The structures and stability of BnNn clusters with octagon(s)†
Li-Hua
Gan
*,
Rui
Li
and
Jie
An
School of Chemistry and Chemical Engineering, Southwest University, 400715, China. E-mail: ganlh@swu.edu.cn
First published on 12th October 2012
Abstract
The structures and stability of (BN)n clusters with alternate B and N atoms containing squares, hexagons and octagons ((BN)n-F4F6F8) are investigated by using density functional theory. The results demonstrate that the isomers of (BN)n-F4F6F8 clusters generally satisfy the isolated-square rule (ISR) and the square adjacency penalty rule (SAPR). The energetically favorable isomers generally have fewer square–square bonds, larger HOMO–LUMO gaps, lower sphericity and asphericity, as well as lower pyramidalization of B and N atoms than other structures. As a whole, the stability of (BN)n-F4F6F8 clusters decreases with the number of octagons. However, four isomers containing one or two octagons in four isomeric clusters (i.e. (BN)n-F4F6F8 (n = 19, 20, 23, and 24) are more thermodynamically stable than their (BN)n-F4F6 counterparts. Further structural analysis demonstrates that octagon(s) of (BN)n-F4F6F8 clusters can release the strain energy by decreasing the pyramidalization angles of the corresponding vertex. Finally, the entropy effect is examined to evaluate the relative stability of (BN)n-F4F6F8 (n = 19, 20, 23, and 24) clusters at high temperatures.
Introduction
Boron-nitrogen fullerene-like clusters (BN)n have been extensively investigated experimentally1–5 and theoretically6–12 after the discovery of C60 in 1985. As iso-electronic analogues to carbon fullerenes, (BN)n clusters with special properties, such as high-temperature stability, low-dielectric constant, large thermal conductivity and good oxidation resistance are intriguing for both scientific research and device applications. It has been reported that in (BN)n clusters the alternate structures constructed by squares and hexagons (for simplification, hereafter named (BN)n-F4F6) are more stable than the structures constructed by pentagons and hexagons.7–9 Moreover, (BN)n-F4F6 clusters satisfy the isolated-square rule (ISR).13 For ISR-violating isomers with square-square bonds (hereafter named B44 bonds), the isomers with fewer B44 bonds are usually more energetically favorable than those with more B44 bonds, and they satisfy the square adjacency penalty rule (SAPR).12 This is similar to C2n-F5F6 fullerenes obeying the powerful isolated pentagon rule14 and the pentagon adjacency penalty rule.15 Furthermore, it is reported that the most favorable isomers of (BN)n-F4F6 clusters usually have large HOMO–LUMO gaps.12,16,17However, recently researchers found that some (BN)n-F4F6 isomers with octagon(s) (hereafter named (BN)n-F4F6F8) are more favorable than the ones only constructed with squares and hexagons, such as (BN)13,12 (BN)20,10 and (BN)24.10,17,18 For (BN)13 clusters, it is reported that the isomer with one octagon in C1 symmetry is more thermodynamically stable than the (BN)13-F4F6 isomers. For (BN)20 clusters, the study shows that one isomer of (BN)20 with two octagons in C4h symmetry is more energetically stable than the (BN)20-F4F6 isomers. For (BN)24 clusters, many researchers found that the isomer with zero B44 bonds constructed by two octagons, eight squares and sixteen hexagons in S8 symmetry is more stable than the (BN)24-F4F6 isomers. Moreover, (BN)24 clusters have been synthesized and detected via laser desorption time-of-flight mass spectrometry.4
So far, systematic studies on (BN)n-F4F6F8 are lacking, and their structures and stabilization mechanism are not well understood. Are there more (BN)n-F4F6F8 structures which are more favorable than their (BN)n-F4F6 counterparts? Do (BN)n-F4F6F8 structures satisfy the ISR and SAPR? How does an octagon change the stability of the (BN)n clusters?
In this work, a systematic density functional theory (DFT) study is performed on all possible isomers of (BN)n-F4F6F8 (n = 15–24) clusters to gain insight into their structures and stability. The calculated results demonstrate that (BN)n-F4F6F8 clusters satisfy the ISR and the SAPR. It is found that four isomers with one or two octagons are more thermodynamically stable than their (BN)n-F4F6 counterparts. The influence of B44 bonds, sphericity (SP), asphericity (AS), pyramidalization of atoms (PA), HOMO–LUMO gaps and the enthalpy-entropy interplay on the stability of (BN)n-F4F6F8 clusters are investigated in detail.
Computational details
The coordinates of all considered (BN)n-F4F6F8 clusters are obtained by running the revised version of CaGe software.19 According to the Euler's theorem, (BN)n-F4F6F8 clusters satisfy the following equations:| n4 − n8 = 6 | (1) |
| n4 + n6 + n8 = n + 2 | (2) |
These isomers are first optimized using the PM3 method to produce the primary classifications of energetic and stability. Then the isomers selected from the PM3 optimization with relative energy (RE) within about 150 kcal mol−1 are refined at the HF/3-21G level, and then the B3LYP/6-31G* method is used to determine the lowest energy isomer. In order to test the reliability of B3LYP/6-31G* method, the calculations with BH and HLYP/6-31G*, B3LYP/6-311+G* and MP2/6-31G* methods are also carried out for the three most stable isomers of (BN)15-F4F6F8 clusters, and the results are listed in the ESI† (S2). It shows that the relative energies with different methods are nearly same. Harmonic vibration frequencies are also calculated to confirm that the optimized structures of the lowest energy isomers are minima on the potential energy surface. All the calculations are carried out with GAUSSIAN 03 software package.21
Results and discussion
A. Results
The complete results of all considered isomers calculated at the B3LYP/6-31G* level are given in the ESI† (S3). Frequency calculations at the same level demonstrate that the most stable isomers of (BN)n-F4F6F8 clusters are the minima on the potential energy surface. The optimized structures of the lowest energy isomers of considered clusters are presented in Fig. 1. | ||
| Fig. 1 The B3LYP/6-31G* optimized structures of the lowest energy isomers of (BN)n-F4F6F8 (n = 15–24) clusters | ||
As shown in S3†, for (BN)15-F4F6F8 clusters, the most stable isomer is (BN)15-0F8-01 with C3h symmetry which has been reported in our previous work,11 followed by (BN)15-1F8-13 in C1 symmetry, which is 55.46 kcal mol−1 higher in energy than the former. For (BN)16 clusters, the most stable isomer is (BN)16-0F8-02 in Td symmetry. The second one is (BN)16-0F8-01 in C1 symmetry, which is 17.10 kcal mol−1 higher in energy than the former. For (BN)17-F4F6F8 clusters, the most stable isomer is (BN)17-0F8-01 in Cs symmetry and the second one is (BN)17-1F8-026 with C1 symmetry, which is 28.88 kcal mol−1 higher in energy than the former; for (BN)18 clusters, the most stable isomer is (BN)18-0F8-02 in S6 symmetry. The second one is (BN)18-1F8-022 in C2 symmetry, and is 9.01 kcal mol−1 higher in energy than the former. For (BN)21-F4F6F8 clusters, the most stable isomer is (BN)21-0F8-01 with C3h symmetry which has been reported,11 followed by (BN)21-1F8-046 in C1 symmetry, which is 7.26 kcal mol−1 higher in energy than the former.
For (BN)19, (BN)20, (BN)23 and (BN)24 clusters, all of their most stable isomers have octagon(s). For (BN)19 polyhedrons, the most stable isomer is (BN)19-1F8-049 with an octagon, which is 15.55 kcal mol−1 lower in energy than the second one (BN)19-0F8-06 without octagon. For (BN)20 polyhedrons, the most stable isomer is (BN)20-2F8-343 with two octagons. The second one is (BN)20-2F8-160, and is 16.14 kcal mol−1 higher in energy than the former. The third one is (BN)20-0F8-06, and its relative energy (RE) is 18.60 kcal mol−1. For (BN)23 clusters, the most stable isomer is (BN)23-1F8-126 with an octagon. The second stable isomer is (BN)23-0F8-03 without an octagon, and it is 11.43 kcal mol−1 higher in energy than the former. The third one is (BN)23-1F8-067 with an octagon, which is 21.78 kcal mol−1 higher in energy than the most stable one. For (BN)24 polyhedrons, the most stable isomer is (BN)24-2F8-1581 with two octagons. The second one is (BN)24-0F8-01, which is 2.08 kcal mol−1 higher in energy than the former. The third one is (BN)24-2F8-3789, and its RE is 18.69 kcal mol−1. Evidently, the isomers containing octagon(s) are energetically competitive with the (BN)n-F4F6 ones.
In order to further investigate the structure of (BN)n-F4F6F8 clusters, we examine the geometrical parameters (bond lengths) of the lowest energy isomers and list them in Table 1. As seen in Table 1, it is evident that the average bond lengths of the lowest energy isomers are all shorter than the B–N (single) bond in H3B–NH3 (1.667 Å), but longer than the B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (double) bond in H2B
N (double) bond in H2B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH2 (1.391 Å) at the same level of theory. As a whole, the order of the average bond lengths for different kinds of bonds is B48 > B46 > B66 > B68.
NH2 (1.391 Å) at the same level of theory. As a whole, the order of the average bond lengths for different kinds of bonds is B48 > B46 > B66 > B68.
| Molecule | B46 | B48 | B66 | B68 |
|---|---|---|---|---|
| B15N15-0F8-01 | 1.478 | — | 1.456 | — |
| 1.469/1.4881 | —/— | 1.433/1.497 | —/— | |
| B16N16-0 F8-02 | 1.473 | — | 1.457 | — |
| 1.473/1.473 | —/— | 1.455/1.460 | —/— | |
| B17N17-0F8-01 | 1.473 | — | 1.462 | — |
| 1.457/1.486 | —/— | 1.396/1.510 | —/— | |
| B18N18-0F8-02 | 1.479 | — | 1.457 | — |
| 1.473/1.487 | —/— | 1.432/1.482 | —/— | |
| B19N19-1F8-049 | 1.476 | 1.482 | 1.458 | 1.424 |
| 1.457/1.499 | 1.481/1.485 | 1.417/1.495 | 1.420/1.434 | |
| B20N20-2F8-343 | 1.477 | 1.482 | 1.451 | 1.422 |
| 1.463/1.482 | 1.482/1.482 | 1.422/1.481 | 1.422/1.422 | |
| B21N21-0F8-01 | 1.478 | — | 1.458 | — |
| 1.474/1.487 | —/— | 1.433/1.476 | —/— | |
| B22N22-0F8-09 | 1.469 | — | 1.461 | — |
| 1.459/1.476 | —/— | 1.419/1.486 | —/— | |
| B23N23-1F8-126 | 1.473 | 1.48 | 1.459 | 1.42 |
| 1.452/1.490 | 1.477/1.484 | 1.418/1.490 | 1.419/1.421 | |
| B24N24-2F8-1581 | 1.474 | 1.479 | 1.458 | 1.419 |
| 1.457/1.483 | 1.479/1.479 | 1.438/1.480 | 1.419/1.419 | |
| Average | 1.475 | 1.481 | 1.458 | 1.421 |
B. Discussion
 | (3) |
 | ||
| Fig. 2 The calculated relative energies (RE) and the numbers of B44 bonds of the considered isomers with different number of octagons at the B3LYP/6-31G* level (“■” “•” “▴” denote the isomers containing none, one and two octagons, respectively). | ||
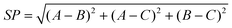 | (4) |
 | (5) |
As shown in S3†, for (BN)15-F4F6F8 clusters, the lowest energy isomer is (BN)15-0F8-01 with the lowest values of SP (0.11) and AS (0.30). This is also true for (BN)16-F4F6F8, (BN)20-F4F6F8, and (BN)22-F4F6F8, (BN)23-F4F6F8, and (BN)24-F4F6F8 clusters. For the other considered clusters, the values of SP and AS of the lowest energy isomers are among the lowest values. The general trend is that the lower the BE, the smaller values of SP and AS the isomers have. As a whole, the order of the values of SP and AS among clusters with different numbers of octagons is (BN)n-0F8 < (BN)n-1F8 < (BN)n-2F8. The introduction of one or two octagon(s) for the same-sized clusters will introduce tension squares, and may lead to more B44 bonds. Consequently, their energies increase. However, for (BN)19-F4F6F8 clusters, the lowest energy isomer is (BN)19-1F8-049 with the values of SP (0.07) and AS (0.39), followed by (BN)19-0F8-06 with the values of SP (0.08) and AS (0.65), but neither of them have a B44 bond. This phenomena also occurs in the three isomeric sets ((i.e. (BN)n-F4F6F8 (n = 20, 23, and 24)). In these cases, the number of B44 bonds for (BN)n-F4F6F8 isomers is not increased compared to the (BN)n-F4F6 isomers after the introduction of one or two octagon(s), and these octagon(s) reduce the local curvature of the cages. Therefore, they have lower values of SP and AS and are lower in energy than others. In summary, the shape of the (BN)n-F4F6F8 clusters plays an important role in determining the stability of (BN)n-F4F6F8 structures.
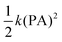 , where k is the force constant. It is reported that for carbon fullerenes, pentagon-pentagon fusions lead to enhanced strain of a cage surface, hence there should be as little curvature as possible so that the σ-skeleton achieves nearly the ideal sp2 geometry and the adjacent p-like orbitals overlap to the utmost extent.33 The PA can be used to measure the deviation of a sp2-hybridized carbon atom from the plane of three adjacent carbon atoms. It is established that a greater PA leads to lower stability of the carbon in sp2 hybridization, and for those carbon fullerenes containing pentagon-pentagon fusions (B55 bonds), the PA of atoms at the B55 bonds are larger than those of others. As iso-electronic analogue to carbon fullerenes, for (BN)n-F4F6 clusters, B atoms prefer the planar geometry (sp2 hybridization) and N atoms prefer the pyramidalization to accommodate a lone pair of electrons.34 We calculate the PAs of all considered isomers and the PA of each atom of all the optimized (BN)n-F4F6F8 isomers at the B3LYP/6-31G* level are obtained by the following eqn (6):32
, where k is the force constant. It is reported that for carbon fullerenes, pentagon-pentagon fusions lead to enhanced strain of a cage surface, hence there should be as little curvature as possible so that the σ-skeleton achieves nearly the ideal sp2 geometry and the adjacent p-like orbitals overlap to the utmost extent.33 The PA can be used to measure the deviation of a sp2-hybridized carbon atom from the plane of three adjacent carbon atoms. It is established that a greater PA leads to lower stability of the carbon in sp2 hybridization, and for those carbon fullerenes containing pentagon-pentagon fusions (B55 bonds), the PA of atoms at the B55 bonds are larger than those of others. As iso-electronic analogue to carbon fullerenes, for (BN)n-F4F6 clusters, B atoms prefer the planar geometry (sp2 hybridization) and N atoms prefer the pyramidalization to accommodate a lone pair of electrons.34 We calculate the PAs of all considered isomers and the PA of each atom of all the optimized (BN)n-F4F6F8 isomers at the B3LYP/6-31G* level are obtained by the following eqn (6):32| PA = θσπ − 90° | (6) |
The average PA of B (denoted as PAB) and N (PAN) atom for each isomer are obtained and also listed in S3†. As shown in S3†, the PAB and PAN of the lowest energy isomer are the lowest of all isomers. For (BN)15-F4F6F8 clusters, the PAB and PAN of the lowest energy isomer (BN)15-0F8-01 are 10.28° and 24.76°, respectively; the values of them for the second isomer (BN)15-1F8-13 are 9.87° and 24.07°, respectively. Evidently, the PAB and PAN of the former are greater than that of the latter, but the former has a larger gap, fewer B44 bond as well as lower SP and AS. This situation also occurs in (BN)21-F4F6F8 clusters. For the other considered clusters, the PAB and PAN of the lowest energy isomer are generally relatively smaller than those of the following ones.
As shown in S3†, the PAB is obviously lower than the PAN for each considered isomer. It demonstrates that the surface of (BN)n-F4F6F8 clusters is crinkly, as also seen from Fig. 1. This is evidently different from the cases in carbon fullerenes, in which the carbon atoms tend to form spherical surfaces and the difference between the PA of adjacent atoms is generally slight. The average PAB and PAN of all considered (BN)n-F4F6F8 (n = 15–24) isomers are 10.45°, 24.69°; 9.98°, 23.65°; 9.62°, 23.00°; 9.30°, 22.27°; 9.01°, 21.80°; 8.73°, 21.21°; 8.53°, 20.73°; 8.70°, 20.78°; 8.16°, 19.81°; 8.04°, 19.54°, respectively, the corresponding differences between PAB and PAN are 14.24°, 13.67°, 13.38°, 12.97°, 12.80°, 12.48°, 12.19°, 12.08°, 11.65°, and 11.50°, respectively. It indicates that the local strain energy decreases as n increases. This is in agreement with the results that (BN)n-F4F6F8 clusters are becoming more favorable than other considered clusters as n increases.
All the PAB and PAN of considered isomers are plotted in the ESI† (S4). As shown in S4†, for each kind of isomer with different numbers of octagons, the lowest energy one has a smaller PAB and PAN than other isomers, and their REs generally increase as the values of PAB and PAN become greater.
| Molecule | V446 | V448 | V466 | V468 | V666 | V668 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAB | PAN | PAB | PAN | PAB | PAN | PAB | PAN | PAB | PAN | PAB | PAN | |
| (BN)15-0F8-1 | — | — | — | — | 10.48 | 25.07 | — | — | 9.46 | 23.52 | — | — |
| 1F8-13 | 17.29 | 37.59 | — | — | 10.59 | 26.64 | 7.99 | 20.32 | 7.01 | 14.57 | — | — |
| 2F8-45 | 15.36 | 36.99 | 15.06 | 33.02 | 10.95 | 27.21 | 7.53 | 19.26 | — | — | 6.04 | 12.77 |
| (BN)16-0F8-02 | — | — | — | — | 10.23 | 24.59 | — | — | 7.70 | 16.50 | — | — |
| 1F8-20 | 15.57 | — | — | 33.73 | 10.49 | 26.27 | 8.08 | — | 7.36 | 12.61 | — | 11.65 |
| 2F8-59 | — | — | — | — | 10.42 | 26.88 | 7.25 | 19.27 | — | — | — | — |
| (BN)17-0F8-1 | — | — | — | — | 10.41 | 25.98 | — | — | 6.95 | 13.88 | — | — |
| 1F8-026 | 15.86 | — | — | 34.09 | 10.33 | 26.00 | 8.05 | 19.82 | 7.16 | 13.56 | — | 9.04 |
| 2F8-050 | 13.46 | 32.93 | 10.59 | 26.64 | 9.87 | 25.76 | 7.87 | 19.72 | 7.44 | 14.97 | 4.79 | 10.06 |
| (BN)18-0F8-02 | — | — | — | — | 10.30 | 26.39 | — | — | 6.63 | 14.03 | — | — |
| 1F8-022 | — | — | — | — | 10.07 | 25.71 | 7.52 | 19.76 | 6.79 | 13.65 | — | — |
| 2F8-085 | — | — | 12.74 | 32.31 | 9.82 | 25.48 | 7.48 | 18.78 | 7.89 | 15.83 | 5.55 | 8.82 |
| (BN)19-0F8-06 | — | — | — | — | 10.54 | 26.52 | — | — | 6.45 | 12.88 | — | — |
| 1F8-049 | — | — | — | — | 9.96 | 25.53 | 7.44 | 19.58 | 6.58 | 13.78 | — | — |
| 2F8-115 | — | — | 11.28 | 28.94 | 9.78 | 24.66 | 7.38 | 19.16 | 6.93 | 15.13 | 5.86 | 12.06 |
| (BN)20-0F8-06 | — | — | — | — | 10.44 | 26.41 | — | — | 6.38 | 12.83 | — | — |
| 1F8-029 | — | — | — | — | 10.17 | 25.71 | 7.10 | 17.66 | 6.73 | 14.21 | 5.16 | 9.47 |
| 2F8-343 | — | — | — | — | 9.21 | 23.72 | 7.32 | 19.44 | 6.97 | 15.02 | — | — |
| (BN)21-0F8-1 | — | — | — | — | 10.37 | 26.48 | — | — | 6.14 | 14.06 | — | — |
| 1F8-046 | — | — | — | — | 10.33 | 25.87 | 7.45 | 17.87 | 6.85 | 13.85 | 4.45 | 10.27 |
| 2F8-1235 | — | — | — | — | 9.75 | 24.49 | 7.60 | 17.78 | 6.82 | 16.93 | 5.26 | 10.22 |
| (BN)22-0F8-09 | — | — | — | — | 10.22 | 25.37 | — | — | 6.17 | 13.46 | — | — |
| 1F8-056 | — | — | — | — | 9.80 | 25.30 | 7.52 | 20.09 | 6.26 | 12.60 | — | — |
| 2F8-355 | — | — | — | — | 9.72 | 25.60 | 7.47 | 19.66 | 6.16 | 12.77 | 3.58 | 6.93 |
| (BN)23-0F8-03 | — | — | — | — | 10.31 | 26.20 | — | — | 5.93 | 12.70 | — | — |
| 1F8-126 | — | — | — | — | 9.69 | 25.21 | 7.44 | 19.88 | 6.03 | 12.65 | — | — |
| 2F8-2469 | — | — | — | — | 9.58 | 24.43 | 7.06 | 17.72 | 6.45 | 13.81 | 5.36 | 10.64 |
| (BN)24-0F8-01 | — | — | — | — | 10.19 | 25.92 | — | — | 5.90 | 12.40 | — | — |
| 1F8-096 | — | — | — | — | 10.12 | 25.71 | 7.03 | 18.65 | 6.01 | 12.17 | 3.73 | 10.00 |
| 2F8-1581 | — | — | — | — | 8.80 | 23.83 | 7.49 | 19.78 | 5.89 | 12.54 | — | — |
| Average | 15.51 | 35.84 | 12.42 | 31.46 | 10.10 | 25.63 | 7.51 | 19.17 | 6.75 | 14.18 | 4.99 | 10.16 |
These findings suggest that the isomers with octagon(s) should be considered during the search for the lowest energy isomer of (BN)n clusters. For the (BN)n-F4F6F8 clusters with n larger than 24, it is highly possible for isomers with octagon(s) to be more energetically favorable than the most stable (BN)n-F4F6 clusters.
 | (7) |
Fig. 3 shows the temperature evolution of the equilibrium concentrations of (BN)n-F4F6F8 (n = 19, 20, 23, and 24) clusters. Evidently, for three isomeric sets (i.e. (BN)n-F4F6F8 (n = 19, 23, and 24)), the lowest energy isomers are prevalent at certain temperatures while the other isomers have a very small proportion, indicating that the lowest energy isomers should be more thermodynamically stable than the other two isomers over a wide range of temperatures. However, in the case of (BN)20-F4F6F8, the second lowest energy isomer eventually surpasses the lowest energy one around 4600 K and becomes the most populated isomer. This situation also occurs in (BN)24-F4F6F8 clusters, in which the third lowest energy isomer exceeds the second lowest energy one at about 2700 K. These results demonstrate that the lowest energy structure is not necessarily the most abundant one at high temperatures.
 | ||
| Fig. 3 B3LYP/6-31G* relative concentrations of the three lowest energy isomers of (BN)n-F4F6F8 clusters (n = 19, 20, 23, and 24, respectively). | ||
4. Conclusions
DFT calculations demonstrate that the isomers of (BN)n-F4F6F8 clusters (n = 15–24) generally satisfy the ISR and the SAPR. The stability of (BN)n-F4F6F8 clusters usually decreases with the number of octagons. The most energetically stable isomers generally have fewer B44 bonds, larger gaps; lower SP and AS, as well as lower PAB and PAN. Four isomers containing octagon(s) in four isomeric clusters (i.e. (BN)n-F4F6F8 (n = 19, 20, 23, and 24)) are predicted to be more thermodynamically stable than their (BN)n-F4F6 counterparts. Further structural analysis demonstrates that introducing an octagon can release strain energy of the cage. It is thought that many more isomers with octagon(s) may exist which are more energetically favorable for (BN)n-F4F6 clusters as n increases.Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (XDJK2010C002), and the National Natural Science Foundation of China (51272216).References
- N. G. Chopra, R. J. Luyken, K. Cherrey, V. H. Crespi, M. L. Cohen, S. G. Louie and A. Zettl, Science, 1995, 269, 966 CAS.
- T. Oku, T. Hirano, M. Kuno, T. Kusunose, K. Niihare and K. Suganuma, Mater. Sci. Eng., B, 2000, 74, 206 CrossRef.
- T. Oku, M. Kuno, H. Kitahara and I. Nartia, Int. J. Inorg. Mater., 2001, 3, 597 CrossRef CAS.
- T. Oku, A. Nishiwaki, I. Narita and M. Gonda, Chem. Phys. Lett., 2003, 380, 620 CrossRef CAS.
- R. T. Paine and C. K. Narula, Chem. Rev., 1990, 90, 73 CrossRef CAS.
- D. L. Strout, J. Phys. Chem. A, 2001, 105, 261 CrossRef CAS.
- H. S. Wu, X. H. Xu, F. Q. Zhang and H. J. Jiao, J. Phys. Chem. A, 2003, 107, 6609 CrossRef CAS.
- D. L. Strout, Chem. Phys. Lett., 2004, 383, 95 CrossRef CAS.
- H. S. Wu, X. H. Xu and D. L. Strout, J. Mol. Model., 2005, 2, 1 CrossRef.
- H. S. Wu and H. J. Jiao, J. Mol. Model., 2006, 12, 537 CrossRef CAS.
- R. Li, L. H. Gan, L. Lin, J. Q. Liu and J. Zhao, THEOCHEM, 2009, 911, 75 CrossRef CAS.
- R. Li, L. H. Gan, Q. Li and J. An, Chem. Phys. Lett., 2009, 482, 121 CrossRef CAS.
- P. W. Fowler, T. Heine, D. Mitchell, R. Schmidtb and G. J. Seifertb, J. Chem. Soc., Faraday Trans., 1996, 92, 2197 RSC.
- H. W. Kroto, Nature, 1987, 329, 529 CrossRef CAS.
- E. Albertazzi, C. Domene, P. W. Fowler, T. Heine, G. Seifert, C. V. Alsenoy and F. Zerbetto, Phys. Chem. Chem. Phys., 1999, 1, 2913 RSC.
- M. L. Sun, Z. Slanina and S. L. Lee, Chem. Phys. Lett., 1995, 233, 279 CrossRef CAS.
- R. R. Zope, T. Baruah, M. R. Pederson and B. I. Dunlap, Chem. Phys. Lett., 2004, 393, 300 CrossRef CAS.
- H. S. Wu and H. J. Jiao, Chem. Phys. Lett., 2004, 386, 369 CrossRef CAS.
- http://www.mathematik.uni-bielefeld.de/~CaGe/ .
- L. H. Gan, R. Li and L. X. Gao, THEOCHEM, 2010, 945, 8 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P. M. W. Gill, B. G. Johnson, W. Chen, M. W. Wong, J. L. Andres, M. Head-Gordon, E. S. Replogle and J. A. Pople, GAUSSIAN03, Revision B. 03, Gaussian, Inc. , Pittsburgh, PA, 2003 Search PubMed.
- L. H. Gan, J. Q. Zhao and Q. Hui, J. Comp. Chem., 2010, 31, 1715 CAS.
- D. E. Manolopoulos, J. C. May and S. E. Down, Chem. Phys. Lett., 1991, 181, 105 CrossRef CAS.
- M. D. Diener and J. M. Alford, Nature, 1998, 393, 668 CrossRef CAS.
- G. Seifert, P. W. Fowler, D. Mitchell, D. Porezag and T. Frauenheim, Chem. Phys. Lett., 1997, 268, 352 CrossRef CAS.
- S. D. Tendero, F. Martin and M. Alcami, ChemPhysChem, 2005, 6, 92 CrossRef.
- P. W. Fowler, T. Heine and F. Zerbetto, J. Phys. Chem. A, 2000, 104, 9625 CrossRef CAS.
- M. Reiher and A. Hirsch, Chem.–Eur. J., 2003, 9, 5442 CrossRef CAS.
- A. Hirsch, Z. Chen and H. Jiao, Angew. Chem., Int. Ed., 2000, 39, 3915 CrossRef CAS.
- X. Lu, Z. Chen, W. Thiel, P. V. R. Schleyer, R. Huang and L. Zheng, J. Am. Chem. Soc., 2004, 126, 14871 CrossRef CAS.
- Z. Chen and R. B. King, Chem. Rev., 2005, 105, 3613 CrossRef CAS.
- R. C. Haddon, Science, 1993, 261, 1545 CAS.
- T. G. Schmalz, W. A. Seitz, D. J. Klein and G. E. Hite, J. Am. Chem. Soc., 1988, 110, 1113 CrossRef CAS.
- H. Y. Zhu, T. G. Schmalz and D. J. Klein, Int. J. Quantum Chem., 1997, 63, 393 CrossRef CAS.
- Z. Slanina, Int. Rev. Phys. Chem., 1987, 6, 251 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra21720a |
| This journal is © The Royal Society of Chemistry 2012 |
