Synthesis of cobalt ferrite nanoparticles in continuous-flow microreactors
Ali
Abou-Hassan
*,
Sophie
Neveu
,
Vincent
Dupuis
and
Valérie
Cabuil
UPMC Univ Paris 6, Laboratoire de Physicochimie des Electrolytes Colloïdes et Sciences Analytiques (PECSA), UMR 7195, équipe Colloïdes Inorganiques, Université Paris 6 (UPMC) Bat F (74), Case 51, 4 place Jussieu, F-75252 Paris Cedex 05, France. E-mail: ali.abou_hassan@upmc.fr; Fax: (+33) 1-44-27-36-75; Tel: (+33) 1-44-27-31-74
First published on 27th September 2012
Abstract
High quality CoFe2O4 nanoparticles were synthesized only in 16 min, continuously in two coupled microreactors. The first microreactor induced the fast homogenization of the reagents mixture at ambient temperature so Fe3+ and Co2+ hydroxides precipitate, while a second microreactor heated at 98 °C allowed the fast aging and the evolution of amorphous hydroxides into faceted and crystalline CoFe2O4.
Due to their small length scales and their large surface to volume ratio, continuous-flow and droplet-based microreactors offer a variety of advantages for optimizing and studying chemical reactions. Compared to conventional reactors, reaction volumes are decreased; thus heat and mass transfer are enhanced allowing a better control of the chemical reactions.1 A large range of nanomaterials such as quantum dots, metallic nanoparticles, oxide nanoparticles and multifunctional core–shell nanoparticles have been synthesized using microreactors.2 Magnetic nanoparticles, and among them spinel ferrite nanoparticles, are a particular class of nanomaterials that have attracted a continuous interest because of their wide applications, especially in the field of medical imaging and therapy, namely magnetic field induced hyperthermia.3 The latter can be significantly improved by using materials with high magnetic anisotropy and large magnetic moments. Cobalt ferrite nanoparticles (CoFe2O4) fulfil these requirements and have been recognized as very promising and efficient in the treatment of tumours by hyperthermia.4 As the magnetic properties of CoFe2O4 nanoparticles are related to their size and shape, controlling the latter is a very important issue. Numerous synthesis methods of such particles have been intensively investigated. These methods include chemical coprecipitation (including in hydrothermal bombs),5 spraying coprecipitation,6 forced hydrolysis in a polyol medium,7 synthesis in oil-in-water micelles8 or thermal decomposition of mixed Co2+ Fe2+ oleate complex.9
Compartmentalization of chemical reactions in microdroplets acting as microreactors by using the “digital-microfluidics”, is a very popular method for the synthesis of nanoparticles.10 However, oil and surfactants are used to generate and stabilize the microdroplets and can interfere with the reaction synthesis thus affecting the nucleation and growth of the nanoparticles.11,12 Compared to microdroplet reactors, continuous-flow reactors are easier to handle and are more representative of the bulk synthesis. The improved homogeneity offers a better reproducibility of the synthesized particles and different reagents can be added downstream to vary the chemical composition of the mixture without any synchronization as in droplets-based microreactors. A direct advantage due to the continuous flow is that individual reactions can be linked into multistep sequences. This enables one reaction to flow into another and thus to combine multiple synthetic steps into a continuous operation.
In this communication, we report the multistep continuous synthesis of cobalt ferrite nanoparticles CoFe2O4 by using a microfluidic device. As CoFe2O4 synthesis is made of two steps, the homogeneous precipitation of iron(+III) and cobalt(+II) hydroxides, followed by the aging of the amorphous precipitate to the spinel type structure. The microfluidic device has been designed in order to separate (in time and space) the Fe3+ and Co2+ hydroxides precipitation and their subsequent growth into CoFe2O4.13 The experimental set-up is shown in Scheme 1 and consists in the association of a coaxial flow microreactor (μR1) with an aging microtubular loop (μR2). While μR1 is kept at room temperature, the temperature of μR2 is maintained at 98 °C by continuous heating in a water bath. pH gradients and mixing in μR1 have been studied and described in details elsewhere.13,14
 | ||
| Scheme 1 Experimental set-up used for the synthesis of cobalt ferrite nanoparticles. TMAOH = tetramethylammonium hydroxide. | ||
In brief, the μR1 is based on a three-dimensional coaxial-flow device where inner and outer streams can be injected respectively with the flow rates Qin and Qout (Scheme 1). The 3D hydrodynamic geometry allows a rapid homogenization of the reactants through flow focalization and avoids problems such as channel clogging or precipitation of the chemical species along the reactor walls. The mixing of reagents in μR1 occurs by diffusion at the point of confluence. Thus the time of mixing can be chosen by simply varying the ratio of the flow rates Qout/Qin. This ratio is the parameter controlling the stability of the inner stream, the mixing times and the pH jump in order to guarantee the coprecipitation of Fe3+ and Co2+ ions and the formation of CoFe2O4 nanoparticles with a high yield. The best mixing time that can be achieved in μR1 is about 80 ms for a Qout/Qin ratio of 400 and was used for the synthesis experiments. In a previous study we have reported a microfluidic approach for the synthesis of elongated goethite α-FeOOH nanoparticles after precipitation of Fe3+ hydroxides and their subsequent aging.15 Compared to this previous work, it is important to note the following differences for the synthesis of cobalt ferrite nanoparticles. Other than the magnetic properties of the cobalt ferrite nanoparticles and their shape, the main difference remains in the mechanisms of precipitation involving the two cations Co2+ and Fe3+ of different reactivity and their transformation to obtain high-quality CoFe2O4 nanoparticles, two steps that are very different and more challenging to control in the case of ferrite synthesis than of goethite.16 To get pure CoFe2O4 ferrite nanoparticles other than an appropriate molar ratio of Co2+ to Fe3+ a fast pH range must be fulfilled to get anticipant coprecipitation of both cations. It is the incorporation of Co2+ that allows transformation of quasi-amorphous ferric hydroxide into ferrite. Therefore, electron transfer between Co2+ and Fe3+ plays a key role in the formation of the CoFe2O4.17 Based on thermodynamic and kinetic considerations, the acidity of water complexes of Fe3+ is higher than those of Co2+ and it is generally accepted that ferrite formation involves the precipitation first of Fe3+ hydroxides followed by the Co2+ hydroxides.18 Thus to increase the incorporation of Co2+, and the selective formation in the transformation phase of cobalt ferrites a fast alkaline jump is required to prevent the formation only of segregated hydroxide phases and their subsequent growth into separated oxides.19
The precipitation of iron(+III) and cobalt(+II) hydroxides in the mixing microreactor is performed by injecting in the inner flow an aqueous mixture of FeCl3 and CoCl2 salts (stoichiometry: 2 Fe3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co2+) and in the outer flow an alkaline solution of tetramethylammonium hydroxide ((CH3)4NOH; TMAOH). TMAOH was chosen prior to any other base as the TMA+ cations afford enhanced stability of colloidal hydroxide dispersion.20 At the outlet of μR1, the colloidal suspension was brown, indicating the formation of (Fe3+, Co2+) hydroxides. The suspended hydroxides were directly injected into the aging coil μR2 (L = 150 cm; 500 μm inner diameter), the latter being continuously heated in a water bath at 98 °C. Under the given flow rate, the hydroxides solution reaches 98 °C within the first centimeter after it has entered the heated zone of the tubing, that is in about 2 s. This was observed qualitatively as the hydroxide brown suspension exiting μR1 instantaneously turned black at the beginning of the coil (μR2). The effective residence time is about 16 min, as estimated from the length of the tubing along which the fluid has reached the stationary temperature of 98 °C.
1 Co2+) and in the outer flow an alkaline solution of tetramethylammonium hydroxide ((CH3)4NOH; TMAOH). TMAOH was chosen prior to any other base as the TMA+ cations afford enhanced stability of colloidal hydroxide dispersion.20 At the outlet of μR1, the colloidal suspension was brown, indicating the formation of (Fe3+, Co2+) hydroxides. The suspended hydroxides were directly injected into the aging coil μR2 (L = 150 cm; 500 μm inner diameter), the latter being continuously heated in a water bath at 98 °C. Under the given flow rate, the hydroxides solution reaches 98 °C within the first centimeter after it has entered the heated zone of the tubing, that is in about 2 s. This was observed qualitatively as the hydroxide brown suspension exiting μR1 instantaneously turned black at the beginning of the coil (μR2). The effective residence time is about 16 min, as estimated from the length of the tubing along which the fluid has reached the stationary temperature of 98 °C.
TEM images of drops collected directly at the exit of μR1 (Fig. 1a) and after aging for 16 min in μR2 (Fig. 1 b) confirmed the transformation of undefined amorphous structure of (Co2+, Fe3+) hydroxides into individual and crystalline CoFe2O4. The obtained CoFe2O4 nanocrystals are faceted with an average length 7.4 ± 3.5 nm. Moreover, the electron diffraction of a large selected zone (inset of Fig. 1b) and the XRD (Fig. 1c) patterns can be easily indexed to CoFe2O4 (JCPDS 22-1086). High-resolution TEM analysis (HRTEM) provided more structural information on the nanoparticles. A representative TEM image of the CoFe2O4 nanoparticles is shown in Fig. 1c, in which the HRTEM image further supports the single-crystalline nature of the nanoparticles.
 | ||
| Fig. 1 TEM images of a) (Co2+, Fe3+) hydroxides formed after mixing in μR1; b) After evolution of (Co2+, Fe3+) hydroxides of image a) into CoFe2O4 nanoparticles and the electron diffraction pattern (inset); c) XRD pattern of CoFe2O4; c) HRTEM image of CoFe2O4 nanoparticles showing the lattice fringes; e) EDX analysis of the CoFe2O4 nanoparticles. | ||
The interlayer distances were calculated to be ≈0.48 nm and ≈0.29 nm, which agrees well with the separation between the (101) and the (220) lattice planes, respectively. Energy dispersive X-ray analysis (EDX) was also measured to determine the chemical composition of the ferrite samples. Results from EDX spectra of many nanoparticles showed that the samples contain only Co, Fe and O for CoFe2O4 (Fig. 1e). Quantitative determination of the chemical composition was obtained by atomic absorption spectroscopy. The atomic ratio Co/Fe was ≈1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, in agreement with the expected stoichiometry of CoFe2O4.
2, in agreement with the expected stoichiometry of CoFe2O4.
Fig. 2 illustrates the advantages to use a microreactor device for mixing and aging compared to the bulk conventional process. The same aqueous mixture of FeCl3 and CoCl2 salts were mixed with an alkaline solution of tetramethylammonium hydroxide under vigorous stirring at room temperature outside the microreactor by using the syringe pumps and with a volumetric flow rate ratio corresponding to Qout/Qin ≈ 400 in the microreactor. The mixture was heated to 98 °C (for a mixture volume of 1 mL it took at least 30 min to reach the stable temperature of 98 °C), then kept at 98 °C for 16 min. The products were analyzed by TEM and compared to those obtained after synthesis in the microreactor (Fig. 2). Only amorphous hydroxides were obtained by this bulk coprecipitation method. When the hydroxide mixture is heated for 16 min at 98 °C in a hydrothermal bomb, the total transformation of amorphous hydroxides into crystalline and well-defined nanoparticles as in the microreactor synthesis cannot be achieved. So, besides being time and energy consuming, bulk conventional synthesis failed after 16 min to induce the total transformation of amorphous hydroxides into crystalline and well-defined nanoparticles as in the microreactor synthesis. It needs 2 h by boiling under reflux at 100 °C,21 or 50 min by heating at 200 °C in a hydrothermal bomb to get CoFe2O4 nanoparticles similar in size to those obtained in 16 min at 98 °C by the microfluidic synthesis.22
 | ||
| Fig. 2 TEM images of the particles obtained at 98 °C after 16 min of aging a) in the microreactor synthesis, b) in bulk synthesis, c) in bulk, in a hydrothermal bomb. | ||
The TEM images corresponding to these samples as well as the size distributions (fitted to a lognormal law) are shown in Fig. 3. Fitting parameters are summarized in Table 1. The magnetic properties (magnetization versus magnetic field) of these three types of ferrite nanoparticles suspended in water were investigated with a commercial squid magnetometer at 300 K and 5 K. While the magnetization process is reversible at 300 K (liquid sample, data not shown) as expected for superparamagnetic particles, hysteresis is observed (Fig. 4) at low temperatures (frozen sample) revealing the ferrimagnetic nature of the cobalt ferrite. The large extracted values of the coercive forces that lie within the same range 10–20 kOe while the ratio of the remanent magnetization Mr in the absence of the magnetic field H (H = 0) on the magnetization at saturation Ms, are between 0.5 and 0.6 (Table 1). These values are characteristics of hard particles suitable for applications such as magnetic recording materials or permanent magnets. They can be estimated here to 10 kOe, 11 kOe and 15 kOe for the particles synthesized using the microfluidic, hydrothermal and precipitation methods, respectively. While the coercive field is expected to be independent of the size of the particle in a simple Stoner–Wohlfarth model, such size dependences indicative of a more complex behaviour are often observed in magnetic nanoparticles. In this respect, our results that point towards a maximum coercive field for CoFe2O4 particles with a diameter around 12 nm are in agreement with the work by Song and Zhang on cobalt ferrite nanoparticles.23
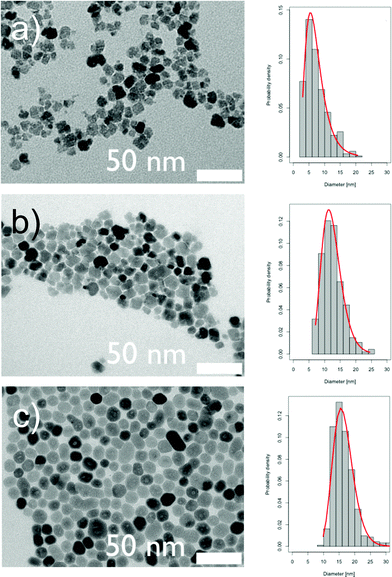 | ||
| Fig. 3 TEM images of the particles obtained at 98 °C after a) 16 min of aging in the microreactor synthesis, b) 2 h at 100 °C in bulk synthesis, c) 50 min at 200 °C in bulk, in a hydrothermal bomb; corresponding size histograms with log-normal fits on the right side. | ||
 | ||
| Fig. 4 Magnetization (normalized to its maximum value) of CoFe2O4 nanoparticles dispersed in water versus magnetic field. | ||
| d 0 (nm) | σ | H c (kOe) | M r/Ms 5 K | |
|---|---|---|---|---|
| Microfluidic | 6.67 | 0.450 | 11 | 0.55 |
| Coprecipitation | 12.11 | 0.261 | 15 | 0.60 |
| Hydrothermal | 16.18 | 0.198 | 10 | 0.6 |
In summary we have demonstrated an efficient, fast and reliable method to accelerate the synthesis of cobalt ferrite CoFe2O4 nanoparticles by using two coupled microreactors. In the first microreactor, the streaming reagents are mixed by molecular diffusion at room temperature in a 3D flow focusing geometry. The coflow geometry allowed the homogeneous and rapid coprecipitation of Co2+ and Fe3+ hydroxides by alkaline solution of TMAOH and avoided the microreactor clogging and precipitation of solids onto its walls. In the second microreactor operating at 98 °C, the evolution of the hydroxides into well defined and crystalline CoFe2O4 nanoparticles was accelerated due to the small dimensions of this aging channel and to the homogeneity of the temperature. Compared to the conventional bulk synthesis methods, our method yields CoFe2O4 nanoparticles that are similar in size and in their magnetic properties to those obtained by these methods. While the conventional synthesis methods are time and energy consuming, and failed to produce at short times of aging crystalline CoFe2O4 nanoparticles, our method required only 16 min of aging (for a velocity of 0.1 cm s−1). Apart from the importance of the different applications of cobalt ferrite nanoparticles, this work adds another question on the use of microreactors for accelerating the aging process of materials dispersed in a fluid carrier.
Experimental section
The coaxial flows micromixer (μR1) is obtained by molding in poly(dimethylsiloxane) (PDMS) and is described in detail in ref. 15. The aging microtubular loop (μR2) consists of a transparent PTFE tube of 1.7 mm inner diameter and 150 cm total length (Upchurch Scientific). Harvard Apparatus syringe pumps (pico 11 plus) controlled the flow rates. As starting materials for the precipitation of CoFe2O4 nanoparticles, we used CoCl2·6H2O salt (VWR), FeCl3·6H2O (27%, VWR), tetramethyl ammonium hydroxide salt (CH3)4NOH·5H2O (97%, Sigma-Aldrich), and hydrochloric acid HCl (37%, VWR) of analytical grade. A solution of total iron and cobalt salts concentration of 10−2 mol L−1 with 0.5 as molar ratio Co2+/Fe3+ in diluted hydrochloric acid (pH = 0.4) and TMAOH (0.172 mol L−1) were used for the synthesis. The precipitate obtained at the end of the microreactor μR2 were isolated, dispersed in aqueous solution of hydrochloric acid (10 min, [HCl] = 0.2 mol L−1) and citrate ions were then added as described in a previous study.20 At the end of this process, the precipitated was washed several times with acetone and ether and finally dispersed in distilled water producing a colloidal dispersion of nanoparticles, stable at pH 7.TEM and HRTEM images were recorded with a JEOL JEM 100 CX transmission electron microscope operating at 100 kV with a point to point resolution of 0.3 nm. The magnetic properties of our particles and in particular their coercive force, were performed on a commercial Quantum Design MPMS SQUID magnetometer at 300 K and 5 K.
References
- V. H. W. Ehrfeld and H. Lowe, Microreactors: New Technology for Modern Chemistry, Wiley-VCH, Weinheim, 2000 Search PubMed.
- Y. Roig, S. Marre, T. Cardinal and C. Aymonier, Angew. Chem., 2011, 123, 12277–12280 Search PubMed.
- G. Baldi, D. Bonacchi, C. Innocenti, G. Lorenzi and C. Sangregorio, J. Magn. Magn. Mater., 2007, 311, 10–16 CrossRef CAS.
- J.-P. Fortin, C. Wilhelm, J. Servais, C. Ménager, J.-C. Bacri and F. Gazeau, J. Am. Chem. Soc., 2007, 129, 2628–2635 CrossRef CAS.
- F. A. Tourhino, R. Franck and R. Massart, Prog. Colloid Polym. Sci., 1990, 79, 128–135 Search PubMed.
- Z. Jiao, X. Geng, M. Wu, Y. Jiang and B. Zhao, Colloids Surf., A, 2008, 313–314, 31–34 Search PubMed.
- S. Ammar, A. Helfen, N. Joini, F. Fievet, I. Rosenman, F. Villain, P. Molinie and M. Danot, J. Mater. Chem., 2011, 11, 186–192 Search PubMed.
- N. Moumen, I. Lisiecki, M. P. Pileni and V. Briois, Supramol. Sci., 1995, 2, 161–168 Search PubMed.
- N. Bao, L. Shen, W. An, P. Padhan, C. H. Turner and A. Gupta, Chem. Mater., 2009, 21, 3458–3468 CrossRef CAS.
- L.-H. Hung, K. M. Choi, W.-Y. Tseng, Y.-C. Tan, K. J. Shea and A. P. Lee, Lab Chip, 2006, 6, 174–178 RSC.
- S.-Y. Teh, R. Lin, L.-H. Hung and A. P. Lee, Lab Chip, 2008, 8, 198–220 RSC.
- S. A. Khan, A. Gunther, M. A. Schmidt and K. F. Jensen, Langmuir, 2004, 20, 8604–8611 CrossRef CAS.
- A. Abou-Hassan, J.-F. Dufrêche, O. Sandre, G. Mériguet, O. Bernard and V. Cabuil, J. Phys. Chem. C, 2009, 113, 18097–18105 CrossRef CAS.
- O. S. A. A. Hassan, V. Cabuil and P. Tabeling, Chem. Commun., 2008, 1783–1785 RSC.
- A. Abou-Hassan, O. Sandre, S. Neveu and V. Cabuil, Angew. Chem., Int. Ed., 2009, 48, 2342–2345 CAS.
- R. M. Cornell and U. Schwertmann, The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses, Wiley-VCH, 2nd edn, 2003 Search PubMed.
- J. P. Jolivet, Metal Oxide Chemistry and Synthesis: From Solution to Solid State, Wiley, 2000 Search PubMed.
- C. F. Baes and R. E. Mesmer, The Hydrolysis of Cations, John Wiley & Sons Inc, 1976 Search PubMed.
- E. Tirosh, G. Shemer and G. Markovich, Chem. Mater., 2005, 18, 465–470 Search PubMed.
- R. Massart and V. Cabuil, J. Chim. Phys., 1987, 7, 84–89.
- S. Neveu, A. Bee, M. Robineau and D. Talbot, J. Colloid Interface Sci., 2002, 255, 293–298 CrossRef CAS.
- V. r. Cabuil, V. Dupuis, D. Talbot and S. Neveu, J. Magn. Magn. Mater., 2011, 323, 1238–1241 Search PubMed.
- Q. Song and Z. J. Zhang, J. Am. Chem. Soc., 2004, 126, 6164–6168 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
