Elaborate fabrication of MOF-5 thin films on a glassy carbon electrode (GCE) for photoelectrochemical sensors†
Chuantao
Hou
a,
Jinyun
Peng
ab,
Qin
Xu
a,
Zhengping
Ji
a and
Xiaoya
Hu
*a
aCollege of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou, Jiangsu, China. E-mail: xyhu@yzu.edu.cn; Fax: +86 0514 87975244; Tel: +86 0514 87971818
bDepartment of Chemistry and Biological Science, Guangxi Normal University of Nationalities, Chongzuo, Guangxi, China
First published on 25th September 2012
Abstract
Continuous MOF-5 thin films were successfully synthesized on a glassy carbon electrode (GCE) with 4-carboxyphenyl as a covalent linker, and their use as a photoelectrochemical sensor for the detection of ascorbic acid was explored.
Metal–organic frameworks (MOFs), as a novel class of crystalline porous materials, have attracted substantial interest because of their potential applications ranging from gas storage, separation, adsorption, catalysis, drug delivery, sensors and so forth.1 Over the past decade, a significant number of MOFs with various structures and topologies have been reported.2 In the meantime, more and more groups have dedicated themselves to exploring the potential applications of these novel materials. Despite being in its infancy, the potential use of MOFs as semiconductors has attracted considerable attention recently.3 Alvaro et al. reported that MOF-5 behaves as a semiconductor and undergoes charge separation (electrons and holes) upon light excitation, which is supported by experimental evidence.4 Moreover, Tachikawa et al. revealed that MOF-5 has a much higher oxidation reaction efficiency than P-25 TiO2 powder, a common photocatalyst, in the one-electron oxidation of several substrates such as aromatic sulfides and amines.5
When it comes to various applications, however, depositing MOF thin films onto a given substrate is usually required.6 Unfortunately, until now, fabricating such a thin film is still a challenge. Despite the development of some methods to fabricate MOF thin films,7–10 it is still difficult to prepare continuous MOF thin films by a simple in situ synthesis route due to the poor heterogeneous nucleation of MOF crystals on supports.
In the field of electroanalytical chemistry, microporous zeolites have been widely used as electrode modifiers to improve the performance of electrodes.11 Metal oxide semiconductors (e.g. TiO2) have also shown clear advantages in photoelectrochemical analysis, which has been reported by our group and others.12 In this respect, compared with zeolites and metal oxides, porous MOFs which behave as semiconductors may find advanced applications in electroanalytical chemistry when they are used as electrode modifiers. With this in mind, we reasoned that if MOF thin films can be deposited evenly onto a glassy carbon electrode (GCE), they might be generally useful for many photoelectrochemical applications.
We now report a novel approach to prepare continuous and steady MOF-5 thin films on a GCE by firstly reducing a 4-carboxyphenyldiazonium salt on the GCE surface to form an organic monolayer, and then immersing this functionalized GCE in pre-prepared MOF-5 mother liquid (Fig. 1).
 | ||
| Fig. 1 Scheme of the fabrication of MOF-5 thin films on a GCE with 4-carboxyphenyl as the covalent linker. | ||
Using this strategy, the fabrication of MOF-5 thin films on a GCE was performed according to the following procedure. Briefly, the completely polished GCE was firstly treated in acetonitrile containing a 4-carboxyphenyldiazonium salt and NBu4BF4 using cyclic voltammetry for two cycles. This treated electrode was then rinsed with copious amounts of acetonitrile and then water before drying under a stream of argon.
In the next step, a fresh MOF-5 mother liquid was prepared. A mixture of Zn(NO3)2·6H2O and terephthalic acid in N,N-dimethylformamide (DMF) was heated at 120 °C for 48 h without stirring before being slowly cooled down to room temperature. Filtering off the colorless crystals gave the fresh MOF-5 mother liquid. After immersing the treated GCE in this clear solution for 24 h, washing with DMF and chloroform, and drying, the MOF-5 thin films were obtained (for details, see ESI†).
It can be seen from the SEM image (Fig. 2a) that the as-prepared MOF-5 films are continuous; no defects or islands are visible. Actually, our original attempts to synthesize continuous MOF-5 thin films on bare GCE supports either by in situ synthesis or by directly immersing the GCE into MOF-5 mother liquid met with failure; the only products we obtained were large cubic-like MOF-5 crystals rather than continuous thin films after several attempts (Fig. 2b). This can be rationalized by assuming that the heterogeneous nucleation of MOF-5 crystals on the bare GCE is very poor.7b,13 Unlike the bare GCE, which has limited oxygen-containing groups,14 the 4-carboxyphenyl modified GCE boasts a high density of carboxylic groups (16.2 × 10−10 mol cm−2, estimated through the integration of the voltammetric signal, see Fig. S1, ESI†),15 which may direct the nucleation and growth of the MOF-5 layers.7b Since this method of functionalization of surfaces by reduction of diazonium salts has been used to modify a series of important materials ranging from carbon, silicon, metals and gallium arsenide to organic materials such as PTFE and polyaniline,16 it is possible that our approach for fabrication of MOF thin films could be extended to use various other materials as substrates.
 | ||
| Fig. 2 SEM images of MOF-5 thin films on a GCE prepared (a) with and (b) without 4-carboxyphenyl as the covalent linker. | ||
The chemical composition of the aforementioned MOF-5 thin films was characterized by SEM-EDX, revealing the expected elemental constituents (e.g., Zn, C, and O). Typical X-ray diffraction (XRD) measurements established that the films comprised MOF-5.
The diffuse reflective spectra (DRS) of MOF-5 indicate that MOF-5 is a semiconducting MOF with a band gap of 3.3 eV (obtained from MOF-5 powder, Fig. S4, ESI†), underlining its potential as a photocatalyst. In order to expand the applicability of our MOF-5 thin films, photoelectrochemical detection of ascorbic acid (AA) was investigated. AA is a widely used, powerful antioxidant with an oxidation potential of −0.185 V (vs. SCE),17 and we predicted that it could be easily oxidized by the holes (E = 3.36 V, vs. SCE) generated by illuminated MOF-5 thin films.4 The proposed photoelectrochemical process for AA oxidation by MOF-5 thin films is shown in Fig. 3. When MOF-5 absorbs photons with energies higher than that of the band gap, electrons are excited from the valence band to the conduction band, forming the electron–hole pairs. The injection of the conduction band electrons into the GCE yields the photocurrent, whereas AA provides the electrons to the valence band holes, completing the photocurrent generation cycle. By measuring the photocurrent originating from the electron transfer process, the concentration of AA at that point may be determined.18
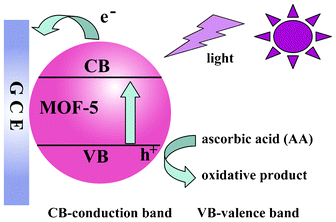 | ||
| Fig. 3 The photocurrent generation mechanism of a MOF-5 modified GCE. | ||
Photoelectrochemical experiments at each fabrication step of the MOF-5 modified GCE were recorded using a Xe lamp source at a given potential of 0 V (vs. SCE). Compared with the bare GCE and the 4-carboxyphenyl modified GCE, a strongly enhanced photocurrent response (Fig. S5, ESI†) was observed for the MOF-5 thin film modified GCE. All three photocurrent responses are higher than that observed for the MOF-5 thin film modified GCE without addition of 1 mM AA. These results confirm that it is the MOF-5 thin films that induce the strong photocurrent for the detection of AA. It is also worth mentioning that the photocurrent response of the MOF-5 thin film modified electrode shows considerable stability over three on–off irradiation cycles for 300 s. As shown in Fig. S6, ESI†, the photocurrent reproducibly increased rapidly with each irradiation and fell back in the dark. Thus, it could be used as a detection signal.
The quantitative behavior of this photoelectrochemical sensor was assessed by measuring the photocurrent response with increasing concentration of AA (Fig. 4). The response displayed a linear increase as the AA concentration increased from 0.05 to 1.4 mM. Since AA has been widely used as an electron donor in photoelectrochemical sensors,19 our MOF-5 modified GCE may be used for the detection of other compounds by enhancing or quenching the photocurrent generated by AA.
 | ||
| Fig. 4 Photocurrent responses at the MOF-5 modified GCE in acetonitrile containing 0.1 M NBu4ClO4 in the presence of 0, 50, 100, 200, 400, 600, 800, 1000, 1200, and 1400 μmol L−1 AA (from bottom to top) at 0 V to a light excitation. Inset: linear curve. | ||
In summary, we report for the first time, to the best of our knowledge, the fabrication of MOF-5 thin films on GCE substrates and provide insight into the application of MOF thin films as photoelectrochemical sensors. The key to prepare such continuous films relies on the introduction of carboxylic groups onto the GCE surface by reduction of a 4-carboxyphenyldiazonium salt. Since this method of functionalization of surfaces has been used to modify a series of important materials, it is possible that our approach to the fabrication of MOF thin films on substrates could be extended to various other material as substrates. We have also demonstrated the preliminary application of the MOF-5 thin film modified GCE as a sensor. The experimental results show that the photocurrent is proportional to the concentration of AA. Since AA has been widely used as an electron donor in photoelectrochemical sensors, our MOF-5 modified GCE may be used for the detection of other compounds by enhancing or quenching the photocurrent generated by AA. The search for stable and more efficient semiconducting MOFs and the preparation of MOF-based photoelectrochemical sensors is in progress in our group.
Acknowledgements
We gratefully acknowledge financial support from the Natural Science Foundation of China (no.21075107, no.21275124, no.21275125) and the Educational Committee of Jiangsu Provincial General Universities Graduate Student Scientific Research Invention Plan (CXZZ12_0893).Notes and References
- (a) J. L. C. Rowsell and O. M. Yaghi, Angew. Chem., Int. Ed., 2005, 44, 4670–4679 CrossRef CAS; (b) D. Farrusseng, A. Aguado and C. Pinel, Angew. Chem., Int. Ed., 2009, 48, 7502–7513 CrossRef CAS; (c) G. Lu and J. T. Hupp, J. Am. Chem. Soc., 2010, 132, 7832–7833 CrossRef CAS; (d) M. D. Allendorf, R. J. T. Houk, L. Andruszkiewicz, A. A. Talin, J. Pikarsky, A. Choudhury, K. A. Gall and P. J. Hesketh, J. Am. Chem. Soc., 2008, 130, 14404–14405 CrossRef CAS; (e) S. T. Meek, J. A. Greathouse and M. D. Allendorf, Adv. Mater., 2011, 23, 249–267 CrossRef CAS; (f) B. S. Zheng, J. F. Bai, J. G. Duan, L. Wojtas and M. J. Zaworotko, J. Am. Chem. Soc., 2011, 133, 748–751 CrossRef CAS.
- (a) M. Eddaoudi, J. Kim, M. Rosi, D. Vodak, J. Wachter, M. O'Keeffe and O. M. Yaghi, Science, 2002, 295, 469–472 CrossRef CAS; (b) D. J. Tranchemontagne, J. L. Mendoza-Cortés, M. O'Keeffe and O. M. Yaghi, Chem. Soc. Rev., 2009, 38, 1257–1283 RSC; (c) D. Zhao, D. J. Timmons, D. Q. Yuan and H. C. Zhou, Acc. Chem. Res., 2011, 44, 123–133 CrossRef CAS.
- (a) H. Khajavi, J. Gascon, J. M. Schins, L. D. A. Siebbeles and F. Kapteijn, J. Phys. Chem. C, 2011, 115, 12487–12493 CrossRef CAS; (b) C. G. Silva, A. Corma and H. García, J. Mater. Chem., 2010, 20, 3141–3156 RSC; (c) D. D. Allendorf, A. Schwartzberg, V. Stavila and A. A. Talin, Chem.–Eur. J., 2011, 17, 11372–11388 CrossRef; (d) M. C. Das, H. Xu, Z. Y. Wang, G. Srinivas, W. Zhou, Y. F. Yue, V. N. Nesterov, G. D. Qian and B. L. Chen, Chem. Commun., 2011, 47, 11715–11717 RSC.
- M. Alvaro, E. Carbonell, B. Ferrer, X. Francesc and H. García, Chem.–Eur. J., 2007, 13, 5106–5112 CrossRef CAS.
- T. Tachikawa, J. R. Choi, M. Fujitsuka and T. Majima, J. Phys. Chem. C, 2008, 112, 14090–14101 CAS.
- (a) A. Shekhah, J. Liu, R. A. Fischer and C. Wöll, Chem. Soc. Rev., 2011, 40, 1081–1106 RSC; (b) A. Bétard and R. A. Fischer, Chem. Rev., 2012, 112, 1055–1083 CrossRef.
- (a) S. Hermes, F. Schröder, R. Chelmowski, C. Wöll and R. A. Fischer, J. Am. Chem. Soc., 2005, 127, 13744–13745 CrossRef CAS; (b) S. Hermes, D. Zacher, A. Baunemann, C. Wöll and R. A. Fischer, Chem. Mater., 2007, 19, 2168–2173 CrossRef CAS.
- (a) O. Shekhah, H. Wang, S. Kowarik, F. Schreiber, M. Paulus, M. Tolan, C. Sternemann, F. Evers, D. Zacher, R. A. Fischer and C. Wöll, J. Am. Chem. Soc., 2007, 129, 15118–15119 CrossRef CAS; (b) O. Shekhah, H. Wang, T. Strunskus, P. Cyganik, D. Zacher, R. A. Fischer and C. Wöll, Langmuir, 2007, 23, 7440–7442 CrossRef CAS.
- J. F. Yao, D. H. Dong, D. Li, L. He, G. S. Xu and H. T. Wang, Chem. Commun., 2011, 47, 2559–2561 RSC.
- M. Y. Li and M. Dincă, J. Am. Chem. Soc., 2011, 133, 12926–12929 CrossRef CAS.
- A. Walcarius, Electroanalysis, 2008, 20, 711–738 CrossRef CAS.
- (a) H. B. Li, J. Li, Z. J. Yang, Q. Xu and X. Y. Hu, Anal. Chem., 2011, 83, 5290–5295 CrossRef CAS; (b) H. B. Li, J. Li, Q. Xu and X. Y. Hu, Anal. Chem., 2011, 83, 9681–9686 CrossRef CAS; (c) G. L. Wang, J. J. Xu and H. Y. Chen, Biosens. Bioelectron., 2009, 24, 2494–2498 CrossRef CAS.
- M. Jahan, Q. L. Bao, J. X. Yang and K. P. Loh, J. Am. Chem. Soc., 2010, 132, 14487–14495 CrossRef CAS.
- W. J. Blaedel and R. A. Jenkins, Anal. Chem., 1974, 46, 1952–1955 CrossRef CAS.
- Y. C. Liu and R. L. McCreery, J. Am. Chem. Soc., 1995, 117, 11254–11259 CrossRef CAS.
- (a) M. Delamar, R. Hitmi, J. Pinson and J. M. Savéant, J. Am. Chem. Soc., 1992, 114, 5883–5884 CrossRef CAS; (b) M. Coates, E. Cabet, S. Griveau, T. Nyokong and F. Bedioui, Electrochem. Commun., 2011, 13, 150–153 CrossRef CAS; (c) M. P. Stewart, F. Maya, D. V. Kosynkin, S. M. Dirk, J. J. Stapleton, C. L. McGuiness, D. L.Allara and J. M. Tour, J. Am. Chem. Soc., 2004, 126, 370–378 CrossRef CAS; (d) G. Liu and M. S. Freund, Chem. Mater., 1996, 8, 1164–1166 CrossRef CAS; (e) D. Bélanger and J. Pinson, Chem. Soc. Rev., 2011, 40, 3995–4048 RSC.
- R. M. C. Dawson, D. C. Elliott, W. H. Elliott and K. M. Jones, Data for Biochemical Research, Oxford Science Publications, Clarendon Press, Oxford, 2nd edn, 1986 Search PubMed.
- (a) J. A. Cooper, K. E. Woodhouse, A. M. Chippindale and R. G. Compton, Electroanalysis, 1999, 11, 1259–1265 CrossRef CAS; (b) J. A. Cooper, M. Wu and R. G. Compton, Anal. Chem., 1998, 70, 2922–2927 CrossRef CAS.
- (a) Z. Qian, H. J. Bai, G. L. Wang, J. J. Xu and H. Y. Chen, Biosens. Bioelectron., 2010, 25, 2045–2050 CrossRef CAS; (b) G. L. Wang, J. J. Xu and H. Y. Chen, Nanoscale, 2010, 2, 1112–1114 RSC; (c) G. L. Wang, P. P. Yu, J. J. Xu and H. Y. Chen, J. Phys. Chem. C, 2009, 113, 11142–11148 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of synthesis and characterization of MOF-5 thin films, additional CVs, EDX, XRD patterns, and photocurrent responses. See DOI: 10.1039/c2ra21848h |
| This journal is © The Royal Society of Chemistry 2012 |
