Investigations on microalgal oil production from Arthrospira platensis: towards more sustainable biodiesel production†
Kate E.
Baunillo
*a,
Roger S.
Tan
a,
Higinio R.
Barros
a and
Rafael
Luque
*b
aXavier University, Ateneo de Cagayan, Philippines. E-mail: kate_ollinuab@yahoo.com
bDepartamento de Quimica Organica, Universidad de Cordoba, Campus de Rabanales, Edificio Marie Curie, Ctra Nnal IV-A, Km 396, Cordoba, E-14014, Spain. E-mail: q62alsor@uco.es; Fax: +34 957212066; Tel: +34 957211050
First published on 25th September 2012
Abstract
Microalgae have emerged as one of the most promising sources for biodiesel production. Arthrospira platensis (Spirulina) is known for its nutritional benefits with very low lipid content. However, among all other algae, spirulina is the most easy to cultivate due to its inherent resistance to contamination and environmental changes. The presented work aims to determine the best culture medium to achieve an optimum lipid content for microalgae in view of a subsequent exploration for biodiesel production. This was conducted by means of a two step microalgal oil extraction followed by a conventional acid-catalysed esterification of the released fatty acids. Algal growth, biomass and lipid content were compared for Spirulina grown under six different culture media. 1) culture medium of complete nutrition (CONTROL); 2) nitrogen and phosphorus-deprived [NP(-)]; 3) nitrogen-deprived [N(-)]; 4) phosphorus-deprived [P(-)]; 5) nitrogen and phosphorus-limited [NPL] and; 6) nitrogen-deprived and phosphorus-limited [N(-)PL]. With the minimum possible dried biomass (0.4780 g L−1), the largest lipid content (ca. 20%) was achieved in spirulina grown under nitrogen-deprived and phosporous-limited conditions [N(-)PL]. Dried biomass from Spirulina grown under two opposite culture media: complete nutrition [CONTROL]; and nitrogen and phosphorous deprived [NP(-)], were utilised for biodiesel preparation after oil extraction. A crude biodiesel yield of 40% and 42%, with a FAME content of 69% and 55% were obtained for CONTROL and NP(-) samples, respectively. Interestingly, the nutrient condition of Spirulina did influence biodiesel yields but not significantly their FAME compositions, which comprised of mostly C16:0 and C18:2, similar to that of conventional biodiesel.
Introduction
Studies on renewable sources and biofuels have been proven to offer promising solutions to replace petroleum-derived fuels and constitute the basis of a more sustainable future society. First generation biodiesel, the world's most widely employed biofuel together with bioethanol, has been extensively produced via transesterification of triglycerides from food crops including rapeseed oil and maize.1,2 Alternative and more sustainable feedstocks, including waste oils and animal fats as well as microalgal oils, have also been recently explored for biodiesel production.3,4 These alternative feedstocks had recently attracted a great deal of interest as food crops cannot realistically satisfy the overall energy demands for biodiesel in the transport sector. This has been due to the competition of food and fibre production for the use of arable lands, regionally constrained market structures, lack of well managed agricultural practices in emerging economies, the need for high water and fertilizer requirements as well as the conservation of bio-diversity together with the food vs. fuel issue.5Among those aforementioned alternative feedstocks, microalgae comprise an important group of photosynthetic, heterotrophic organisms which have an extraordinary potential for cultivation as energy crops. Microalgae are chosen in the production of biodiesel because of their capability to accumulate and to store lipids in large quantities. Compared with terrestrial plants, microalgae have high oil content and growth rate (with the possibility to exceed 50 wt.% oil content by weight of dry biomass for maximum microalgal yields of 136![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 litres per hectare).6,7 Microalgae can also be cultivated under difficult agro-climatic conditions in open ponds or closed bioreactors, thus avoiding competitions for limited arable lands. Apart from accumulating oil, microalgae are able to produce a wide range of commercially interesting byproducts including fats, oils, sugars and functional bioactive compounds.8 Culture of specific microalgae is however currently restricted to open ponds due to the high associated costs of photobioreactors.9 Sustained open pond production has been successful for a limited number of microalgal species including Spirulina, Chlorella and Dunaliella.10 Maximum production of these microalgae is achieved under tropical or subtropical conditions which indicate that these could suitably be cultivated under Philippine settings as well as countries including Hong Kong, Southern China, Indonesia and South America.
900 litres per hectare).6,7 Microalgae can also be cultivated under difficult agro-climatic conditions in open ponds or closed bioreactors, thus avoiding competitions for limited arable lands. Apart from accumulating oil, microalgae are able to produce a wide range of commercially interesting byproducts including fats, oils, sugars and functional bioactive compounds.8 Culture of specific microalgae is however currently restricted to open ponds due to the high associated costs of photobioreactors.9 Sustained open pond production has been successful for a limited number of microalgal species including Spirulina, Chlorella and Dunaliella.10 Maximum production of these microalgae is achieved under tropical or subtropical conditions which indicate that these could suitably be cultivated under Philippine settings as well as countries including Hong Kong, Southern China, Indonesia and South America.
Arthrospira platensis, commonly known as Spirulina, is a photosynthetic, filamentous, spiral-shaped, multicellular cyanobacteria whose metabolism generates important metabolites including fatty acids, proteins and bioactive compounds.11 Spirulina large-scale culturing and harvesting is currently feasible as the species is resistant to contamination. Despite its low fatty acid content as compared to other algae, spirulina is highly productive in terms of biomass generation.12 In any case, its composition, including fatty acids and proteins profiles, together with ways to improve its cultivation, have been extensively investigated due to the many nutritional benefits these algal species can provide.13–16 Some of the most common and largely produced fatty acids in spirulina metabolism include γ-linolenic, linoleic and palmitic acids as well as oleic acid in some cases.13 Interestingly, these fatty acids are comparable to those found in different oilseeds such as soybean and palm oil that have been extensively utilised for biodiesel production.17 This makes spirulina oil an attractive alternative candidate to be transesterified to biodiesel.
In this work, we aim to investigate the effect of different culture media in the cell growth and lipid content of Arthrospira platensis, followed by extraction and evaluation of the fatty acid profile in the obtained microalgal oil for its subsequent transesterification to biodiesel.
Experimental
Cultivation
The Arthrospira platensis starter culture was purchased from the Philippine National Collection of Microorganisms (PNCM), National Institute of Molecular Biology and Biotechnology (BIOTECH), University of the Philippines Los Baños. A schematic representation of the complete cultivation methodology has been included in Scheme 1. A 100 mL starter culture (part A) was diluted with 250 mL culture medium (with complete nutrition as shown in Table 1) in a well washed transparent plastic container covered with a net to prevent contact from insects or any external items (part B). Tap water (obtained from the laboratory) was used as solvent for the chemicals needed and to provide calcium chloride and other micronutrients for the culture medium. The culture was initially grown under laboratory conditions and then placed in a brighter place but without direct sunlight since Spirulina under highly diluted conditions has been reported to be fragile against external parameters including direct light exposure and violent shaking.18 The culture was stirred at least three times a day to ensure that all cells of the population were equally exposed to light and nutrients as well as to improve gas exchange between the culture medium and the air.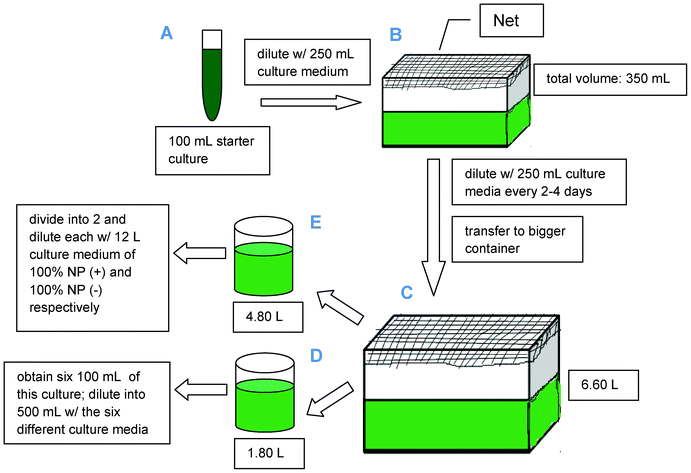 | ||
| Scheme 1 Schematic diagram for the cultivation of Spirulina. | ||
| Ingredient | Amount (g) per liter of medium |
|---|---|
| a To prevent precipitation of Iron sulfate; One bag of green tea was used per preparation and soaked in a 50 mL beaker with 25 mL water. | |
| Sodium bicarbonate | 16 |
| Potassium nitrate | 2 |
| Sea Salt | 1 |
| Ammonium phosphate | 0.1 |
| Strong green teaa | 1 mL |
| Iron sulfate | 0.01 |
| Magnesium sulfate | 0.1 |
The dense culture was further diluted with its culture medium until the volume has reached 6.60 L in volume (at the same time transferred into a bigger transparent container, part C). After 17 days, the optical density of the culture was obtained (0.708 nm) through UV-Vis spectrophotometry at λmax = 680 nm. The total solution (6.60 L) was then divided into two solutions (4.80 and 1.80 L, respectively, parts D and E) which were processed separately. Firstly, the solution of 4.80 L from the culture was subdivided into two portions and each of them were diluted with 12 L of the corresponding culture medium to further observe the effects of: 1) culture medium of complete nutrition [CONTROL] and; 2) nutrients deprivation/starvation [NP(-)] towards lipid and FAME content. CO2, obtained directly from the environment, was used as carbon source for microalgal growth.
On the 27th day of culture, the remaining 1.80 L solution (part D) was divided into six 100 mL of dense Arthrospira platensis culture which were each diluted to a total final volume of 500 mL via addition of the 400 mL culture medium of the six investigated media: 1) culture medium of complete nutrition (CONTROL); 2) nitrogen and phosphorus-deprived [(NP(-)]; 3) nitrogen-deprived [N(-)]; 4) phosphorus-deprived [P(-)]; 5) nitrogen and phosphorus-limited [NPL] and; 6) nitrogen-deprived and phosphorus-limited [N(-)PL]. The missing possible combinations (nitrogen limited as well as nitrogen-limited and phosphorous deprived) have not been included in the manuscript for the sake of simplicity as they provided essentially no new insights or results worth mentioning in this work.
The depleted conditions were first obtained by excluding the unwanted nutrients from the medium (as described in Table 1), except that, when potassium nitrate was excluded, potassium sulfate (0.5 g L−1) was added to compensate the nutrient -potassium.
Sample preparation
After 22 days of cultivation, the optical densities for CONTROL and NP(-) were of 0.505 nm and 0.290 nm, respectively. The cells were harvested through vacuum filtration and were re-suspended with less amount of distilled water on a pre-weighed evaporating dish. Samples were dried in an oven overnight at 100 °C, cooled, weighed and finally pulverised.To assess cell growth, regular measurements of the optical density at 680 nm were conducted in a UV-Vis Spectrophotometer (UV-1700). The six cultures as well as the culture medium (blank test) were stirred prior to measuring the optical density. Eventually, the cells were harvested on the 36th day, oven dried and subsequently pulverised in the same procedure as above. The basis of a 36-day cultivation media can be justified according to previous literature reports that showed a continue increase of biomass production over 31 days.12 We decided to extend the study to some extra days of cultivation to ascertain any relevant changes in biomass production for any of the treatment combinations. However, as per observation, none of the treatments showed any appreciable change in biomass growth (or drop) after 36 days.
Lipid extraction
Dried samples were separately extracted using a mixture of chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (2
methanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v). Ca. 50 mL of solvents were employed for every gram of dried sample in each extraction step. After stirring the sample using a magnetic stirrer bar for 5 h, samples were centrifuged at 3000 rpm for 10 min.19 The solid phase was carefully separated via filtration wherein two pieces of filter papers were applied to provide complete separation. The solids left were re-extracted three times using the same procedure. The combined solvent layers were then passed through a 2.5 cm thick layer of anhydrous sodium sulfate into a pre-weighed container for rotary evaporation. The solvent phase was removed under vacuum at 65 °C. Lipids weight measurements were conducted in triplicates (see supporting information†) and the lipid content (%) by weight difference were then calculated.20
1 v/v). Ca. 50 mL of solvents were employed for every gram of dried sample in each extraction step. After stirring the sample using a magnetic stirrer bar for 5 h, samples were centrifuged at 3000 rpm for 10 min.19 The solid phase was carefully separated via filtration wherein two pieces of filter papers were applied to provide complete separation. The solids left were re-extracted three times using the same procedure. The combined solvent layers were then passed through a 2.5 cm thick layer of anhydrous sodium sulfate into a pre-weighed container for rotary evaporation. The solvent phase was removed under vacuum at 65 °C. Lipids weight measurements were conducted in triplicates (see supporting information†) and the lipid content (%) by weight difference were then calculated.20
Biodiesel production
The transesterification of the algal oil for the CONTROL and NP(-) samples was carried out under acid-catalysed conditions adapting the method of Johnson et al.21An acid-catalysed method was selected due to the large presence of free fatty acids in the algal oil that will originate soaps and emulsions under otherwise more efficient based-catalysed conditions.17 A mixture of 3.4 mL methanol, 0.6 mL sulfuric acid and 4.0 mL chloroform was added to the algal oil produced per gram of the algal biomass. The mixture was heated at 90 °C for 3 h. The samples were thoroughly mixed during heating. After the reaction was completed, the samples were cooled down to room temperature, quenched with 2 mL distilled water and left overnight to allow phase separation. The lower phase which contained the biodiesel (FAME) was collected and transferred to a pre-weighed glass vial. The solvent was evaporated using nitrogen gas and then the final product quantified, analysed and characterised accordingly.FAME characterization
The FAME composition contained in the crude biodiesel samples was further analysed upon trimethylsilyl (TMS) derivatisation at Pilipinas Kao, Inc., via GC. An amount of 0.03 g from each sample was weighed and added to 2 mL TMS, bath at 60 °C for 1 min, added to 1.5 mL hexane, and then diluted to 10 mL with water. The upper layer which contained the FAME was then collected and subsequently analysed by GC and GC-MS fitted with a FID detector and an Ultra-Alloy column (15 m × 250 μm × 0.15 μm). Table 2 provides an overview of all the instrument parameters utilised. The FAME percentages were worked out by means of area normalization.| Carrier Gas | Helium |
|---|---|
| Oven Program Initial Temp. | 60 °C |
| Hold Time 1 | 2 min |
| Ramp 1 | 10 °C min−1 |
| Oven Program Final Temp. | 350 °C |
| Hold Time 2 | 15 min |
| Equilibration Time | 0.5 min |
| H2 Flow | 40 mL min−1 |
Results and discussion
Effect of nutrient starvation/limitation to algal growth, biomass and lipid content
Apart from carbon, Spirulina requires the usual major biological nutrients: nitrogen, phosphorus, potassium, sulfur, magnesium, calcium, iron, plus a number of micronutrients. In many cases, the micronutrients and calcium need not to be fed in the culture, being supplied as natural impurities contained in the make-up water and the chemicals used as nutrients for the algae.18 Algae physiology can be manipulated in order to achieve an optimum accumulation of lipids as well as to investigate its effect on algal growth. Algal metabolism can be directed to the accumulation of lipids in spite of cellular duplication and protein synthesis by imposing nutrient limiting conditions. Various combinations of starvation have been designed to turn the metabolism into an anabolic lipid accumulating phase. Nitrogen and phosphorus starvation have both been proven to increase lipid accumulation.22 While nitrogen limitation has been shown to improve lipid production,22,23 phosphorus changing conditions have been investigated to change the lipid composition.22,24 The presence of phosphorus in the lipids used for biodiesel production gives a high concentration in the final biofuel which is deleterious and completely undesirable for the quality of biodiesel.22An optical density monitoring was performed from each culture through UV-Vis at 680 nm to assess cell growth (Fig. 1 and ESI†). The optical density of spirulina was not significantly affected at different culture media having different nutrient limiting conditions (p > 0.05). However results from Fig. 1 pointed out that phosphorus is probably the most important nutrient for algal growth as confirmed by further investigations on lipid content. In fact, phosphates are widely accepted as the main nutrient controlling the development of natural populations of cyanobacteria in many freshwater environments.25 Low levels of P could limit the growth of many algal species. Inorganic phosphorus has been reported to help in nitrogen fixation,26 with nitrogenase activity gradually increasing and reaching a plateau to maintain the enhanced activity for a longer period. On the other hand, the organic phosphorus (which is readily available for the culture media either deprived or P limited), also helped in the normal growth and metabolism right from the beginning, thus resulting in a normal nitrogenase activity.
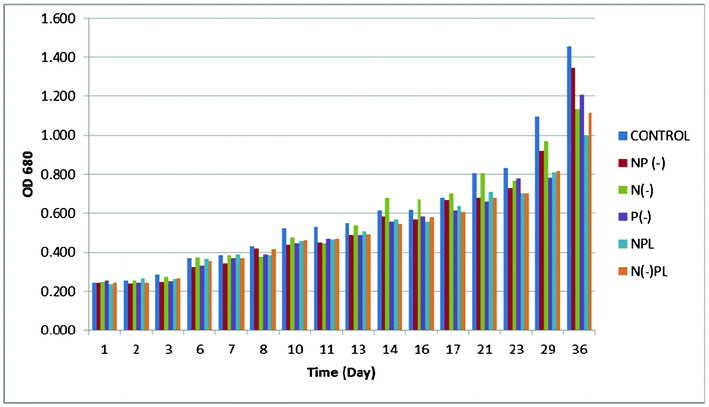 | ||
| Fig. 1 Optical density of A. platensis at different culture media. | ||
Biomass of each culture (g of dry weight per liter) were collected after 36 days of culture followed by lipids extraction (Fig. 2 and 3, ESI†). The harvested dry biomass was very similar for all cultures (and only slightly lower to that of complete nutrition conditions, Fig. 2). Interestingly, the lipid contents obtained for each culture were remarkably different (Fig. 3) and, most importantly, strongly dependent on phosphorous conditions, in good agreement with previous observations.24
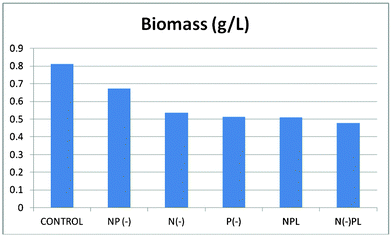 | ||
| Fig. 2 Dry weight/biomass of Spirulina at different culture media. | ||
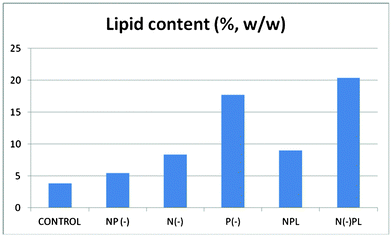 | ||
| Fig. 3 Lipid Content of Spirulina at different culture media. | ||
In fact, the lipid content of the algal cells should not be correlated to the generated biomass, but to the nutrient conditions. The phosphorous limited culture exhibited by far the largest lipid content (ca. 20 wt%) associated to the lowest generated biomass, only followed by the phosphorous deprived culture (> 15 wt%). Interestingly, the lowest yield of lipids was observed for the complete nutrition cultured microalgae which obviously possessed the largest biomass generation (CONTROL). These findings confirm that phosphorus limitation or starvation can significantly contribute to the accumulation of lipids in Arthrospira platensis algal cells as compared to no appreciable effects found for nitrogen in terms of lipids generation.
Cyanobacteria are considered to be an efficient sink for P, thus continuously causing P-transformations. P limitation is also known to cause a dramatic decrease in photosynthetic oxygen evolution, ATPase, nitrogenase and other enzymes activity. In fact, phosphorous is as an essential element for all living cells, component of DNA, RNA, ATP as well as membrane phospholipids that organisms tend to accumulate and concentrate. Phosphorous absence or limitation consequently forces these organisms (e.g. microalgae) to accumulate massive amounts of carbon in the form of carotenoids and lipids in order to survive.27,28
Comparably, nitrogen is more important for protein production. It plays a pivotal role in many critical functions (e.g. photosynthesis) and is a major component of amino acids.29 Algal cells under nitrogen starvation/limitation did not have larger lipid contents as compared to those under phosphorus-starved/limited conditions. Cyanobacteria including Arthrospira platensis are capable of supplying the lacking nitrogen through nitrogen fixation and thus, are still able to reproduce without changing its composition.
Upon microalgal oil extraction, CONTROL and NP(-) extracted oils were selected to be esterified to biodiesel despite their lowest lipid yield as compared to the rest of the cultures. The reason for this was the interest in the evaluation of the effect of both Nitrogen and Phosphorus starvation on FAME profile, content and composition in the final biodiesel sample. Interestingly, biodiesel yields for both complete nutrition and nitrogen/phosphorous deprived cultures were relatively similar (ca. 40–42%), in good agreement with the similarly extracted microalgal oil for both cultures (3.8 vs. 5.5 wt% microalgal oil, Fig. 3). The FAME profiles are shown in Table 3 (see also ESI). As clearly seen from results, some interesting differences were observed in terms of FAME distribution from complete nutrition vs. N/P deprived cultures.
| FAME Composition (wt.%) | CONTROL | NP(-) |
|---|---|---|
| C10:0 (methyl decanoate) | 1.0 | 1.0 |
| C14:0 (methyl myristate) | 9.2 | — |
| C16:0 (methyl palmitate) | 46.6 | 34.3 |
| C18:2 (methyl linoleate) | 11.9 | 14.4 |
| C18:1 (methyl oleate) | — | 1.0 |
| C18:0 (methyl stearate) | — | 0.5 |
| C20:0 (methyl arachidate) | — | 0.5 |
| FAME Content in biodiesel (%) | 68.7 | 54.7 |
| Monoglycerides (MAG) Total (%) | 26.7 | 21.0 |
| Diglycerides (DAG) Total (%) | — | 3.6 |
| Triglycerides (TAG) Total (%) | — | — |
| Others Total (%) | <5 | <20 |
Major FAMEs contained in the CONTROL-converted biodiesel were C10:0 (methyl decanoate), C14:0 (methyl myristate), C16:0 (methyl palmitate), C18:2 (methyl linoleate). These FAMEs were also present in the NP(-) sample, with the absence of C14:0. Importantly, C18:1 (methyl oleate), C18:0 (methyl stearate) and C20:0 (methyl arachidate) were also found, even at small quantities, in the NP(-) medium. The obtained FAME compositions were comparable to those of biodiesel-derived from conventional feedstocks, particularly related to C16:0 and C18:2 content.
Traces of monoacylglycerides (MAG) and some diacylglycerides (DAG) as intermediates from the transesterification reaction as well as other contaminants including traces of glycerol, FFA and other co-extracted compounds were also detected in both biodiesel samples, especially for the NP(-) sample (Table 3). This may indicate that some of these detected impurities could have been released from the microalgae species under the stress caused by nutrient deprivation/limitation. Unexpectedly, no polyunsaturated fatty acids (PUFA) typically found in microalgal species [e.g. ω-3 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (EPA) and 22
5 (EPA) and 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (DHA)] were detected in appreciable quantities in any of the biodiesel samples. These findings indicate these are not accumulated by the algae under the investigated conditions. The presence of these PUFA is highly undesirable in biodiesel due to their detrimental properties and negative impact of the polyunsaturations on oxidation stability.17,30,31
6 (DHA)] were detected in appreciable quantities in any of the biodiesel samples. These findings indicate these are not accumulated by the algae under the investigated conditions. The presence of these PUFA is highly undesirable in biodiesel due to their detrimental properties and negative impact of the polyunsaturations on oxidation stability.17,30,31
These remarkable findings point to the fact that microalgae grown under P deprived/limited conditions (which are able to accumulate up to 20 wt% lipid content into their cells) can have a highly compatible FAME profile for biodiesel production. An almost negligible production of undesirable polyunsaturated fatty acids (PUFA) were obtained in the final biofuel.
Conclusions
Arthrospira platensis (spirulina) was investigated as renewable feedstock for the production of microalgal oil in an attempt to achieve a more sustainable biodiesel preparation. Several conditions including N and P deprivation and limitation in spirulina cultivation were screened to evaluate biomass growth and lipid accumulation. P was found to be the critical element to control to maximise the lipid content in spirulina. In this way, a maximum of 20 wt% microalgal oil could be obtained upon extraction of the algal cells cultivated under nitrogen deprivation and phosphorous limitation (N(-)PL). Interestingly, biomass growth followed a completely opposite trend, with N(-)PL showing the lowest generated biomass as compared to the maximum biomass production achieved in complete nutrition (CONTROL) cultures.Extracted CONTROL and NP(-) spirulina oils were subsequently converted into biodiesel which showed similar biodiesel yields (ca. 40–42%) could be obtained by conventional acid catalysis. A similar FAME profile was also found for both biodiesel samples, comprising C16:0, C18:2 as major compounds, which is almost comparable to that of conventional biodiesel from oil crops. PUFA content in the microalgal oils was negligible under the investigated conditions, which confirms the potential of biodiesel production from spirulina. Further studies are currently ongoing in our groups to investigate the economic and environmental feasibility of biodiesel production from the optimised cultures (N(-)PL, containing ca. 20 wt.% algal oil) using a one-pot two step direct extraction/transesterification process that will be reported in due course.
Acknowledgements
Kate E. Baunillo thanks the Chemistry Department of Xavier University, Ateneo de Cagayan. Rafael Luque gratefully acknowledges support from the Spanish MICINN via the concession of a Ramon y Cajal contract (ref. RYC-2009-04199) and funding under projects P10-FQM-6711 (Consejeria de Ciencia e Innovacion, Junta de Andalucia) and CTQ2011 28954-C02-02 (MICINN).References
- G. Antolin, Y. Tinaut, V. Castano, C. Perez and A. I. Ramirez, Bioresour. Technol., 2002, 83, 111–114 CrossRef CAS.
- X. Lang, A. Dalai, N. Bakhshi, M. Reaney and P. Hertz, Bioresour. Technol., 2001, 80, 53–62 CrossRef CAS.
- A. B. M. H. Sharif, A. B. Nasrulhaq, H. A. M. Majid, S. Chandran and R. Zuliana, Asia Biofuel Conference Book, 2007, Singapore Search PubMed.
- E. G. Shay, Biomass Bioenergy, 1993, 4, 227–242 CrossRef CAS.
- K. Vijayaraghavan and K. Hemanathan, Energy Fuels, 2009, 23, 5448–5453 CrossRef CAS.
- Y. Chisti, Biodiesel from microalgae. Biotechnol. Adv., 2007, 25, 294–306 CAS.
- T. M. Mata, A. A. Martins and N. S. Caetano, Renewable Sustainable Energy Rev., 2010, 14, 217–232 CrossRef CAS.
- E. Ibañez, M. Herrero, J. A. Mendiola and M. Castro-Puyana, Marine Bioactive Compounds: Sources, Characterization and Applications, Ed. M. Hayes, Springer Science, The Netherlands, 2012, 55-98 Search PubMed.
- A. L. Mascarelli, Environ. Sci. Technol., 2009, 43, 7160–7161 CrossRef CAS.
- L. Reijnders, Energies, 2009, 2, 48–56 CrossRef CAS.
- J. Falquet, Antenna Technologies, http://antenna.ch/en/documents/AspectNut_UK.pd (accessed: August 2012) Search PubMed.
- L. Uslu, O. Isik, K. Koc and T. Goksan, African J. Biotechnol., 2010, 10, 386–389 Search PubMed.
- Compositional guideline, Arthrospira platensis. Department of Health and Ageing Threapeutic Goods Administration, http://www.tga.gov.au/docs/html/ compguid/ platensis.htm Search PubMed.
- P. Chung, W. G. Pond, J. M. Kingsbury, E. F. Walker and L. Krook, J. Anim. Sci., 2006, 319–330 Search PubMed.
- G. Andrich, A. Zinnai, U. Nesti, F. Venturi and R. Florentini, Acta Aliment., 2006, 35, 195–203 CrossRef CAS.
- R. Chaiklahana, N. Chirasuwan, V. Lohab and B. Bunnag, ScienceAsia, 2008, 34, 299–305 Search PubMed.
- R. Luque, L. Herrero-Davila, J. M. Campelo, J. H. Clark, J. M. Hidalgo, D. Luna, J. M. Marinas and A. A. Romero, Energy Environ. Sci., 2008, 1, 542–564 CAS.
- http://www.antenna.ch/en/documents/Jourdan_UK.pdf .
- A. Widjaja, C. Chien and Y. Ju, J. Taiwan Inst. Chem. Eng., 2009, 40, 13–20 CrossRef CAS.
- F. Shahidi, Food Analytical Chemistry., 2001, http://www.nshtvn.org/ebook/molbio/Current%20Protocols/CPFAC/fad0101.pdf Search PubMed.
- M. B. Johnson and Z. Wen, Energy Fuels, 2009, 23, 5179–5183 CrossRef CAS.
- M. Scarsella, G. Belotti, P. De Filippis and M. Bravi, Chem. Eng. Trans., 2010, 20, 85–90 Search PubMed.
- A. M. Illman, A. H. Scragg and S. W. Shales, Enzyme Microb. Technol., 2000, 27, 631–635 CrossRef CAS.
- I. K. Goldberg and Z. Cohen, Phytochemistry, 2006, 67, 696–701 CrossRef.
- J. E. G. Raymont, Plankton and productivity in the oceans, Second Edition, Vol. 1Phytoplankton, Pergamon Press, Oxford-New York, 1980 Search PubMed.
- United States Environmental Protection Agency. Phosphorus. Water Monitoring and Assessment. http://water.epa.gov/type/rsl/monitoring/vms56.cfm.
- M. S. Yandigeri, A. K. Yadav, K. K. Meena and S. Pabbi, Antonie van Leeuwenhoek, 2010, 97, 297–306 CrossRef CAS.
- M. N. Merzlyak, O. B. Chivkunova, O. A. Gorelova, I. V. Reshetnikova and A. E. Solovchenko, J. Phycol., 2007, 43, 833–843 CrossRef CAS.
- H. S. Misra and R. Tuli, Microbiology, 1994, 140, 971–976 CrossRef CAS.
- J. D. McCurry, EN-15779 – Gas chromatographic analysis of polyunsaturated FAME in biodiesel made from algae and marine oils, http://www.chem.agilent.com/Library/applications/5990-8875EN.pdf Search PubMed.
- R. Luque, Energy Environ. Sci., 2010, 3, 254–257 CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra21796a |
| This journal is © The Royal Society of Chemistry 2012 |
