Dependence of pretilt angle on orientation and conformation of side chain with different chemical structure in polyimide film surface†
Xu
Wang
,
Huina
Wang
,
Longbo
Luo
,
Jieyang
Huang
,
Jie
Gao
and
Xiangyang
Liu
*
State Key Laboratory of Polymer Materials Engineering, College of Polymer Science and Engineering, Sichuan University, Chengdu, Sichuan 610065, P. R. China. E-mail: lxy6912@sina.com; Fax: +86 28 85405138; Tel: +86 28 85400377
First published on 29th August 2012
Abstract
A series of polyimide films were synthesized based on various side-chained diamines, 4-phenylphenyl-3,5-diaminobenzoate (DABB), 6-(4-phenylphenoxy)hexyl-3,5-diaminobenzoate (C6BB), 6-(4-(4-butoxylphenyl)phenoxy)hexyl-3,5-diaminobenzoate (C6BBC4) and 4-(4-butoxylphenyl-phenyl-3,5-diaminobenzoate (BBC4), the side chains of which contain a rigid biphenyl linked with flexible alkyl. The pretilt angle of liquid crystal molecules on the side-chained polyimide films based on C6BBC4 or BBC4 (PI-C6BBC4 or PI-BBC4) increased obviously with increasing side chain content and even achieved 87–89°. In contrast, the polyimide film based on C6BB or BB (PI-C6BB or PI-BB) even with a high side chain content could only achieve the homogeneous alignment. The properties and microscopic structure of the functionalized polyimide films were analyzed by polarized ATR-FTIR, computer simulations and surface energy etc. It was found that the side chains with different chemical structure get the different orientation and conformation in the outmost layer of polymer film, affecting the pretilt angle of liquid crystal molecules on this film surface. For PI-BBC4 and PI-C6BBC4, the side chains with the non-polar group at the tail take the vertical conformation in the outer layer of the polymer surface, which benefited the liquid crystal to get a large pretilt angle, even vertical alignment on the film surface. The side chain of PI-C6BB with the flexible alkyl spacer is equipped with good mobility. It is easy for its polar mesogen groups at the surface to take the conformation parallel to the polymer film, which leads to the formation of homogeneous alignment of LC molecules.
Introduction
For the conventional aromatic polyimides, the low pretilt angle, poor processability and low transmittance in the visible region limits its application in electro-optial devices, such as liquid crystal displays (LCDs).1,2 The introduction of side chain groups,3–8 fluorinated groups9,10 or a twisted structure11 could effectively improve the optical property and pretilt angle of the PI films, among which the side-chained PI alignment films play an important role in meeting the requirements of the high performance LCD for pretilt angle due to the high pretilt angle, excellent transmittance and good processing performance.In order to follow the scientific advancement demands, a great deal of research is being carried out to further perfect the performance of side-chained polyimide films. The angle between the orientation of liquid crystal molecules and alignment of the film plane is called the pretilt angle, which is an important parameter for LC displays. Some polyimide films with long alkyl groups as side chains achieve a large pretilt angle,7 but their alignment stability was so bad that the high temperature and rubbing process could reduce the pretilt angle obviously. When only rigid mesogen groups instead of the alkyl chain was introduced as the side chain, the alignment stability of polyimide films was improved, while the low pretilt angle of this polyimide film could not meet the requirements of a high performance display.12 However, when side chains introduced a rigid mesogen group and flexible alkyl group together, the side-chained PI films achieved excellent stability and a large pretilt angle, even up to 90°.3–6
We studied the conformation of side chains of PI-C6BBC4-85 at the film surface by polarized attenuated total reflection Fourier transform infrared (polarized-ATR-FTIR) in a recent paper, which proved the alignment consistence between LC molecules and chemical units on the surface of PI alignment film.13 This was consistent with the reported alignment mechanism that the pretilt angle was affected by the geometric structure of side chains in the alignment film surface.14–17 However, the dependence of the pretilt angle on the chemical structure of the side chain and what factors determine the conformation of the side chain in the outmost layer of alignment film surface are still unclear.
Therefore, a series of functionalized diamines containing various side-chains were synthesized, 4-phenylphenyl-3,5-diaminobenzoate (DABB), 6-(4-phenylphenoxy)hexyl-3,5-diaminobenzoate (C6BB), 6-(4-(4-butoxylphenyl)phenoxy)hexyl-3,5-diaminobenzoate (C6BBC4) and 4-(4-butoxylphenyl-phenyl-3,5-diaminobenzoate (BBC4). The corresponding polyimide was synthesized from one kind of functionalized diamine and biphenyltetracarboxylicdianhydride (BPDA) and 4,4′-oxydianiline (ODA) in different ratios, which was abbreviated PI-BB, PI-C6BB, PI-C6BBC4 and PI-BBC4, respectively. As shown in Scheme 1, the alkyl and biphenyl were linked in the different arrangements in the side chain. The alignment property, surface energy, bulk structure and some macroscopic properties of the functionalized polyimide films were studied. The microscopic structure of the surface area was analyzed by polarized ATR-FTIR and computer simulations. The focus has especially been on exploring the orientation and conformation of side chains in the outmost layer of the alignment film surface. The effect of the chemical structure of side chains on the pretilt angle of liquid crystal molecules was discussed, which would provide theoretical guidance for molecular design of side-chained PI alignment film with high performance.
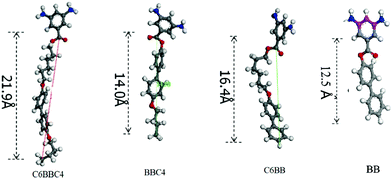 | ||
| Scheme 1 Energy-minimized structures of functionalized diamines. | ||
Results and discussion
2.1 Monomer synthesis
The diamine, DABB, was synthesized according to ref. 18. The other three kinds of functional diamines with side-chains were synthesized following the synthesis routes shown in Scheme 2.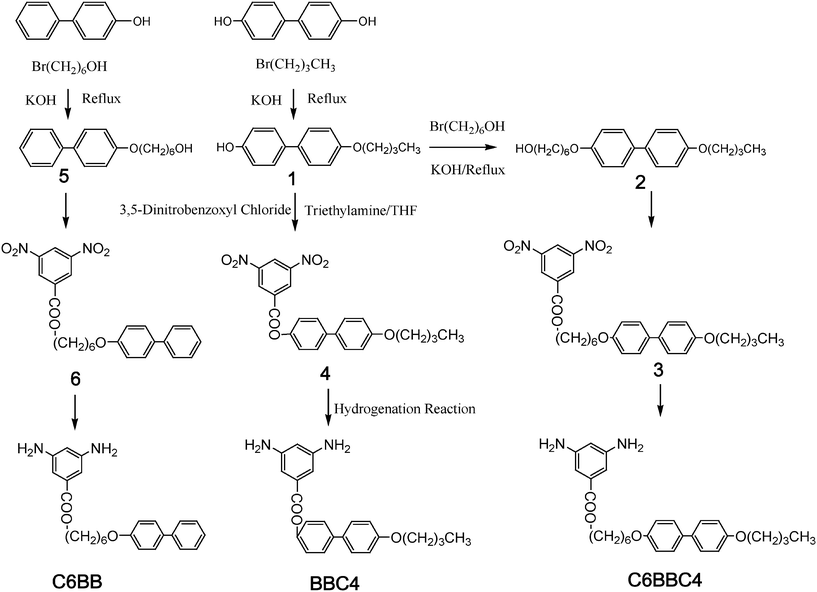 | ||
| Scheme 2 Synthesis routes of three kinds of functionalized diamines. | ||
As shown in Scheme 1, the energy-minimized structures of the functional diamines were calculated by the Materials Studio 4.0 software of Accelrys Inc. The length of the side-chains was 12.5 Å, 15.0 Å, 16.4 Å and 21.9 Å for BB, BBC4, C6BB and C6BBC4, respectively, which were in the order of C6BBC4 > C6BB > BBC4 > BB.
2.2 Polyimide synthesis
Poly(amic acid) was synthesized from one kind of side-chained diamine, BPDA and ODA in different ratios, for which the corresponding polyimide was abbreviated as PI-C6BB, PI-C6BBC4 and PI-BBC4, respectively. The synthesis route is shown in Scheme 3. By controlling the molar content of the functional diamine, polyimides with different side chain content were prepared. For example, when the poly(amic acid) was synthesized in the molar ratio of C6BB:ODA:BPDA fixed at 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, the side chain content of the corresponding polyimide was considered to be 50%, which was abbreviated as PI-C6BB-50; when the molar ratio was fixed at 10
10, the side chain content of the corresponding polyimide was considered to be 50%, which was abbreviated as PI-C6BB-50; when the molar ratio was fixed at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, the side chain content was 100% and the polyimide was abbreviated as PI-C6BB-100.
10, the side chain content was 100% and the polyimide was abbreviated as PI-C6BB-100.
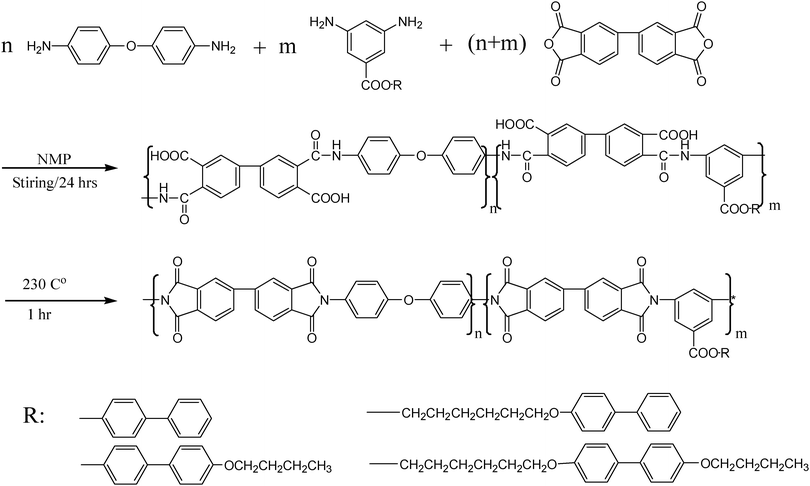 | ||
| Scheme 3 Synthesis routes of polyimides based on functionalized diamine, ODA and BPDA. | ||
The intrinsic viscosities of the poly(amic acid)s were measured by using a Ubbelhode-type viscometer in NMP. As shown in Fig. 1, the intrinsic viscosities of these PAA solutions all decreased with increasing side chain content. This mainly resulted from that the low reaction activity of the side-chained diamine reduced the molecular weight of the polymer.19 The FT-IR spectra of polyimide films indicated that the poly(amic acid)s had been turned into the polyimides completely after the imidization process (as shown in the supporting information†). The corresponding polyimide films had good flexibility and mechanical properties, indicating that the macromolecules of polyimides had a good molecular weight.21
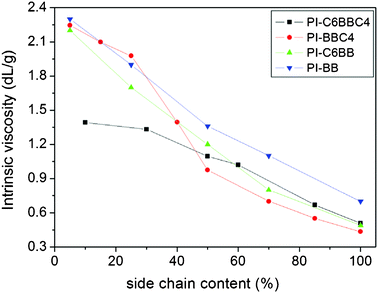 | ||
| Fig. 1 The dependence of intrinsic viscosities of functionalized polyimides with different side chains on side-chain content. | ||
2.3 Pretilt angle
Fig. 2 shows the pretilt angle values of liquid crystal molecules on the side-chained polyimide films PI-BB, PI-BBC4, PI-C6BB and PI-C6BBC4 with different side-chain content. It can be seen that the liquid crystal molecules could only achieve homogeneous alignment on the surface of all of the polyimide films with low side-chain content below 25%. The pretilt angles were lower than 10°. With increasing side chain content, the pretilt angles grew obviously.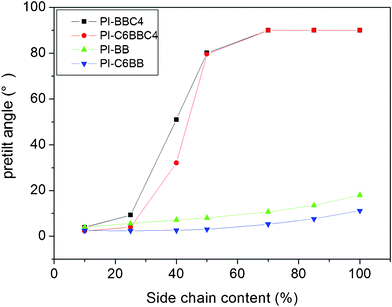 | ||
| Fig. 2 Pretilt angles of LC molecules on PI films with different side chains. | ||
For PI-BB, a rigid biphenyl group was introduced into the polyimide backbone as the side chain. This resulted in an increase of the pretilt angle, but the effect was not satisfactory. Even with 100% side-chain content, the pretilt angle of liquid crystal molecules on this polyimide film was 17.5°. Side chains containing alkyl and biphenyl groups were designed, like PI-BBC4, PI-C6BB and PI-C6BBC4. The alkyls were connected to biphenyl in a different way in the side chain.
As shown in Fig. 2, the pretilt angle of PI-C6BB was always lower than that of PI-BB. The maximum value of the pretilt angle was only 11°, with 100% side chain content. Therefore, the introduction of an alkyl spacer linked biphenyl and molecular backbone for PI-C6BB did not get better alignment properties.
In the cases of PI-BBC4 and PI-C6BBC4, the effect was remarkable for improvement of the pretilt angle. With the side chain content of PI-BBC4 and PI-C6BBC4 films increasing, the corresponding pretilt angles rose significantly. When the side chain content was 70%, the pretilt angles achieved 87–89°. Thus, a pretilt angle between 2–90° could all be achieved just by changing the content of the side chains. For these LC cells, the conoscopic POM images and study results of alignment stability are shown in the supporting information†.
2.4 Polarized ATR-FTIR analysis
The previous work had confirmed the enrichment of side chains with low polarity groups into the surface of polyimide film, which reflected that the side chains played an important role in determining the size of the pretilt angle of a liquid crystal.13,20 The focus was on studying the effect of the chemical structure on the orientation and conformation of side chains in the polyimide film surface.Polarized ATR-FTIR spectra were obtained using polarized IR radiation with different electric vectors. By changing the electric vector, the orientation groups had different adsorption strengths in the polarized ATR-FTIR spectra.22,23 The angle of polarization corresponds to the angle between the electric vector of incident light and X-axis direction. It was set to 0° with the electric vector parallel to the X-axis direction and 90° with the electric vector vertical to the X-axis direction, as shown in Fig. 3.
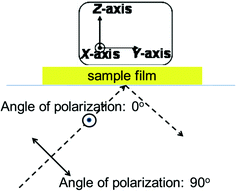 | ||
| Fig. 3 A scheme of ATR-FTIR with different angles of polarization. | ||
According to our previous work, the peaks at 1605.5 cm−1 were assigned to the 1,4-disubstituted C–C stretching vibration of the biphenyl group in the side chain, ν(C–C)S.13 The averaged direction of transition moments of ν(C–C)S was parallel to the para-direction of the biphenyl group in the side chain. The conformation of side chains is mainly determined by the arrangement of its phenyl rings.
Polarized ATR-FTIR was used to study the conformation and orientation of the side chains in the surface of PI-BB-85, PI-C6BB-85, PI-BBC4-85 and PI-C6BBC4-85 films. Fig. 4 shows polar diagrams of absorbance of peaks at 1605.5 cm−1 as a function of the angle of polarization of incident polarized light, obtained using linearly polarized IR spectroscopy.
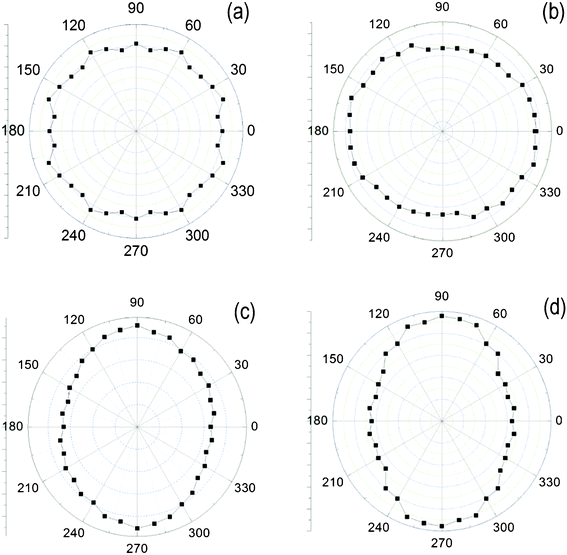 | ||
| Fig. 4 Polar diagrams of the absorbance of peaks at 1605.5 cm−1 as a function of the angle of polarization of incident polarized light for four kinds of aromatic polyimide films, (a) PI-BB-85, (b) PI-C6BB-85, (c) PI-BBC4-85 and (d) PI-C6BBC4-85. | ||
The polar diagrams of PI-BBC4 and PI-C6BBC4 films at 1605.5 cm−1 exhibit a maximum intensity of absorbance peak along the direction 90°↔270° and a minimum intensity along the direction 0°↔180°. In addition, the Z-axial component of incident polarized light achieves the maximum value with an angle of polarization of 90° or 270° and the minimum value with an angle of polarization of 0° or 180°. Thus, in the PI alignment films, the transition moments ν(C–C)S in the surface of PI-BBC4 and PI-C6BBC4 films orient parallel to the plane of the film.
The polar diagrams of PI-C6BB exhibit a maximum intensity of absorbance peak along the direction 0°↔180° and a minimum intensity along the direction 90°↔270°, which indicates that the transition moment ν(C–C)S in the surface of PI-C6BB films favorably orient parallel to the plane of film.
There are not distinct maximum and minimum values in the polar diagrams of PI-BB.
The dichroic ratio, RATR, is defined in eqn (1). The data is shown in Table 1.
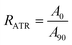 | (1) |
| Functionalized Polyimide | RATR |
|---|---|
| PI-BB-85 | 1.02 |
| PI-C6BB-85 | 1.23 |
| PI-BBC4-85 | 0.72 |
| PI-C6BBC4-85 | 0.67 |
Here, A90 and A90 represent the absorption strength in the IR spectrum with angle of polarization of 0° and 90°, respectively.
The dichroic ratio of PI-BB was 1.02, which indicated that the biphenyl groups of the PI-BB side chain exhibited an isotropic orientation in the film surface. The rigid biphenyl group with low motility was directly linked with the molecular backbone, resulting in it being restricted by the molecular backbone. Thus, the conformation of the random polyimide molecular backbone determined the disorder of the rigid biphenyl side chain.
In contrast, the dichroic ratio of PI-C6BB at 1605.5 cm−1 is 1.23, which shows that the averaged directions of transition moments of ν(C–C)S oriented parallel to the plane of the film. The biphenyl groups in the side chain of PI-C6BB exhibit higher degrees of in-plane orientations.
For PI-C6BBC4 and PI-BBC4, the dichroic ratio was 0.67 and 0.72, respectively. It was indicated that the rigid biphenyl groups in the side chain of PI-BBC4 and PI-C6BBC4 films were in some order in the surface of the PI film, with the orientation perpendicular to the surface of the PI alignment film.
In cases of PI-C6BBC4 and PI-BBC4, the side chains with the non-polar group at the tail take a vertical conformation in the surface. This was easy for the liquid crystal to get a large pretilt angle, even vertical alignment, according to the reported alignment mechanism where the pretilt angle was affected by the geometric structure of the side chain in the surface of the alignment film.
As PI-C6BB, the side chain with the flexible alkyl spacer is equipped with good mobility. It is easy for the polar mesogen groups of the side chain in the surface to take a conformation parallel to the polymer film under the attractive forces between them, which leads to the formation of a homogeneous alignment of LC molecules. Besides, the pretilt angle of PI-C6BBC4 was lower than that of PI-BBC4 before 50% side chain content, which might be due to the existence of an alkyl spacer in the side chain of PI-C6BBC4.
2.5 Characterization of the bulk structure of polyimide film
In order to study the aggregated structure variation of PI films after the introduction of side chains, the WXRD spectra of PI-C6BBC4-100, PI-C6BB-100, PI-BBC4-100 and PI-m-PDA (synthesized based on BPDA and m-phenylenediamine) was measured in the reflection mode. The obtained spectra were analyzed, as shown in Fig. 5. Judging from the peak shape, it can be seen that these PI films are amorphous. This polyimide film reveals only one broad peak, which corresponds to the mean interchain distance.24,25 The values of this distance, calculated with the formula 2dsinθ = λ, are 3.6 Å, 4.0 Å and 4.0 Å for PI-C6BB, PI-BBC4 and PI-C6BBC4, respectively, which all are larger than that of PI-m-PDA without a side chain, 3.4 Å. Thus, the value of mean interchain distance is in the order: PI-m-PDA < PI-C6BB-100 < PI-BBC4-100 ≈ PI-C6BBC4-100. The steric effect of the side chain is considered to result in an increased mean interchain distance and determine the increasing range. The steric effect of the side chain should be in the order of PI-BBC4 ≈ PI-C6BBC4 > PI-C6BB. Therefore, it was easy for the side chain of PI-BBC4 and PI-C6BBC4 to take the conformation vertical to the molecular backbone compared with that of PI-C6BB.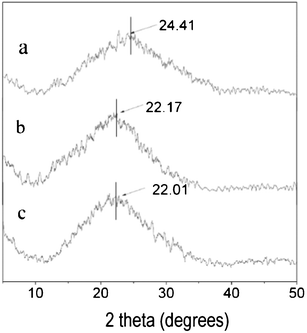 | ||
| Fig. 5 XRD spectra of PI-C6BB-100 (a), PI-BBC4-100 (b) and PI-C6BBC4-100 (c). | ||
The Tgs of PI-C6BB, PI-BBC4 and PI-C6BBC4 dependant on the content of side chain are shown in Fig. 6. The Tgs of PI-C6BB and PI-C6BBC4 had been decreasing following the increasing of side chain content, which was 224 °C and 203.5 °C for PI-C6BB and PI-C6BBC4, respectively, with 100% side chain content. This was mainly due to the mobility of the macromolecules, which was related to the free volume of polymer bulk and the steric effect of the side chain.26,27
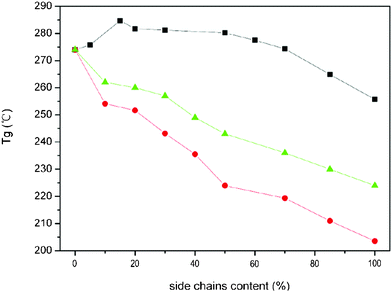 | ||
| Fig. 6 The Tgs of PI-BBC4 and PI-C6BBC4 depend on the content of the side chain. Triangle: PI-C6BB; square: PI-BBC4; circle: PI-C6BBC4. | ||
The Tg of PI-C6BBC4 had been lower than that of PI-C6BB. This was considered to result from the larger free volume of PI-C6BBC4 compared with PI-C6BB. The introduction of a flexible alkyl tail lengthened the PI-C6BBC4 side chains and enhanced these steric effects, resulting in the larger free volume of polymer bulk, which has been confirmed by the results of XRD and densities measurements. The density was 1.3569 g cm−1, 1.3014 g cm−1, 1.2965 g cm−1 and 1.4016 g cm−1 for PI-C6BB-100, PI-BBC4-100, PI-C6BBC4-100 and PI-m-PDA, respectively, which was in the order of PI-m-PDA > PI-C6BB > PI-BBC4 ≈ PI-C6BBC4.
In the case of PI-BBC4, the Tg temperature first increased with the side chain content increasing and then decreased. The maximum value is 284 °C with side chain content at 15%. The free volume of PI-BBC4 films was also enlarged due to the introduction of side chains. The variation of free volume failed to explain the increase of Tg with side chain content between 0–15%. By comparing PI-C6BBC4 and PI-C6BB, the rigid biphenyl in the side chain was directly linked to the molecular main chain of PI-BBC4 without a flexible alkyl spacer, which restricts the movement of the whole macromolecule. It can be seen that the mobility of the macromolecules decreased due to the absence of a flexible alkyl spacer, resulting in the increase of Tg temperature.
Thus, it was confirmed that the side chains of PI-BBC4 and PI-C6BBC4 more easily took the vertical conformation to the macromolecular backbone compared with that of PI-C6BB. Due to the PI molecular backbones exhibiting higher degrees of in-plane orientation, it was possible for the side chain to take the vertical orientation to the plane of the PI film, which supported the polarized ATR-FTIR results. Also, by comparing PI-BBC4 and PI-C6BBC4 with PI-C6BB, it can be seen that the alkyl tail group plays an important role in determining the conformation of the side chain.
2.6 Computer simulation
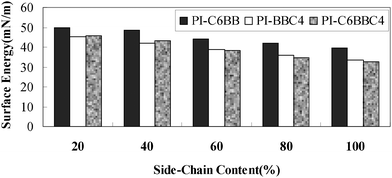
The densities of the side-chained polyimide films were measured, giving 1.346 g cm−1, 1.357 g cm−1, 1.301 g cm−1 and 1.297 g cm−1 for PI-BB-85, PI-C6BB-85, PI-BBC4-85 and PI-C6BBC4-85, respectively. Also, the surface energy of PI-BB-85, PI-C6BB-85, PI-BBC4-85 and PI-C6BBC4-85 was 43.3 mN m−1, 41.5 mN m−1, 37.2 mN m−1 and 36.8 mN m−1, respectively. Based on the above data, the surface area was simulated using Materials Studio software by employing a Dreiding force field. The simulation detail is given in ref. 28. A schematic diagram of the surface areas obtained by computer simulation is shown in Fig. 7.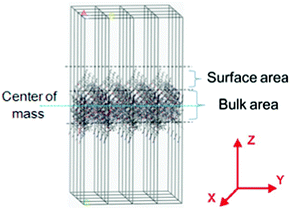 | ||
| Fig. 7 A schematic diagram of surface areas obtained by computer simulation. | ||
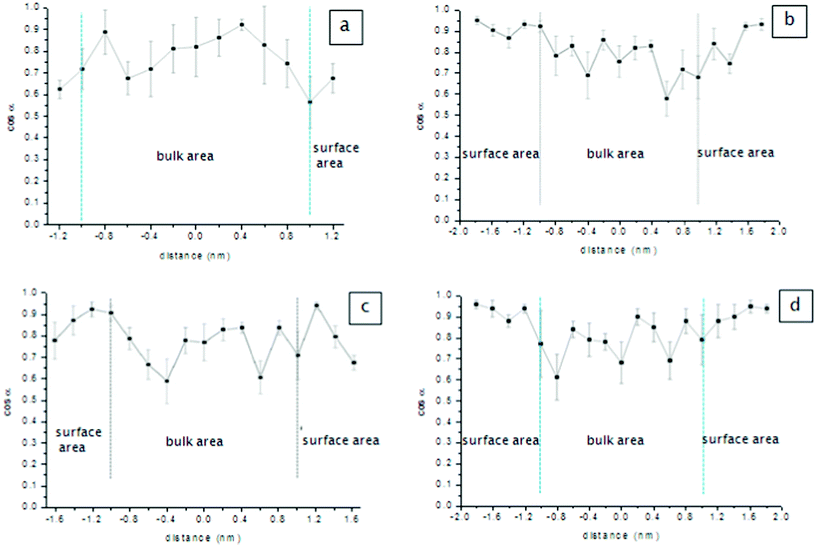
For side-chained PIs, the surface conformation of side chains is mainly determined by the arrangement of phenyl rings, and it is evaluated using the angle α, between the z-axis and the phenyl ring plane. If the cosα is 1 or −1, the phenyl ring is parallel with the z-axis, namely vertical to the x–y plane. The simulation results are presented in Fig. 8.
 | ||
| Fig. 8 Optimized structures of molecular modeling for PI-BB-85, PI-C6BB-85, PI-BBC4-85 and PI-C6BBC4-85. | ||
 | ||
| Fig. 9 Surface energies of different PI films as a function of side chain content. | ||
The values of cosα in the surface area were even higher at 0.95 in the systems PI-BBC4 and PI-C6BBC4, indicating that most of the phenyl rings in the surface area were vertical to the x–y plane. These results were in reasonable agreement with the results of polarized ATR-FTIR. The vertical conformation of the phenyl ring in the side chain of PI-BBC4 and PI-C6BBC4 accounted for their high pretilt angles according to the previously reported results.
In contrast, the phenyl rings of PI-BB were randomly arranged in the bulk for their large deviation of cosα, while being arranged parallel in the surface region because of their decrement in cosα compared with the bulk state. For the PI-C6BB system, its side chains were also parallel to the x–y plane in the surface region. The flexible alkyl chain was used to connect the biphenyl group with the molecular main chain, resulting in the biphenyl being able to take the conformation parallel to the polymer bulk in the outer surface under the attractive forces between them.
The biphenyl groups of the PI-CBBC4 and PI-C6BBC4 side chains took the vertical orientation in the surface of the PI alignment film, which should be due to the introduction of the alkyl group at the end of the side chain. It is easy for the side chains of PI-BBC4 and PI-C6BBC4 to get an “standing up” conformation in the surface under the repulsive force between the alkyl tail and the main chain.
The alkyl chain, C4, was introduce to the side chain as a tail for the functionalized polyimide PI-BBC4 and PI-C6BBC4. Actually, the alkyl tail was regarded as a nonpolar group. The repulsive force from the polarity difference of the nonpolar groups with the polar polyimide backbone resulted in the enrichment of the side chain into the surface of the polyimide film and determined the vertical conformation of the side chain. While the flexible alkyl was used as the spacer connecting the biphenyl and molecule backbone not the tail of the side chain, it was hard to for this nonpolar group to move towards the outer surface, and the attractive forces between the biphenyl and polymer bulk played a decisive role in determining the in-plane orientation of the biphenyl group of the side chain in the outermost layer.
For PI-BBC4 and PI-C6BBC4, the nonpolar groups are enriched in the outermost layer of the polymer film, resulting in the low surface energy. The increase of side chain content would lower the surface energy still further.
As shown in Fig. 9, surface energies of PI-C6BBC4 and PI-BBC4 films have been obviously lower than that of the corresponding PI-C6BB film. The surface energy data of these film materials was consistent with the above supposition.
Taken together, side chains with different chemical structures get different orientations and conformations in the outmost layer of a polymer film, affecting the pretilt angle of liquid crystal molecules on this film surface. The side chains with a non-polar group at the tail take the vertical conformation in the outer layer of the polymer surface, which benefits the liquid crystal to get a large pretilt angle, even vertical alignment on the film surface. Side chains with a flexible alkyl spacer are equipped with good mobility. It is easy for the polar mesogen groups of side chains in the surface to take the conformation parallel to the polymer film under the attractive forces between them, which leads to the formation of homogeneous alignment of LC molecules. It was confirmed that the polarity of the tail group in the side chain played a decisive role in determining the orientation and conformation of groups in the side chain in the film surface.
3. Experimental
3.1 Material
4,4′-dihydroxybiphenyl (Henan Yanhua Chemical Corp. China), 4-diphenol, 1-bromobutane, triethylamine (TEA, Chengdu Kelong Chemical Reagent Corp China), 6-chlorohexanehexol (Acros Organics, New Jersey), 3,5-dinitrobenzoyl chloride (Taixing Refined Chemical Co. Ltd. Jiangsu China) and 5% palladium on activated carbon (Pd/C, Acros Organics, New Jersey) were used as received. 4,4′-diaminodiphenyl ether (ODA, Shanghai Institute of Synthetic Resins, China) was purified by recrystallization from ethanol. 3,3′,4,4′-biphenyl dianhydride (BPDA, Acros Organics, New Jersey) was dried for 5 h at 180 °C prior to use. N-Methyl-2-pyrrolidinone (NMP, Pu Yang MYJ Technology, China) was distilled under reduced pressure after drying by P2O5. The indium tin oxide (ITO)-coated glass was obtained from CSG Holding Co., Ltd, China. 4-pentyl-4′-cyanobiphenyl (5CB, no = 1.53, ne = 1.74, Td = 24 °C, Ti = 34 °C) was supplied by Beijing Tsinghua Yawang Liquid Crystal Materials Co., Ltd, China.3.2 Diamine monomer synthesis
4′-butoxydiphenol and equimolar KOH mixture were added into heated ethanol with stirring. After refluxing for 30 min, equimolar 6-bromo-n-hexanol was added into the reaction mixture and stirred for 24 h at reflux. After cooling, the crude product was filtered and washed with 20 ml DMF to get a white powder of 6-(4-phenylphenol)-n-hexanol.
C6BBC4 was synthesized by a hydrogenation reaction of 6-(4-(4-butoxylphenyl)phenoxy)hexyl-3,5-diaminobenzoate (C6BBC4), which was synthesized from 3,5-dinitrobenzoxyl chloride and 6-(4-(4-butoxyphenyl)phenol)-n-hexanol, as shown in Scheme 2. Compound 2 was dissolved in 100 ml THF and 10 ml triethylamine. 3,5-dinitrobenzoxyl chloride dissolved in 30 ml THF and was dripped into the THF solution with ice cooling. After 12 h stirring, the precipitate was filtered, washed with deionized water and dried under vacuum at 80 °C to get a crude yellow compound. The crude product was recrystallized from ethanol to get the yellow crystals of compound 3. Compound 3 (7 g), 5% palladium on activated carbon (0.7 g, Pa/C) and alcohol (70 ml) were added into an autoclave equipped with a magnetic stirrer. Hydrogenation reaction was conducted for 2 h at 70 °C under a hydrogen atmosphere of 1.2 MPa. Then, the mixture was added into 150 ml THF. The solution was filtered for removing Pd/C, which was evaporated to remove the solvent. The crude product was recrystallized from methanol to get a yellowish compound of C6BBC4. The mp was 196.1 °C. Yield: 49%. FTIR: 3458.72, 3369.04 (υH–N), 2955.87, 2936.65, 2866.19 (fatty group υCH), 1697.15 (υC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1604.27, 1498.58 (υ C–C of aromatic ring), 1271.17, 1245.55 and 1178.29 (υC–O–C). 1H-NMR (CDCl3, 400 MHz, δ (ppm)): 0.99 (t, 3H, –CH3), 1.52 (m, 6H, CH2 between two methylenes), 1.79 (m, 6H, CH2 between methylene and oxymethylene), 3.66 (s, 4H, –NH2), 4.00 (m, 4H, –CH2O–Ar–), 4.28 (t, 2H, –CH2O–C
O), 1604.27, 1498.58 (υ C–C of aromatic ring), 1271.17, 1245.55 and 1178.29 (υC–O–C). 1H-NMR (CDCl3, 400 MHz, δ (ppm)): 0.99 (t, 3H, –CH3), 1.52 (m, 6H, CH2 between two methylenes), 1.79 (m, 6H, CH2 between methylene and oxymethylene), 3.66 (s, 4H, –NH2), 4.00 (m, 4H, –CH2O–Ar–), 4.28 (t, 2H, –CH2O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 6.18 (m, 1H, aromatic H ortho to two amino groups), 6.78 (d, 2H, aromatic H ortho to carboxyl group), 6.94 (m, 4H, aromatic H ortho to alkoxyl group), 7.46 (d, 4H, aromatic H meta to alkoxyl group).
O), 6.18 (m, 1H, aromatic H ortho to two amino groups), 6.78 (d, 2H, aromatic H ortho to carboxyl group), 6.94 (m, 4H, aromatic H ortho to alkoxyl group), 7.46 (d, 4H, aromatic H meta to alkoxyl group).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1608.36, 1498.34 (υ C–C of aromatic ring), 1236.16 (υ C–O–C). 1H-NMR (CDCl3, 400 MHz, δ (ppm)): 1.55 (m, 4H, CH2 between two methylenes), 1.95 (m, 4H, CH2 between methylene and oxymethylene), 3.58 (s, 4H, –NH2), 3.97 (m, 2H, –CH2O–Ar–), 4.24 (t, 2H, –CH2O–C
O), 1608.36, 1498.34 (υ C–C of aromatic ring), 1236.16 (υ C–O–C). 1H-NMR (CDCl3, 400 MHz, δ (ppm)): 1.55 (m, 4H, CH2 between two methylenes), 1.95 (m, 4H, CH2 between methylene and oxymethylene), 3.58 (s, 4H, –NH2), 3.97 (m, 2H, –CH2O–Ar–), 4.24 (t, 2H, –CH2O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 6.16 (m, 1H, aromatic H ortho to two amino groups), 6.77 (d, 2H, aromatic H ortho to carboxyl group), 6.92 (m, 2H, aromatic H ortho to alkoxyl group), 7.21 (t, 2H, aromatic H para to Ar–OR), 7.43 (m, 2H, aromatic H ortho to –Ar–OR) 7.50 (d, 2H, aromatic H meta to alkoxyl group), 7.58 (d, 2H, aromatic H ortho to –Ar–OR).
O), 6.16 (m, 1H, aromatic H ortho to two amino groups), 6.77 (d, 2H, aromatic H ortho to carboxyl group), 6.92 (m, 2H, aromatic H ortho to alkoxyl group), 7.21 (t, 2H, aromatic H para to Ar–OR), 7.43 (m, 2H, aromatic H ortho to –Ar–OR) 7.50 (d, 2H, aromatic H meta to alkoxyl group), 7.58 (d, 2H, aromatic H ortho to –Ar–OR).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1605.27, 1500.58 (υC–C of aromatic ring), 1271.34, 1245.87 and 1178.56 (υC–O–C). 1H-NMR (CDCl3, 400 MHz, δ (ppm)): 0.95 (t, 3H, –CH3), 1.48 (m, 2H, CH2 between two methylenes), 1.71 (m, 2H, CH2 between methylene and oxymethylene), 4.01 (m, 4H, –CH2O–Ar–), 5.11 (s, 4H, –NH2), 6.11 (m, 1H, aromatic H ortho to two amino groups), 6.60 (d, 2H, aromatic H ortho to carboxyl group), 7.02 (m, 2H, aromatic H ortho to alkoxyl group), 7.24 (m, 2H, aromatic H ortho to carboxyl ester group), 7.59 (d, 2H, aromatic H meta to alkoxyl group), 7.65 (d, 2H, aromatic H meta to carboxyl ester group).
O), 1605.27, 1500.58 (υC–C of aromatic ring), 1271.34, 1245.87 and 1178.56 (υC–O–C). 1H-NMR (CDCl3, 400 MHz, δ (ppm)): 0.95 (t, 3H, –CH3), 1.48 (m, 2H, CH2 between two methylenes), 1.71 (m, 2H, CH2 between methylene and oxymethylene), 4.01 (m, 4H, –CH2O–Ar–), 5.11 (s, 4H, –NH2), 6.11 (m, 1H, aromatic H ortho to two amino groups), 6.60 (d, 2H, aromatic H ortho to carboxyl group), 7.02 (m, 2H, aromatic H ortho to alkoxyl group), 7.24 (m, 2H, aromatic H ortho to carboxyl ester group), 7.59 (d, 2H, aromatic H meta to alkoxyl group), 7.65 (d, 2H, aromatic H meta to carboxyl ester group).
3.3 Synthesis of PIs
The polyimides were synthesized from one kind of side chained diamine, BPDA and ODA in different ratios, which are abbreviated to PI-C6BB, PI-C6BBC4 and PI-BBC4, respectively. To a 50 ml of a reactor equipped with a mechanical stirrer and a nitrogen inlet, the side-chained diamine with different ratios of ODA copolymerized with equimolar BPDA in the solvent of NMP, getting the poly(amide acid) (PAA) solutions. The solid content was 10%. The PAAs were coated on ITO-coated glass plates and thermally imidized into the corresponding PIs at 230 °C for 1h. The polymerization procedure is shown in Scheme 3.3.4 Characterization
FT-IR spectra were recorded on a Nicolet 560 Fourier transform spectrometer with KBr pellets. ATR-FTIR spectra were recorded on a Nicolet 560 Fourier transform spectrometer using PI films coated on glass. Proton nuclear magnetic resonance (1H-NMR) spectra were obtained using a Bruker-ACE 400 MHz NMR spectrometer. Inherent viscosities were measured at 0.5 g dL−1 concentration in NMP at 25 °C using an Ubbelohde viscosimeter. LC cell fabrication and pretilt angle measurements were made as follows. The prepared PI films on the ITO-coated glass were subsequently rubbed with a roller covered commercial rubbing cloth, and the rubbing strength L was calculated as follows: L = lN(2πrn/60υ − 1), where L (mm) is the total length of the rubbing cloth that touches a certain point of the film; l (0.3 mm) is the contact length of the rubbing roller circumference; N is the cumulative number of rubbings; υ (17.2 mm s−1) is the velocity of the substrate stage; and n (700 rpm) and r (22.5 mm) are the rubbing roller speed and radius, respectively. LC cells were fabricated from two pieces of rubbed fluorinated PI films assembled in an antiparallel rubbing direction with 43 μm (cell gap) thick spacers and filled with 4-pentyl-4′-cyanobiphenyl (5CB) by the capillary method. The pretilt angles of the fabricated LC cells were measured by modified crystal rotation method with a PAT-20 measurement device (Chanchun Liancheng Instrument Co., Ltd.). Large pretilt angles near 90° were confirmed by conoscopy using a Leica DM 130 LB polarised light microscopy (Leica Microsystems, Wetzlar, Germany). Polarizing microscopy from Shanghai Millimeter Precision Instrument Co. Ltd. (Shanghai China) was used to evaluate the alignment behavior of the LC. To test the surface energy, the contact angles of deionised water and methylene iodide on the surface of the PI films were measured by a Kruss DSA100 (Krüss, Hamburg, Germany) measurement device. Each sample was tested three times and mean contact angles were obtained. Wide angle X-ray diffraction analysis of PI films was carried out on a Philips X' PertPRO MPD. Glass transition temperature was characterized by Differential Scanning Calorimetry (DSC), performed on Netzsch 204 DSC with a ramping rate of 5 °C min−1. A Young's harmonic mean equation was applied to predict the surface energies from the contact angles. Computer simulations were performed using the Materials Studio 4.0 software of Accelrys Inc. Molecular mechanics and molecular dynamics simulation were performed using Materials Studio software by employing a Dreiding force field. Film density measurements were performed using the sinkfloat technique described by Numata et al.29.4. Conclusions
Several kinds of side-chained polyimide alignment films, PI-BB, PI-C6BB, PI-BBC4 and PI-C6BBC4, were synthesized from one kind of functionalized diamine, BPDA and ODA in different ratios. For PI-C6BBC4 and PI-BBC4 films, the pretilt angle of liquid crystal molecules increased obviously with increasing side chain content and even achieved 87–89°. In contrast, the LC molecules on PI-BB and PI-C6BB films even with a high side chain content could only achieve horizontal alignment. The polarized ATR-FTIR and computer simulation results show that the rigid biphenyl groups in the side chains took the vertical orientation to the film plane in the surface for PI-BBC4 and PI-C6BBC4 and the in-plane orientation for PI-BB and PI-C6BB when the side chain content was 100%. It was confirmed that the polarity of the tail group in the side chain played a decisive role in determining the orientation and conformation of groups in the side chain.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 50973073/50773044) and by the Combination Project of Guangdong Province and Ministry of Education (No. 2011A090200014). We acknowledge Analytical & Testing Center Sichuan University, P. R. China for characterization.References
- M. K. Ghosh, K. L. Mittal, ed. in Polyimides, fundamentals and applications, Marcel Dekker, New York, 1996 Search PubMed.
- C. E. Sroog, J. Polym. Sci., Macromol. Rev., 1976, 11, 161 CrossRef CAS.
- H. Lai, L. Qin, X. Y. Liu and Y. Gu, Eur. Polym. J., 2008, 44, 3724–3730 CrossRef CAS.
- L. Li, J. Yin, Y. Sui, H. J. Xu, J. H. Fang, Z. K. Zhu and Z. G. Wang, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 1943–1949 CrossRef CAS.
- S. B. Lee, G. J. Shin, J. H. Chi, W. C. Zin, J. C. Jung, S. G. Hahm and M. Ree, Polymer, 2006, 47, 6606–6611 CrossRef CAS.
- T. Ichino, S. Sasaki, T. Matsuura and S. Nishi, J. Polym. Sci., Part A: Polym. Chem., 1990, 28, 323 CrossRef CAS.
- H. Kang, J. S. Park, E. H. Sohn, D. Kang, C. Rosenblatt and J. C. Lee, Polymer, 2009, 50, 5220 CrossRef CAS.
- S. I. Kim, M. Ree, T. J. Shin and J. C. Jung, J. Polym. Sci., Part A: Polym. Chem., 1999, 37, 2909 CrossRef CAS.
- T. Matsuura, Y. Hasuda, S. Nishi and N. Yamada, Macromolecules, 1991, 24, 5001 CrossRef CAS.
- T. Matsuura, M. Ishizawa, Y. Hasuda and S. Nishi, Macromolecules, 1992, 25, 3540 CrossRef CAS.
- S. Z. D. Cheng, F. Li, E. P. Savitski, F. W. Harris, Molecular design of aromatic polyimide films as uniaxial negative birefringent optical compensators in liquid crystal displays, Elsevier, Cambridge, U. K., 1997 Search PubMed.
- Z. J. Liu, F. Yu, Q. Zhang, Y. Zeng and Y. H. Wang, Eur. Polym. J., 2008, 44, 2718 CrossRef CAS.
- X. Wang, P. Zhang, Y. Chen, L. B. Luo, Y. W. Pang and X. Y. Liu, Macromolecules, 2011, 44, 9731 CrossRef CAS.
- Y. J. Lee, J. G. Choi, I. Song, J. M. Oh and M. H. Yi, Polymer, 2006, 47, 1555 CrossRef CAS.
- R. Arafune, K. Sakamoto and S. Ushioda, Appl. Phys. Lett., 1997, 71, 2755 CrossRef CAS.
- Y. T. Chern and M. H. Ju, Macromolecules, 2009, 42, 169 CrossRef CAS.
- M. Han and K. Ichimura, Macromolecules, 2001, 34, 90 CrossRef CAS.
- H. Fan and Y. Gu, Acta. Polym. Sinica., 2001, 4, 28 Search PubMed.
- T. Gupta and B. Adhikari, J. Polym. Sci., Part A: Polym. Chem., 2001, 39, 2978 CrossRef CAS.
- J. V. Facinelli, S. L. Gardner, L. Dong, C. L. Sensenich, R. M. Davis and J. S. Riffle, Macromolecules, 1996, 29, 7342–7350 CrossRef CAS.
- X. Wang, S. L. Chen, Y. J. Yang, Y. Chen, M. Li and X. Y. Liu, Polym. Int., 2010, 59, 1622 CrossRef CAS.
- M. Niwa, H. Kawakami, T. Kanamori, T. Shinbo, A. Kaito and S. Nagaoka, Macromolecules, 2001, 34, 9039 CrossRef CAS.
- C. T. Johnston and G. S. Premachandra, Langmuir, 2001, 17, 3712 CrossRef CAS.
- X. Y. Liu, L. H. Guo and Y. Gu, Polymer, 2005, 46, 11949 CrossRef CAS.
- X. Y. Liu, J. Yang, L. H. Guo and Y. Gu, Macromol. Rapid Commun., 2005, 26, 1682 CrossRef CAS.
- W. M. Li, G. Chen, S. B. Zhang, H. Wang and D. H. Yan, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 3550 CrossRef CAS.
- S. H. Hsiao and Y. H. Chang, J. Polym. Sci., Part A: Polym. Chem., 2004, 37, 1255 CrossRef.
- M. Li, H. Lai, B. Chen, X. Y. Liu and Y. Gu, Liq. Cryst., 2010, 37, 149 CrossRef CAS.
- S. Numata, K. Fujisaki and N. Kinjo, Polymer, 1987, 28, 2282–2288 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra21647g/ |
| This journal is © The Royal Society of Chemistry 2012 |
