Energy production, use and saving in a bioelectrochemical desalination system†
Bo
Zhang
and
Zhen
He
*
Department of Civil Engineering and Mechanics, University of Wisconsin-Milwaukee, Milwaukee, Wisconsin 53211. E-mail: zhenhe@uwm.edu (Z. He); Fax: (414) 229-6958; Tel: (414) 229-5846
First published on 17th September 2012
Abstract
This study experimentally and quantitatively investigated the energy benefit of a bioelectrochemical desalination system consisting of microbial desalination cells as pre-desalination units before electrodialysis (ED). The pre-desalination in an upflow microbial desalination cell (UMDC) could reduce ED energy consumption and desalination time by 45.3% and 25.0%, respectively. Both serial and parallel connections of three UMDCs achieved comparable performance in desalination and energy production. Direct charging in a serial connection transferred 86.6% of the energy from the UMDC system to a rechargeable battery, and 41.8% of the energy to an ultracapacitor, suggesting that ultracapacitors that are designed for quick charging may not be suitable for energy extraction from bioelectrochemical systems. About half of the stored energy in the rechargeable battery or ultracapacitor was lost during discharging to power the ED unit. The parallel connection aided by a DC-DC converter did not successfully charge either the rechargeable battery or the ultracapacitor.
1. Introduction
Brackish water has a salt concentration between that of freshwater and seawater. Desalinating brackish water offers an opportunity to significantly increase the freshwater supply for drinking and other purposes;1 however, the intensive consumption of energy by current desalination technologies is a major drawback, resulting in high operating costs and water prices.2 The use of renewable energy to drive desalination helps to build a sustainable desalination approach in terms of energy resources and environmental effects, but the high costs associated with renewable energy remains a significant challenge.3 As a result, the shortage of freshwater resources and high cost of existing desalination processes have created a demand for new desalination technologies with both environmental and economic benefits.Recent advances in bioelectrochemical systems introduced the concept of using the microbial desalination cell (MDC) as an alternative desalination method,4–6 which has received great attention.7,8 Derived from microbial fuel cells (MFCs), MDCs use electric potential generated from the microbial metabolism of organic compounds to drive desalination, similar to electrodialysis (ED). The advantages of an MDC include less external energy for the desalination process and simultaneous wastewater treatment, and researchers have further developed the MDC concept in several ways. For example, a ferricyanide cathode was replaced by an air cathode with a reduced amount of anolyte required for desalination.9 Continuously-operated MDCs were developed with upflow configuration at different scales and salt removal could be achieved from either NaCl solution or seawater.10,11 Desalination efficiency improved with multiple membrane pairs, mimicking ED.12,13 When oxygen is removed from the cathode and an external potential is applied to MDCs, hydrogen gas can be produced during desalination.14,15
Although MDCs are promising as a low-energy desalination method, their limitations must be understood to find a suitable application niche. It was believed that the primary function of MDCs is wastewater treatment,11 and desalination is a “bonus” effect that takes advantage of bioenergy production during the treatment process. Research demonstrates that MDCs can generally remove salts well at the expense of a lengthy retention time of several days,11 which will require a large reactor volume as compensation, thereby increasing capital investment. The low desalination efficiency (in terms of retention time) has two implications for MDC application: first, MDCs may be more appropriate as pre-desalination units in connection with conventional desalination processes downstream. Partial reduction of salinity could result in significant energy savings in downstream desalination, and this concept has been proposed9 and theoretically analyzed,11 but there has not been experimental verification. Second, MDCs may be more suitable for desalinating brackish water instead of seawater. It is known that the salt concentration of feeding water at the economic limit of standard ED applications is below 10 g TDS L−1,16 within the range of brackish water. A lower salinity in feeding water will lead to a shorter period of desalination time in MDCs, compensating for slow salt removal.
Another issue regarding MDC operation raised by our previous studies is the production of electrical energy vs. electrical current.10 MDCs can produce electricity like that in MFCs. Contradictory results are obtained when evaluating electricity generation and desalination: high-current generation can remove more salt, but little power is harvested; high-power production can produce more electric energy that may offset energy consumption by downstream desalination, but desalination efficiency is low in MDCs. Although our previous study concluded that high-power operation could be beneficial because of energy production, the analysis was based on the assumption that 100% of energy produced in MDCs could be used by the downstream desalination process, and actual operation will expect a large loss of energy during transfer, storage, and use.11 Therefore, it is necessary to examine this phenomenon experimentally.
In this study, a bioelectrochemical desalination system was investigated for energy production, use and saving. The system consisted of the previously developed upflow MDCs (UMDCs) coupled to a lab-scale ED unit. The ED unit acted as an example of a downstream desalination unit that could be other processes like reverse osmosis. Experiments were conducted to perform three tasks: 1) investigate the energy benefits of using an UMDC as a pre-desalination unit before an ED unit; 2) examine energy production and desalination performance of the UMDCs operation under high-current, high-power, and charging conditions; and 3) study charging and discharging using rechargeable batteries and ultracapacitors to harvest energy from the UMDCs.
2. Materials and methods
2.1 UMDC setup and operation
The previously developed UMDCs were used in this study. Briefly, the UMDCs are tubular reactors made of ion exchange membranes. Carbon brush and carbon cloth (with Pt as catalysts) were used as the anode and the cathode electrodes, respectively. The liquid volume is 1.9 L for the anode compartment and 0.85 L for the salt compartment. More details of UMDC construction and schematics can be found in the supplementary data or our previous publication.11 A single UMDC or three UMDCs were employed in this study for different purposes. When three UMDCs were operated, they were electrically connected either in series or in parallel.The UMDCs were operated under a room temperature of ∼20 °C. The anolyte contained (per L of tap water): sodium acetate, 2 g; NH4Cl, 0.15 g; NaCl, 0.5 g; MgSO4, 0.015 g; CaCl2, 0.02 g; KH2PO4, 0.53 g; K2HPO4, 1.07 g; yeast extract, 0.1 g; and trace elements, 1 mL.17 The anolyte was fed at 4 mL min−1, resulting in a hydraulic retention time (HRT) of ∼8 h, and was recirculated at 150 mL min−1. The initial anode inocula were a mixture of aerobic and anaerobic sludge from South Shore Wastewater Treatment Plant (Milwaukee, WI). Artificial brackish water was prepared by dissolving NaCl in tap water (6 g L−1). For single-UMDC operation, brackish water was continuously fed into the salt compartment of the UMDC in an upflow mode and the flowrate was adjusted to obtain the desired HRTs. For three-UMDC system operation, brackish water was fed as sequential batches in which brackish water was completely replaced after 18 h desalination. Continuous feeding of brackish water was also examined with the three-UMDC system. The catholyte was the acidified water (adjusted with sulfuric acid) at a pH of 2.5 and was used to rinse the cathode electrode from the top to the bottom at a flowrate of 4 mL min−1. The use of the acidified water benefited electricity generation11 and had a much lower cost (3%) compared to the phosphate buffer solution that is commonly used in bioelectrochemical studies. The “HRT” in the results and discussion refers to the retention time of brackish water, unless stated elsewhere.
2.2 Electrodialysis operation
A commercially available lab-scale electrodialysis (ED) unit (64002, PCCell GmbH, Heusweiler, Germany) was operated at room temperature as an example of a downstream desalination unit in this study. The ED contains 10 cell pairs, each of which is assembled with standard ion exchange membranes (PC-SK and PC-SA) and spacer. The active surface area of each membrane is 64 cm2. The anode electrode is Pt/Ir-MMO-coated Ti-stretched metal, and the cathode electrode is stainless steel. Electrolytes for both the anode and the cathode are Na2SO4 (100 mM), and were recalculated at a rate of 100 mL min−1. The effluent from the UMDC operation, as the feeding water to the ED, was equally divided into two parts, which were then pumped into the ED as concentrated and diluted solutions (recirculated at 100 mL min−1), respectively; therefore, the water recovery rate was 50%. A power supply (3465A, Circuit Specialists Inc., Mesa, AZ, USA) provided voltage for the ED operation (5 V for the effluent from the single-UMDC operation and 10 V for the effluent from the three-UMDC system), unless a rechargeable battery or an ultracapacitor was used.2.3 Charging and discharging
Two approaches were adopted for extracting electric energy from the UMDCs. The first approach was direct charging. When the three UMDCs were connected in series, the rechargeable battery or ultracapacitor was directly connected to the UMDC circuit for direct charging to a voltage of 1.2 V (rechargeable battery) or 2.7 V (ultracapacitor) (Fig. 1A). When the three UMDCs were connected in parallel, a DC-DC converter was applied to the electric circuit to increase the cell voltage to 3.3 V for charging (Fig. 1B). The DC-DC converter (TPS61200, Texas Instruments, Dallas, TX, USA) was successfully applied in our previous study of sediment MFC18 and proved effective in voltage elevation. The ultracapacitor (Maxwell Technology, Inc., San Diego, CA, USA) has a capacitance of 350 F and voltage rating of 2.7 V. The energy stored in an ultracapacitor was calculated according to the equation in the supplementary data or our previous publication.18 The rechargeable battery has a capacity of 1000 mAh (DC2400 NiMH rechargeable AAA battery, Duracell, Bethel, CT, USA). Before charging, the rechargeable battery or ultracapacitor was discharged through the ED unit until the condition that electrical current generation in the ED unit became zero, indicating that the rechargeable battery or ultracapacitor did not have enough energy to activate the ED process. The discharging of a rechargeable battery or an ultracapacitor to the ED was conducted with the aid of the DC-DC converter (Fig. 1C).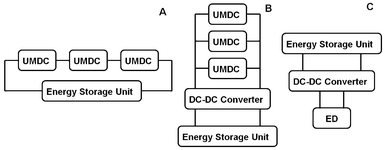 | ||
| Fig. 1 Connections of electric circuits during charging and discharging: (A) direct charging with serial connection of the UMDCs; (B) indirect charging with parallel connection of the UMDCs; and (C) discharging with the aid of a DC-DC converter to power the ED unit. | ||
2.4 Measurement and analysis
The cell voltage was recorded every 60 s by a digital multimeter (2700, Keithley Instruments, Inc., Cleveland, OH, USA). The pH was measured using a benchtop pH meter (Oakton Instruments, Vernon Hills, IL, USA). The electrical conductivity was measured by a benchtop conductivity meter (Mettler-Toledo, Columbus, OH, USA). The polarization curve was performed by a potentiostat (Reference 600, Gamry Instruments, Warminster, PA, USA) at a scan rate of 0.2 mV s−1. Energy consumption by the ED unit was calculated by integrating power consumption with time. The charging efficiency was defined as the ratio between energy delivered into the energy-storage units and theoretic energy produced in the UMDC system (computed from high-power operation). The discharging efficiency was the ratio between the energy released to the ED and energy charged into the energy-storage units. The overall efficiency, or energy recovery efficiency, was the ratio between energy released to the ED unit and energy produced in the UMDC system. When a rechargeable battery or an ultracapacitor was used to power the ED unit, the (additional) ED energy requirement was calculated by the difference between the energy release from those energy-storage units to the ED unit when desalinating one litre of the UMDC effluent and the energy consumption of the ED unit (operated by a power supply) when desalinating one litre of the same effluent.3. Results and discussion
3.1 Single UMDC as pre-desalination before ED
A single UMDC was operated and its saline effluent was transferred to an ED unit for further desalination. The results clearly demonstrated the benefit of using MDCs as pre-desalination units (Fig. 2). At an HRT of 13.75 h and an external resistance of 0.1 Ω, the UMDC reduced the electrical conductivity of brackish water from 10.89 to 6.28 mS cm−1. When this water was further desalinated by the ED at an applied voltage of 5 V, it consumed 0.88 ± 0.01 kWh m−3 and took 90 min to decrease the electrical conductivity to 1.08 mS cm−1. For comparison, the same amount of original brackish water without the UMDC pre-desalination required 1.60 ± 0.01 kWh m−3 and 120 min to reach 1.19 mS cm−1 in the ED—a saving of 45.3% in energy consumption and 25.0% in desalination time.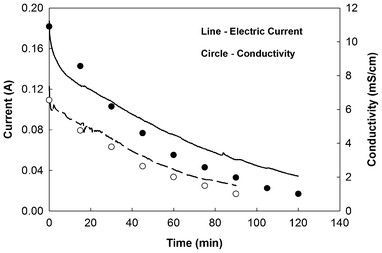 | ||
| Fig. 2 Comparison of electrical current generation and solution conductivity in the ED between desalinating the original brackish water (solid line and dark circle) and the UMDC effluent (dashed line and white circle). | ||
The performance of the UMDC directly affected the energy consumption by the ED unit. The UMDC was operated at three different HRTs, 18, 13.75, and 6 h (at an external resistance of 0.1 Ω), and two external resistances, 0.1 and 10 Ω (at an HRT of 13.75 h) (Fig. 3). The HRT of 18 h resulted in the lowest electrical conductivity of 4.52 ± 0.40 mS cm−1 in the brackish water, and a shorter HRT of 6 h doubled the effluent electrical conductivity to 9.35 ± 0.08 mS cm−1. Accordingly, the energy consumption by ED to desalinate the UMDC effluent increased from 0.59 ± 0.04 kWh m−3 for the effluent at the HRT of 18 h to 1.39 ± 0.15 kWh m−3 for the effluent at 6 h. Compared with the energy consumed by the ED treating brackish water without UMDC pre-desalination, the energy savings varied from more than 63.2 ± 2.2% for the condition of HRT 18 h to 13.5 ± 9.6% for the lowest HRT. Increasing the external resistance from 0.1 to 10 Ω at the HRT of 13.5 h also increased the effluent electrical conductivity to 9.20 ± 0.28 mS cm−1; consequently, the energy consumption by ED treatment of this effluent increased to 1.29 ± 0.03 kWh m−3 and energy savings decreased to 19.4 ± 1.8%. It is worth noting that altering HRTs did not obviously change electrical current generation and electrical current of the UMDC at 0.1 Ω varied between 90 and 100 mA under three HRTs; however, increasing the external resistance to 10 Ω significantly decreased the current generation to ∼38 mA.
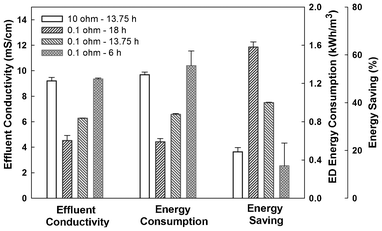 | ||
| Fig. 3 Effects of HRTs and external resistance on the UMDC performance and energy consumption in ED. | ||
Those results have demonstrated experimentally and quantitatively the energy benefits of a bioelectrochemical desalination system using MDCs as pre-desalination units before ED, although the conclusion is straightforward. The lower salinity of the UMDC effluent reduced both energy consumption and desalination time by ED. Electrical current, or electron flow, is the key factor to salt removal in MDCs. Although previous studies suggested that salinity reduction in MDCs was caused by multiple factors, including electrical current, diffusion, ion exchange, and water osmosis, electrical current generation is the most important among them.9,11 A higher electrical current improved salt removal, as indicated by the comparison of desalination results between 0.1 and 10 Ω at the same HRT; however, the effluent salinity is determined by both current generation and total salt input. A higher salt input tends to cause a higher effluent salinity, which explains why different effluent salinities were obtained at 0.1 Ω, although current generation was similar among three HRTs. It also explains why the desalinated waters at two different current generations (0.1 Ω/HRT 6 h and 10 Ω/HRT 13.75 h) had a similar effluent electrical conductivity, even though more salts were actually removed under the condition of 0.1 Ω/HRT 6 h.
3.2 Operating conditions of the three-UMDC system
The operating conditions, high-current vs. high-power, were investigated with three UMDCs electrically connected either in series or in parallel at an HRT of 18 h. The ohmic resistance of the UMDC system was determined as 30 Ω (serial connection) or 5 Ω (parallel connection) using polarization curves (Fig. S1†); thus, the UMDC system was operated at the ohmic resistance as the high-power condition. The high-current condition was achieved with an external resistance of 0.1 Ω. In addition, the UMDC system was operated under the mode of charging a rechargeable battery or an ultracapacitor. The saline effluents collected from those operations were further desalinated by an ED.When three UMDCs were connected in series, the open circuit potential (OCP) reached 3.25 V (Fig. S1†). High OCPs (>1 V) have been reported in our previous studies of both MFCs and MDCs.11,19 The maximum power output of the UMDC system was 72 mW and the short circuit current was 108 mA. During the 18 h test, the current generation with the high-current operation decreased from 94 to 66 mA because of the reduced salinity in the salt solution by desalination, while the high-power operation decreased the electrical current from 57 to 37 mA (Fig. 4). Likewise, current generation under the charging mode also exhibited a decreasing trend, although there was a difference between charging the rechargeable battery and the ultracapacitor. When charging the rechargeable battery, the current profile was similar to that of the high-power operation, but electrical current dramatically decreased to ∼3.6 mA when charging an ultracapacitor during the 18 h period. Total coulomb output depended on current generation. The high-current operation produced the highest coulomb output of 4611 C, and the ultracapacitor-charging operation yielded the lowest of 820 C. The total coulombs from the high-power and battery-charging operations were 2535 and 2150 C, respectively. The conductivities of the UMDC effluent under those four conditions are shown in Fig. 5. As expected, the lowest electrical conductivity was achieved from the high-current operation that produced the highest electrical current and coulombs among the four. When one litre of those effluents was further desalinated by the ED individually, the lowest energy consumptions (excluding the energy release from the rechargeable battery or ultracapacitor) were from the rechargeable battery-charging operation (1.03 ± 0.16 kWh m−3) and the high-current operation (1.06 ± 0.02 kWh m−3). When brackish water was fed continuously into the UMDC system, current generation was relatively stable at ∼100 mA with the high-current operation, resulting in a low-effluent conductivity of 5.73 ± 0.14 mS cm−1 that consumed 0.75 ± 0.18 kWh m−3 by the ED to desalinate one litre of this effluent.
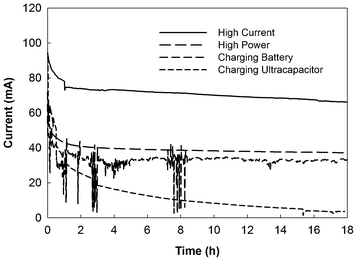 | ||
| Fig. 4 Electrical current generation of the UMDC system in serial connection under different conditions. | ||
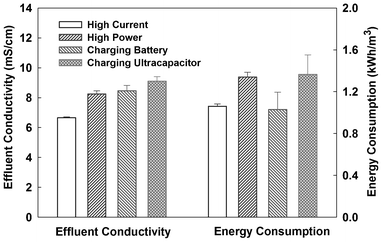 | ||
| Fig. 5 Conductivities of the effluents from the UMDC system in serial connection and the (additional) energy consumption by the ED to desalinate those effluents. | ||
The UMDC system in parallel connection produced an open circuit voltage of 1.14 V, a maximum power output of 62 mW, and a short circuit current of 230 mA (Fig. S1†). The effluent conductivities and the ED energy consumption under both high-current and high-power conditions were similar to those in the serial connection (Fig. S2†). The current generations under both conditions are shown in Fig. S3†. The pHs of the anode effluent and salt effluent under parallel connection were also similar to those under serial connection (Fig. S4†). Because charging with either the rechargeable battery or ultracapacitor was not successful, no comparison was made for ED energy consumption under the conditions of the energy harvest for parallel connection.
The low energy requirement by ED treating UMDC effluents under the charging-battery condition (serial connection) suggests that high-power operation could be beneficial because of the produced energy, confirming the finding in our previous publication, which theoretically analyzed the energy benefits of high-power operation with reverse-osmosis as a post-desalination process.11 This result could be attributed to the use of a NiMH rechargeable battery with an operating voltage of 1.2 V, very close to the UMDC voltage of 1.5 V where the maximum power output was achieved. A similar current generation between the charging-battery and high-power conditions (Fig. 4) also indicated that the rechargeable battery was charged under a condition similar to high-power production. Thus, charging this battery could extract most of the UMDC energy (86.6% in the present study). The results have revealed that bioenergy produced in MDCs could be potentially useful. High-current operation, on the other hand, does not require energy transfer and thus simplifies the system operation. Although the energy requirement with the high-current operation did not outperform that of the charging-battery condition in batch (brackish water) operation, continuous feeding of brackish water with the high-current operation led to a lower energy requirement by ED. If the discharging efficiency of the rechargeable battery can be improved beyond 52% of the present study (which seems likely20), high-power operation will be more advantageous. When the efficiency of energy-storage units is low, the simple system with high-current operation (no need for charging and discharging) should be considered because it can save capital investment in infrastructure and energy-storage units.
3.3 Charging and discharging
The charging and discharging of the electric energy produced in the UMDC system was examined with two different energy-storage units, a rechargeable battery, and an ultracapacitor. Under the serial connection, current generations of the UMDC system with both energy storage units during the charging process are shown in Fig. 4. The charging voltages behaved differently: the voltage of the rechargeable battery was constantly at 1.2 V, while the voltage of the ultracapacitor increased from 0.34 to 2.67 V in 18 h (Fig. 6A). As a result, the total energy charged into the rechargeable battery was 2582 J, and there was 1248 J delivered into the ultracapacitor by the same UMDC system during the same period of time. For comparison, the UMDC system could produce 2983 J with the high-power operation. Therefore, the rechargeable battery had extracted 86.6% of the theoretic energy produced in the UMDC system and the ultracapacitor had a charging efficiency of 41.8%. When the two energy-storage units were discharged at 3.3 V (via a DC-DC converter) to drive ED treating the effluents from the charging operation, the energy released was 1333 and 780 J from the rechargeable battery and the ultracapacitor, respectively. Thus, the discharging efficiency of the rechargeable battery was 51.6% and the ultracapacitor discharged 62.5% of its stored energy. The difference was also shown in the discharging time (Fig. 6B). The overall energy recovery efficiency was 44.6% for the rechargeable battery and 26.1% for the ultracapacitor.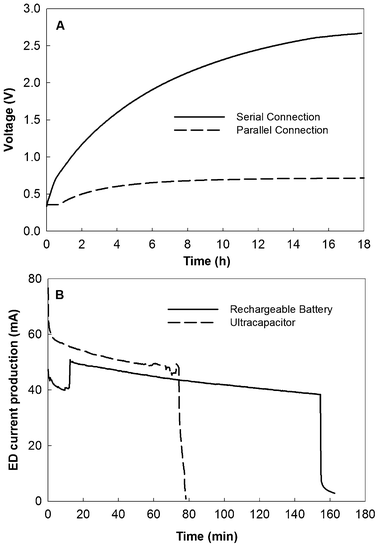 | ||
| Fig. 6 Voltage curves of an ultracapacitor (350 F, 2.7 V) charged by the UMDC system in serial and parallel connections (A) and electrical current productions in the ED powered by the rechargeable battery and the ultracapacitor with the serial UMDC operation (B). | ||
The parallel connection did not achieve a comparable charging to the serial connection. Because the voltage of the UMDC system under parallel connection was low, a DC-DC converter was linked to the system to boost the voltage for charging. After 18 h charging, the voltage of the ultracapacitor reached 0.72 V (Fig. 6A), representing an energy content of 91 J, significantly lower than 1248 J from the serial connection charging. The battery charging (two rechargeable batteries connected in series) was not successful. No discharging test was performed with either ultracapacitor or rechargeable batteries because of low energy extraction from the UMDC system under the parallel connection.
A critical element in high-power operations is the transfer, storage, and use of electric energy produced from MDCs, and several losses of energy are involved in this process. The actual charging condition has a current generation lower than high-power operations; therefore, energy is lost during the charging process. In this study, the rechargeable battery could extract more than 86% of the energy, but the ultracapacitor could only achieve 42%. Energy loss during storage can be minimized, assuming brief storage is needed (longer storage times will increase the loss of electric charge due to self-discharge). Another major loss occurs during discharging; although the rechargeable battery obtained the most of energy from the UMDC system, it lost nearly 50% during discharging through powering ED. An efficient electric storage unit and circuit may help to reduce this loss, and it is found that the discharging efficiency of some energy-storage units can be more than 60%.20
The charging/discharging issues are important to the high-power operation of MDCs and could also have some implications for MFCs, which are designed to produce electric power. The research on MFC charging has been limited to power delivery from sediment MFCs,21,22 but there is a significant difference in energy harvest between sediment MFCs and reactor-type MFCs for wastewater treatment. The goal of power production in sediment MFCs is to support remote sensors that require low power input. Energy loss is acceptable and not a critical issue during the process of storage and use, as long as it can accumulate enough energy within a designated period of time. Reactor-type MFCs, on the other hand, are expected to deliver as much power as possible, and energy loss should be minimized. In previous studies, power management systems (PMS) were usually employed to extract energy from MFC,18,22 but a significant energy loss occurred, for example, in which only 15% of the energy from an MFC could be used to power a hydrophone, possibly due to the low efficiency of the charge pump.23
The direct charging (in serial connection of the UMDCs) and use of a DC-DC converter to discharge in our study yielded the overall energy recovery efficiency between 26.1% and 44.6%, with an inferior performance from the ultracapacitor, which could be due to a large self-discharge during the charging process. Ultracapacitors are designed for quick charging (from seconds to minutes vs. hours for charging batteries). The low electricity production in the UMDC system resulted in a long charging-period in hours and thus could have increased energy loss in the ultracapacitor via self-discharge. Therefore, according to the results of the present study, rechargeable batteries may be more appropriate for extracting energy from reactor-type bioelectrochemical systems. The indirect charging with the aid of a DC-DC converter (in parallel connection) did not successfully charge either the ultracapacitor or rechargeable battery, indicating that the DC-DC converter may have consumed a large amount of electric energy.24 However, a power management system (including a DC-DC converter) may still be necessary for harvesting energy from large-scale bioelectrochemical systems, in which the energy loss to a power management system could be insignificant compared with energy production.
3.4 Perspective
Considering that MDCs do not require external energy to run desalination, except the energy for pumping at a normal pressure, the use of MDCs as pre-desalination units could save a considerable amount of energy; however, challenges remain in developing a well-functioning MDC-ED system. First, MDC performance should be further improved. The results clearly indicate that better-performing MDCs can save more energy because more salt is removed before ED. Second, the MDC-ED system requires a supply of wastewater; therefore its application is limited to the areas that have access to both wastewater and brackish water. The use of organic compounds, instead of wastewater, to feed the anode of MDCs could make the system more flexible, but its economic feasibility needs investigation. Third, it requires coordinating the operation between MDCs and ED; for example, in this study, the desalination process in the UMDC was much slower than that of ED (18 h vs. 1–3 h for treating the same amount of water), causing the ED unit to be on standby most of time. This problem may be solved by increasing the volume of MDCs or using MDCs to desalinate a portion of feeding water. Last, when evaluating MDCs, one should have wastewater treatment as a primary goal and desalination as a beneficial addition.4. Conclusion
The results demonstrated that using UMDCs as a pre-desalination process saved both energy and desalination time in a bioelectrochemical desalination system. Bioenergy was harvested from the UMDCs and applied to power the ED. The choice of energy storage units and collecting system clearly affected energy transfer and use; this aspect was not sufficiently explored here. The ones used in this study (350 F ultracapacitor and 1000 mAh rechargeable battery) were arbitrarily selected as examples to interpret energy issues in the desalination system. Future studies will expand energy devices to ultracapacitors with different capacitances and rechargeable batteries with different energy densities for an optimal choice.Acknowledgements
This study was financially supported by a research grant from Gannett Fleming, Inc., and a grant from Binational Agricultural Research and Development Fund (BARD- US-4455-11). The authors thank Michelle Schoenecker (UW-Milwaukee) for her assistance with manuscript proofreading.References
- J. A. Redondo, Desalination, 2001, 138, 29–40 CrossRef CAS.
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Marinas and A. M. Mayes, Nature, 2008, 452, 301–310 CrossRef CAS.
- C. Charcosset, Desalination, 2009, 245, 214–231 CrossRef CAS.
- X. Cao, X. Huang, P. Liang, K. Xiao, Y. Zhou, X. Zhang and B. E. Logan, Environ. Sci. Technol., 2009, 43, 7148–7152 CrossRef CAS.
- H. Luo, P. Xu, T. M. Roane, P. E. Jenkins and Z. Ren, Bioresour. Technol., 2012, 105, 60–66 CrossRef CAS.
- Y. Qu, Y. Feng, X. Wang, J. Liu, J. Lv, W. He and B. E. Logan, Bioresour. Technol., 2012, 106, 89–94 CrossRef CAS.
- J. L. Schnoor, Environ. Sci. Technol., 2010, 44, 2219–2219 CrossRef.
- F. Zhang, M. Chen, Y. Zhang and R. J. Zeng, J. Membr. Sci., 2012 DOI:10.1016/j.memsci.2012.06.009.
- M. Mehanna, T. Saito, Y. Jingling, M. A. Hickner, X. Cao, X. Huang and B. E. Logan, Energy Environ. Sci., 2010, 3, 1114–1120 CAS.
- K. S. Jacobson, D. Drew and Z. He, Bioresour. Technol., 2011, 102, 376–380 CrossRef CAS.
- K. S. Jacobson, D. Drew and Z. He, Environ. Sci. Technol., 2011, 45, 4652–4657 CrossRef CAS.
- X. Chen, X. Xia, P. Liang, X. Cao, H. Sun and X. Huang, Environ. Sci. Technol., 2011, 45, 2465–2470 CrossRef CAS.
- Y. Kim and B. E. Logan, Environ. Sci. Technol., 2011, 45, 5840–5845 CrossRef CAS.
- H. Luo, P. E. Jenkins and Z. Ren, Environ. Sci. Technol., 2011, 45, 340–344 CrossRef CAS.
- M. Mehanna, P. D. Kiely, D. F. Call and B. E. Logan, Environ. Sci. Technol., 2010, 44, 9578–9583 CrossRef CAS.
- T. Hayes and D. Arthur, in The 11th Annual International Petroleum Environmental Conference, Albuquerque, NM, 2004 Search PubMed.
- Z. He, N. Wagner, S. D. Minteer and L. T. Angenent, Environ. Sci. Technol., 2006, 40, 5212–5217 CrossRef CAS.
- F. Zhang, L. Tian and Z. He, J. Power Sources, 2011, 196, 9568–9573 CrossRef CAS.
- F. Zhang, K. S. Jacobson, P. Torres and Z. He, Energy Environ. Sci., 2010, 3, 1347–1352 CAS.
- S. Foster, C. Calwell, T. Reeder and R. Neugebauer, Battery chargers and energy efficiency: summary of findings and recommendations, Natural Resources Defence Council, 2003 Search PubMed.
- M. E. Nielsen, C. E. Reimers, H. K. White, S. Sharma and P. R. Girguis, Energy Environ. Sci., 2008, 1, 584–593 CAS.
- C. Donovan, A. Dewan, H. Peng, D. Heo and H. Beyenal, J. Power Sources, 2011, 196, 1171–1177 CrossRef CAS.
- A. Meehan, H. Gao and Z. Lewandowski, IEEE Trans. Power Electron., 2011, 26, 176–181 CrossRef.
- Y. Kim, M. C. Hatzell, A. J. Hutchinson and B. E. Logan, Energy Environ. Sci., 2011, 4, 4662–4667 CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Fig. S1–4 show polarization curves, data from the UMDC system in parallel connection, effluent pH, and ultracapacitor charging. See DOI: 10.1039/c2ra21779a |
| This journal is © The Royal Society of Chemistry 2012 |
