lower than [IMes·H]+.22 Our own studies in DMSO indicate a ΔpKa of 3.5,23 however attempts to directly ascertain the position of the equilibrium were hampered by difficulties with overlapping 1H NMR resonances. When carried out in benzene (see ESI†), the position of the equilibrium could be determined more precisely. Although the equilibrium constant determined is not directly applicable to the ionic liquid reaction studied here, it is noteworthy that, under the same reaction conditions, a benzene solution of [bmim][NTf2] and catalytic IMes 6 (same molar ratio as actual experiment) would contain 6% of the original IMes 6 after equilibration. This is consistent with the marked decrease in catalytic activity for IMes 6 in [bmim][NTf2] 1vis-à-vis THF.
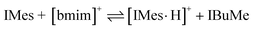 | (1) |
![Extent of conversion of the reaction outlined in Scheme 1 carried out in either THF (squares), [bmim][NTf2] (1, open triangles) or [bm2im][NTf2] (7, solid triangles) using carbene 6 as the catalyst at 24.9 °C.](/image/article/2012/RA/c2ra21889e/c2ra21889e-f2.gif) | ||
| Fig. 2 Extent of conversion of the reaction outlined in Scheme 1 carried out in either THF (squares), [bmim][NTf2] (1, open triangles) or [bm2im][NTf2] (7, solid triangles) using carbene 6 as the catalyst at 24.9 °C. | ||
To further evaluate the role of proton exchange between the ionic liquid 1 and the carbene 6, the lactones 5 were prepared in the 2-methylimidazolium ionic liquid; 1-butyl-2,3-dimethylimidazolium bis(trifluoromethanesulfonyl)imide ([bm2im][NTf2], 7), which lacks the acidic C2-proton of [bmim]+. This afforded a moderate increase in reaction rate relative to [bmim][NTf2] 1 (Fig. 2), but remained far slower than reactions conducted in THF. This suggests that the relative acidity of the imidazolium C2 proton is not solely responsible for the observed decrease in reaction rate upon moving the preparation of the lactones 5 from THF to the ionic liquid 1.§
The diastereoselectivity of the preparation of the lactones 5, which produces the isomers cis-5 and trans-5, was followed concurrently to the studies outlined in Fig. 1 and 2 (Table 1). It is noteworthy that the diastereoselectivities of all reactions do not alter throughout each process, as is consistent with literature reports for this reaction.
As outlined in Table 1, the decreased steric bulk of IBuMe 2 relative to IMes 6 leads to decreased stereoselectivity. Moreover, despite promising increases in diastereoselectivity for some heterocycle-forming reactions,10,14 most likely due to the increased internal pressure of solvation in ionic liquids resulting from their significant cohesive energies,24 the effect of changing from THF to [bmim][NTf2] 16,7 for the preparation of the lactones 5 is to markedly decrease selectivity. Interestingly, replacement of the ionic liquid with [bm2im][NTf2] 7 substantially increases diastereoselectivity.
In conclusion, whilst ionic liquids offer many advantages, their use as solvents for NHC organocatalysis may be limited by the deleterious effects of the solvent on catalyst activity and selectivity. Drawing on reactions conducted in THF and the ionic liquids 1 and 7, it is reasonable to suggest that (i) interactions between the ionic liquid cation and IMes 6, and (ii) [where possible] proton exchange between the ionic liquid cation and IMes 6, and the poor catalytic activity of the resultant carbene 2, result in a reduction in the catalytic activities of carbenes in the preparation of the lactones 5 in ionic liquid reaction media. This is in contrast to the increased efficacy of transition metal catalysts in ionic liquids, which can be attributed in part to effects of the solvent.15 Further investigations of organocatalysis and homogeneous catalysis using carbenes in ionic liquid reaction media are ongoing in our laboratories.
Notes and references
- Ionic Liquids in Synthesis, ed. P. Wasserscheid and T. Welton, Wiley-VCH, Weinheim, Germany, 2008 Search PubMed.
- For example see S. Toma, M. Meciarová and R. Sebesta, Eur. J. Org. Chem., 2009, 321–327 CrossRef CAS.
- D. W. C. MacMillan, Nature, 2008, 455, 304–308 CrossRef CAS.
- N. Marion, S. Díez-González and S. P. Nolan, Angew. Chem., Int. Ed., 2007, 46, 2988–3000 CrossRef CAS.
- M. Feroci, I. Chiarotto, M. Orsini, R. Pelagallia and A. Inesi, Chem. Commun., 2012, 48, 5361–5363 RSC.
- C. E. Rosella and J. B. Harper, Tetrahedron Lett., 2009, 50, 992–994 CrossRef CAS.
- S. R. D. George, G. L. Edwards and J. B. Harper, Org. Biomol. Chem., 2010, 8, 5354–5358 CAS.
- S. G. Alexander, M. L. Cole, S. K. Furfari and M. Kloth, Dalton Trans., 2009, 2909–2911 RSC.
- S. G. Alexander, M. L. Cole and J. C. Morris, New J. Chem., 2009, 33, 720 RSC.
- B. Y. W. Man, J. M. Hook and J. B. Harper, Tetrahedron Lett., 2005, 46, 7641–7645 CrossRef CAS.
- H. M. Yau, S. A. Barnes, J. M. Hook, T. G. A. Youngs, A. K. Croft and J. B. Harper, Chem. Commun., 2008, 3576–3578 RSC.
- H. M. Yau, S. J. Chan, S. R. D. George, J. M. Hook, A. K. Croft and J. B. Harper, Molecules, 2009, 14, 2521–2534 CrossRef CAS.
- H. M. Yau, A. G. Howe, J. M. Hook, A. K. Croft and J. B. Harper, Org. Biomol. Chem., 2009, 7, 3572–3575 CAS.
- S. G. Jones, H. M. Yau, E. Davies, J. M. Hook, T. G. A. Youngs, J. B. Harper and A. K. Croft, Phys. Chem. Chem. Phys., 2010, 12, 1873–1878 RSC.
- M. R. Gyton, M. L. Cole and J. B. Harper, Chem. Commun., 2011, 47, 9200–9202 RSC.
- H. M. Yau, A. K. Croft and J. B. Harper, Faraday Discuss., 2012, 154, 365–371 RSC.
- C. Burstein and F. Glorius, Angew. Chem., Int. Ed., 2004, 43, 6205–6208 CrossRef CAS.
- S. S. Sohn, E. L. Rosen and J. W. Bode, J. Am. Chem. Soc., 2004, 126, 14370–14371 CrossRef CAS.
- S. Urban, M. Tursky, R. Frohlich and F. Glorius, Dalton Trans., 2009, 6934–6940 RSC.
- M. K. Denk and J. M. Rodezno, J. Organomet. Chem., 2000, 608, 122–125 CrossRef CAS.
- M. L. Cole, C. Jones and P. C. Junk, New J. Chem., 2002, 26, 1296–1303 RSC.
- E. M. Higgins, J. A. Sherwood, A. G. Lindsay, J. Armstrong, R. S. Massey, R. W. Alder and A. C. O'Donoghue, Chem. Commun., 2011, 47, 1559–1561 RSC.
- M. H. Dunn, M. L. Cole and J. B. Harper, unpublished results Search PubMed.
- L. M. N. B. F. Santos, J. N. C. Lopes, J. A. P. Coutinho, J. M. S. S. Esperanca, L. R. Gomes, I. M. Marrucho and L. P. N. Rebelo, J. Am. Chem. Soc., 2007, 129, 284–285 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Preparation of the ionic liquids 1 and 7, preparation of the carbenes 2 and 6, representative stacked plots showing reaction progress, conversion vs. time plots for IMes 6 catalysed process in THF at lower T values, plot demonstrating autocatalytic effects of products 5, demonstration of presence of IBuMe 2 in ionic liquid 1, deuterium exchange experiments, equilibrium determination for [bmim]+/IMes 6 in benzene-d6. See DOI: 10.1039/c2ra21889e |
| ‡ In a typical experiment, the starting materials trans-cinnamaldehyde 3 (ca. 0.23 mmol) and p-bromobenzaldehyde 4 (ca. 0.24 mmol) were added to a solution of the carbene catalyst (either 2 or 6, 10 mol%) in the appropriate solvent (0.6 mL) under argon in NMR tubes fitted with J. Youngs valves. Reactions were held at 24.9 °C and followed using 1H NMR spectroscopy. Spectra were recorded at regular intervals until consumption of the limiting reagent 3 was noted. T1 tests were carried out to ensure sufficient delay times between scans such that all nuclei had relaxed before subsequent excitation. Analysis of the spectra allowed the extent of conversions to be determined by considering the integrals of the signals corresponding to aldehyde proton resonance of trans-cinnamaldehyde 3 and the C5 proton resonance of each of the diastereomeric pairs of the products 5. Comparison of product integrals with the sum gave the extent of conversion. All reactions were carried out in triplicate. |
| § Electrospray MS experiments¶ were carried out to determine if in the ionic liquid cases there was significant build-up of the charged intermediate in the catalytic cycle. The intermediate could be readily identified in both the THF and ionic liquid cases, however no build-up was observed in the ionic liquid process relative to the THF case. |
| ¶ Mass spectrometric results were obtained at the Bioanalytical Mass Spectrometry Facility within the Mark Wainwright Analytical Centre of the University of New South Wales with the technical support of Mr Lewis Adler. This work was undertaken using infrastructure provided by NSW Government co-investment in the National Collaborative Research Infrastructure Scheme (NCRIS) and subsidised access to this facility is gratefully acknowledged. |
| This journal is © The Royal Society of Chemistry 2012 |
