Production of value-added chemicals from bio-oil via acid catalysis coupled with liquid–liquid extraction†
Xun
Hu
,
Daniel
Mourant
,
Richard
Gunawan
,
Liping
Wu
,
Yi
Wang
,
Caroline
Lievens
and
Chun-Zhu
Li
*
Fuels and Energy Technology Institute, Curtin University of Technology, GPO Box U1987, Perth, WA 6845, Australia. E-mail: chun-zhu.li@curtin.edu.au; Fax: (+) 618 9266 1138; Tel: (+) 618 9266 1131
First published on 20th August 2012
Abstract
Sugar/sugar derivatives in bio-oil can be effectively separated from aromatics via water/chloroform extraction. Subsequent acid-treatment in methanol converts the sugars into levulinic acid/ester and the sugar derivatives into fuel additives. Further extraction with CH2Cl2/CHCl3 could efficiently separate methyl levulinate and levulinic acid from other products.
Bio-oil, the liquid product from pyrolysis of solid biomass, is a sustainable feedstock for renewable liquid fuels and chemicals.1 However, the heating value of bio-oil is much lower than that of fossil fuels due to the high oxygen content in bio-oil.2 Thus, bio-oil has to be upgraded to reduce the oxygen content. Nevertheless, upgrading of bio-oil in current fossil oil refineries is rather difficult due to the high tendency of bio-oil towards polymerization.3
Various organics with quite different oxygen-containing functional groups mix together in bio-oil, leading to the high instability of bio-oil.4 However, if we take bio-oil as a source of chemicals, then we do not have to take a long way or pay a high price to remove the substantial amount of oxygen in bio-oil. A lot of oxygen-containing chemical stocks now are produced from fossil fuels via oxidation or hydration of olefins to introduce oxygen-containing functional groups.5 In contrast, these functional groups are already present in bio-oil.6 Thus, production of value-added chemicals from bio-oil can be an economical way to utilize bio-oil.
How to selectively produce targeted chemicals from bio-oil and effectively separate them are two main challenges due to the complexity of bio-oil. Aromatics, sugars, and sugar derivatives are the main components of bio-oil, which can be potentially hydrogenated to fuel.7 However, some components especially sugars are relatively difficult to process via hydrotreating due to their high tendency to polymerize.8 Moreover, sugars are quite reactive during the heating of bio-oil, which could condense together with sugar derivatives and aromatics to form polymers.9
Instead of hydrogenation, C6 sugars could be efficiently converted into the platform chemical, methyl levulinate, with little polymer formation in a methanol-rich medium.10 Thus, it is worthwhile to convert the “troublesome” sugars in bio-oil into value-added chemicals via acid-catalyzed conversion, as yields of only the anhydrosugars such as levoglucosan from biomass pyrolysis could be up to 27%.11 Hence, this study proposed a strategy of converting sugars and aromatics in bio-oil via different processes (acid-catalyzed versus hydrogenation). The two processes with different treatments require the separation of sugars and aromatics from each other, which could be potentially be accomplished by extraction of bio-oil with solvents due to the different polarities of sugars and aromatics, as is shown in Scheme 1. This study focused on the separation of sugars from aromatics, the transformation of sugars and sugar derivatives into platform chemicals, and the separation of the chemicals produced.
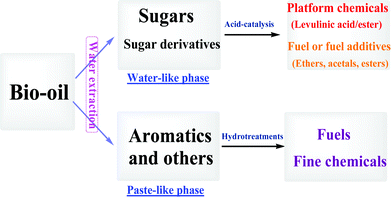 | ||
| Scheme 1 Utilisation of different fractions of bio-oil with different strategies. | ||
The acid-treatment of bio-oil in alcohol could effectively convert sugars but does not work on most aromatics.12 Aromatics are undesirable during the acid-catalyzed conversion of sugars as they would interfere with separation of the products. The separation of sugars from aromatics allows us to remove the “troublesome” sugars from bio-oil and convert the other components such as aromatics into fuels via hydrotreating.
Sugars and aromatics have distinct polarities. By adding polar water, bio-oil can be separated into two phases.13 The bio-oil from mallee wood is naturally one phase, but it also can be separated into an upper polar water-like phase and a lower less polar paste-like phase by adding water. Distributions of some typical compounds in the two phases were shown in Table 1 (Table S1 in the ESI shows full list of the compounds in the two phases†). Levoglucosan together with other sugars and sugar derivatives were the main components in the water-like phase, while ca. 70 to 80% of the aromatics enriched the paste-like phase.
| Typical compounds in bio-oil | Abundance (%) | Overall concentration (wt%) | |
|---|---|---|---|
| Water-like phase | Paste-like phase | ||
| a By adding water to increase the water content in bio-oil to ca. 41 wt%, the bio-oil separated into a water-like phase and a paste-like phase automatically. | |||
| Levoglucosan | 83.4 | 16.6 | 5.5 |
| Phenol | 27.2 | 72.8 | 0.1 |
| Eugenol | 17.4 | 82.6 | 0.1 |
| Vanillin | 28.6 | 71.4 | 0.1 |
| p-Methylguaiacol | 19.1 | 80.9 | 0.1 |
| 2-Methoxyphenol | 27.0 | 73.0 | 0.2 |
| Furfural | 31.1 | 68.9 | 0.8 |
| 2-Furanmethanol | 54.4 | 45.6 | 0.1 |
| Acetic acid | 73.6 | 26.4 | 6.0 |
| Propanoic acid | 79.7 | 20.3 | 0.2 |
| Hydroxyacetone | 70.0 | 30.0 | 2.9 |
| Hydroxyacetaldehyde | 73.7 | 26.3 | 7.3 |
Some furan components such as furfural have a very high tendency towards polymerization in the presence of an acid catalyst,14 majority of which was extracted into the paste-like phase. This could avoid the polymerization of furfural during acid-treatment of the water-like phase of bio-oil, and furfural together with the aromatics in the paste-like phase can be further hydrogenated to fuel additives. The carboxylic acids are relatively difficult to be hydrogenated,15 but the extraction of them into the water-like phase and the followed acid-treatment in methanol would convert them into esters, which are fuel additives. Similarly, hydroxyl aldehyde and hydroxyl acetone also could be converted into their acetals or ethers which are fuel additives or pharmaceutical intermediates.16
Table 2 shows conversion of the typical compounds under acid-treatment of the water-like phase of bio-oil and the formation of some main products at different temperatures with a residence time of 180 min. The anhydrosugars in bio-oil could be easily converted at the various reaction temperatures. Methyl α-D-glucopyranoside (MGP) was the main product from methanolysis of levoglucosan at 135 °C and it further dehydrated to 5-(hydroxymethyl)furfural (HMF) and the ether or acetal of HMF (not shown) at the elevated temperatures. Simultaneously, HMF together with its ethers and acetals were further converted into methyl levulinate and levulinic acid. Relatively more methyl levulinate was formed at 165 °C while more levulinic acid at 180 °C was produced. The detailed results for the conversion of levoglucosan and the formation of levulinic acid/ester and other intermediates versus reaction time was shown in Figure S1 in the ESI.† The formation of more levulinic acid than methyl levulinate at 180 °C was mainly due to the formation of more water at this temperature. Etherification reactions between methanol produces water, which changes the equilibrium between the acid and ester (Figure S2 in the ESI†).
| Compounds | Reaction temperatures (°C) | ||||
|---|---|---|---|---|---|
| RT | 135 | 150 | 165 | 180 | |
a Experimental conditions: bio-oil/methanol mass ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4; catalyst loading: 13.3 wt%; stirring rate = 600 rpm; residence time at the given reaction temperatures = 180 min. “RT” means the reaction mixture at room temperature.
b The data for methyl α-D-glucopyranoside (MGP), levulinic acid, and methyl levulinate means their concentrations in bio-oil (wt%), while the data for the others are their conversion (%) versus reaction temperatures. 4; catalyst loading: 13.3 wt%; stirring rate = 600 rpm; residence time at the given reaction temperatures = 180 min. “RT” means the reaction mixture at room temperature.
b The data for methyl α-D-glucopyranoside (MGP), levulinic acid, and methyl levulinate means their concentrations in bio-oil (wt%), while the data for the others are their conversion (%) versus reaction temperatures.
|
|||||
| Levoglucosan | 0 | 95.1 | 94.7 | 97.8 | 100 |
| Anhydro-d-mannosan | 0 | 100 | 100 | 100 | 100 |
| Dianhydro-d-glucopyranose | 0 | 100 | 100 | 100 | 100 |
| HMF | 0 | 91.4 | 82.1 | 84.8 | 100 |
| 2-Furanmethanol | 0 | 100 | 100 | 100 | 100 |
| MGPb | 0 | 3.7 | 2.5 | 0.1 | 0 |
| Levulinic acidb | 0.1 | 0.1 | 0.4 | 1.3 | 2.9 |
| Methyl levulinateb | 0 | 0.9 | 1.9 | 4.7 | 4.0 |
| Guaiacol | 0 | 3.2 | 8.6 | 9.9 | 13.7 |
| Isocreosol | 0 | 14.9 | 32.0 | 35.6 | 40.9 |
| Hydroxyl aldehyde | 0 | 93.8 | 94.8 | 97.0 | 99.1 |
| Hydroxyl acetone | 0 | 35.2 | 65.3 | 81.1 | 82.8 |
| Acetic acid | 0 | 81.8 | 71.9 | 46.8 | 31.0 |
Some HMF was present in the raw bio-oil, and it also was converted into levulinic acid/ester during acid-treatment of the water-like phase of bio-oil. Other furans such as 2-furylmethanol also could be converted into levulinic acid/ester.17 The big sugar oligomers in bio-oil could decompose into the small sugars such as levoglucosan18 and hence contributed to levulinic acid/ester formation. Reaction network for levulinic acid/ester formation from the sugars and furans in the water-like phase bio-oil was summarized in Scheme 2. Depending on requirements, the sugars could be converted into methyl glucoside, the pharmaceutical intermediate19 at 135 °C, or levulinic acid/ester, the building blocks for chemical diversity20 at 165 °C.
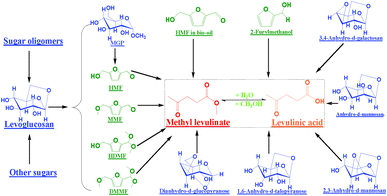 | ||
| Scheme 2 Reaction network for the formation of levulinic acid/ester from the sugars and furans in water-like phase of bio-oil. MMF: 5-(methoxymethyl)-2-furancarboxadhyde; HDMF: 5-(hydroxymethyl)-2-(dimethoxymethyl)furan; DMMF: 2-(dimethoxymethyl)-5-(methoxymethyl)furan. | ||
Some aromatics in the water-like phase of bio-oil such as guaiacol were not converted much while others such as isocreosol were converted to some extent (Table 2). Their reaction pathways in the methanol-rich medium is not clear. The presence of the remaining aromatics in the water-like phase of bio-oil is undesirable as they would interfere with the following separation of levulinic acid/ester, which will be discussed later. The sugar derivatives such as hydroxyl aldehyde were converted into 1,1,2-trimethoxyl ethane with 1,1-dimethoxyl ethanol formed as the intermediate at 135 °C (Figure S3, ESI†). Hydroxyl acetone was converted into methoxyacetone (Figure S4, ESI†), while acetic acid was converted into methyl acetate. These ethers, acetals and esters could be fuel additives or starting materials for fine chemicals.
The effects of methanol/bio-oil mass ratio on sugar conversion are presented in Figure S5, ESI.† Hydrolysis and methanolysis reactions occurred in parallel in the acid-treatment. The higher water content favored the hydrolysis reactions, producing more levulinic acid. The higher methanol ratio content favored the methanolysis reactions, producing more MGP, the ether of HMF, and methyl levulinate.
The sum of levulinic acid and methyl levulinate produced (Fig. 1a) was remarkably lower at lower methanol ratios, which was caused by polymerization reactions. The solid polymer formed upon catalysis was shown in Fig. 1b. The polymer was found even at 135 °C and increased at the elevated temperatures and was significant at the low methanol ratios such as at the bio-oil/methanol mass ratio of 1 in Fig. 1b. Sugars and HMF have a high tendency towards polymerization in aqueous medium in the presence of an acid catalyst.21 The conversion of sugars to MGP and HMF to its ethers and acetals could suppress the polymerization.10 The abundance of MGP and MMF were much lower at the low methanol ratios (Figure S5, ESI†), leading to the formation of more polymers.
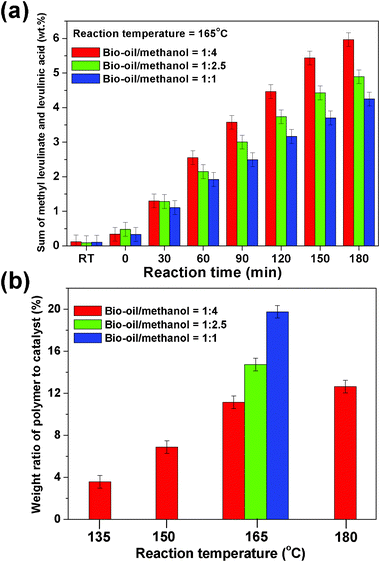 | ||
| Fig. 1 (a) Sum concentrations of methyl levulinate and levulinic acid versus bio-oil/methanol mass ratios and reaction time at 165 °C. Catalyst loading: 13.3 wt%; stirring rate = 600 rpm. “RT” in x-axis means the reaction mixture at room temperature. “0 min” means that the temperature just reached 165 °C. (b) Formation of polymer versus reaction temperatures and bio-oil/methanol mass ratios. | ||
In the acid-treatment of the water-like phase of bio-oil, the polymer came from not only sugars but also sugar derivatives. At the low methanol ratios, the conversions of hydroxyl acetone and hydroxyl aldehyde were similar to that at the high methanol ratios (Figure S6 and S7, ESI†), but their methanolysis products formed were much less at the low methanol ratios. The acid-treatments of hydroxyl aldehydes and hydroxyl acetone in water showed that they polymerized to heavy compounds. These sugar derivatives contain active α-H, hydroxyl, and carbonyl groups, making them easy to polymerize with themselves, or with the intermediates from sugars via an electrophilic reaction and/or aldol condensation reaction.
Selective production of chemicals from bio-oil is half the work to obtain chemicals from bio-oil while separation of them from the complex bio-oil mixture is the another half. After the acid-treatment in methanol, the product mixture contains the desirable products (methyl levulinate, levulinic acid, light esters, or MGP) and the undesirable ones (aromatics and others). Via distillation it was difficult to separate them effectively as their boiling points are close. Extraction is a potential way to obtain the specific chemical as polarities of the chemicals are distinct. To choose a solvent that can selectively extract methyl levulinate and levulinic acid, a water solution containing the targeted compounds and others were prepared and extracted with twelve solvents.
As is shown in Table 3 (Table S2 and S3 showing detailed results, ESI†), dichloromethane and chloroform showed the best capacity to extract levulinic esters from water but with a low affinity for levulinic acid, which provides a potential way to separate the levulinic esters from levulinic acid. In addition, these two solvents also showed minimal affinity for the polar levoglucosan and MGP. The similar polarity was supposed to be the main reason for the high efficiency of CH2Cl2/CHCl3 to extract levulinic esters. However, the polarity of CHCl3 was 4.1, which is not far away from that of 1-butanol (3.9) and ethyl acetate (4.4), but the latter two showed much lower affinity for methyl levulinate. The underlying reason is not yet known. CHCl3 is very volatile (boiling point: 61 °C), which can be easily separated from methyl levulinate.
| Compounds | Extraction percentage in various solvents (%) | |||
|---|---|---|---|---|
| Petroleum ether | Ethyl Acetate | Dichloromethane | Chloroform | |
| a To extract the compounds, a water solution containing the chemicals with the concentration of 1 wt% each were prepared and were extracted with the solvents with an equal volume ratio. The numbers in the Table means percentages of the compounds extracted into the solvents. | ||||
| Methyl levulinate | 17.6 | 79.7 | 95.0 | 96.4 |
| Ethyl levulinate | 39.4 | 90.2 | 98.1 | 98.9 |
| Levulinic acid | 0.7 | 43.0 | 19.0 | 14.9 |
| Guaiacol | 59.7 | 96.7 | 97.3 | 97.2 |
| Phenol | 40.1 | 97.3 | 86.6 | 82.9 |
| Eugenol | 92.6 | 99.5 | 99.7 | 99.7 |
| Vanillin | 12.0 | 94.5 | 94.0 | 94.7 |
| Levoglucosan | 0.0 | 0.0 | 0.0 | 0.0 |
| MGP | 0.0 | 0.0 | 0.0 | 0.0 |
Although CH2Cl2/CHCl3 showed high affinity to extract levulinic esters, they also showed a similar affinity for the aromatics (Table 3). Some aromatics remained in the water-like phase of bio-oil after the water extraction (Table 1), which have similar boiling points to levulinic acid/esters and thus have to be removed to avoid their interference in the followed separation. From Table 3 it was known that the aromatics were highly soluble in CH2Cl2/CHCl3 but the sugars were not. Thus, it was possible to extract the aromatics for the water-like phase of bio-oil with CH2Cl2/CHCl3. CHCl3 was used to extract the water-like phase of bio-oil. The distribution of typical compounds in the upper water-like phase and lower CHCl3 phase are shown in Table 4. As expected, ca. 90% of the aromatics could be transferred into the CHCl3 phase. After water extraction, only ca. 20% of the aromatics remained in the water-like phase of bio-oil, which can be further removed via the CHCl3 extraction. Thus, the interference of aromatics could be minimized via water extraction followed by CHCl3 extraction of the water-like phase of bio-oil. In addition, the furans and the cyclopentenones also could be efficiently extracted to the CHCl3 phase (Table 4). These compounds contain the conjugated π bonds and are highly reactive to polymerization, the removal of which via the extraction contributed to the suppression of polymerization. The whole process for production/separation of the platform chemicals from bio-oil is summarized in Scheme 3.
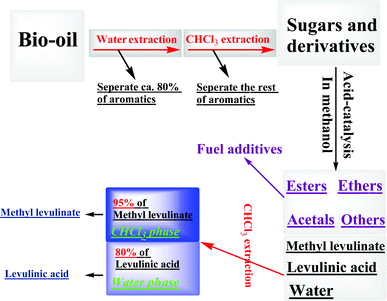 | ||
| Scheme 3 The production and separation of levulinic acid/ester via the liquid–liquid extraction coupled with acid catalysis. | ||
| Typical compounds in the water-like phase of bio-oil | Abundance distribution (%) | |
|---|---|---|
| In water phase | In CHCl3 phase | |
| a V water-like phase of bio-oil/VCHCl3 = 1. The mixture was shaken vigorously for 30 min and then processed with ultrasonic irradiation for another 30 min and then kept still at room temperature for 12 h for analysis. | ||
| Levoglucosan | 97.0 | 3.0 |
| Eugenol | 4.0 | 96.0 |
| 2-Methoxy-4-methyl-phenol | 4.8 | 95.2 |
| Acetoguaiacone | 11.4 | 88.6 |
| Furfural | 21.2 | 78.8 |
| 5-Methyl-2(5H)-furanone | 15.9 | 84.1 |
| 2-Methyl-2-cyclopenten-1-one | 12.9 | 87.1 |
| Hydroxyl acetone | 80.3 | 19.7 |
| Hydroxyl aldehyde | 97.5 | 2.5 |
Conclusions
Selective production of value-added chemicals and the following separation are the keys to utilize bio-oil as a feedstock for chemical production. Extraction of bio-oil with water followed by CH2Cl2/CHCl3 could effectively separate sugars from aromatics. Further acid-treatment of the sugars converted them into the value-added building blocks, levulinic acid/ester. The levulinic acid and methyl levulinate could be further separated via the extraction with CH2Cl2 or CHCl3. The transformation/separation process developed here make the “troublesome” sugars in bio-oil become a valuable feedstock for platform chemicals. In addition, separation of aromatics from bio-oil opens another gate to utilize the aromatics in bio-oil as a feedstock for fine chemicals or fuels.References
- (a) G. W. Huber and A. Corma, Angew. Chem., Int. Ed., 2007, 46, 7184 CrossRef CAS; (b) W. Li, C. Pan, Q. Zhang, Z. Liu, J. Peng, P. Chen, H. Lou and X. Zheng, Bioresour. Technol., 2011, 102, 4884 CrossRef CAS; (c) X.-L. Du, Q.-Y. Bi, Y.-M. Liu, Y. Cao and K.-N. Fan, ChemSusChem, 2011, 4, 1838 CrossRef CAS; (d) M. G. Al-Shaal, W. R. H. Wright and R. Palkovits, Green Chem., 2012, 14, 1260 RSC; (e) M. J. Climent, A. Corma and S. Iborra, Green Chem., 2011, 13, 520 RSC; (f) S. Dee and A. T. Bell, Green Chem., 2011, 13, 1467 RSC.
- (a) D. Mohan, C. U. Pittman Jr. and P. H. Steele, Energy Fuels, 2006, 20, 848 CrossRef CAS; (b) G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044 CrossRef CAS; (c) J. Nie, H. Chen, Q. Song, B. Liao and Q. Guo, Energy Fuels, 2010, 24, 5722 CrossRef CAS.
- (a) T. R. Carlson, G. A. Tompsett, W. C. Conner and G. W. Huber, Top. Catal., 2009, 52, 241 CrossRef CAS; (b) D. C. Elliott, T. R. Hart, G. G. Neuenschwander, L. J. Rotness and A. H. Zacher, Environ. Prog. Sustainable Energy, 2009, 28, 441 CrossRef CAS.
- (a) X. Jiang and N. Ellis, Energy Fuels, 2010, 24, 2699 CrossRef CAS; (b) O. D. Mante and F. A. Agblevor, Waste Manage., 2012, 32, 67 CrossRef CAS; (c) R. N. Hilten and K. C. Das, Fuel, 2010, 89, 2741 CrossRef CAS.
- M. G. Clerici, Appl. Catal., 1991, 68, 249 CrossRef CAS.
- (a) S. Czernik and A. V. Bridgewater, Energy Fuels, 2004, 18, 590 CrossRef CAS; (b) S. Miao and B. H. Shanks, Appl. Catal., A, 2009, 359, 113 CrossRef CAS.
- J. Zakzeski, P. C. A. Bruijnincx, A. L. Jongerius and B. M. Weckhuysen, Chem. Rev., 2010, 110, 3552 CrossRef CAS.
- (a) D. C. Elliott, T. R. Hart, G. G. Neuenschwander, L. J. Rotness and A. H. Zacher, Environ. Prog. Sustainable Energy, 2009, 28, 441 CrossRef CAS; (b) A. Oasmaa, E. Kuoppala, A. Ardiyanti, R. H. Venderbosch and H. J. Heeres, Energy Fuels, 2010, 24, 5264 CrossRef CAS.
- X. Hu, Y. Wang, D. Mourant, R. Gunawan, C. Lievens, W. Chaiwat, M. Gholizadeh, L. Wu, X. Li and C.-Z. Li, AIChE J., 2012 DOI:10.1002/aic.13857.
- X. Hu and C.-Z. Li, Green Chem., 2011, 13, 1676 RSC.
- G. Dobele, G. Rossinskaja, T. Dizhbite, G. Telysheva, D. Meier and O. Faix, J. Anal. Appl. Pyrolysis, 2005, 74, 401 CrossRef CAS.
- X. Li, R. Gunawan, C. Lievens, Y. Wang, D. Mourant, S. Wang, H. Wu, M. Garcia-Perez and C.-Z. Li, Fuel, 2011, 90, 2530 CrossRef CAS.
- N. M. Bennett, S. S. Helle and S. J. B. Duff, Bioresour. Technol., 2009, 100, 6059 CrossRef CAS.
- (a) G. Marcotullio and W. D. Jong, Green Chem., 2010, 12, 1739 RSC; (b) A. S. Diasa, S. Limaa, D. Carriazob, V. Rivesb, M. Pillingera and A. A. Valente, J. Catal., 2006, 244, 230 CrossRef; (c) D. M. Alonso, J. Q. Bond and J. A. Dumesic, Green Chem., 2010, 12, 1493 RSC.
- D. C. Elliott, Energy Fuels, 2007, 21, 1792 CrossRef CAS.
- A. Hull, I. Golubkov, B. Kronberg and J. Stam, Int. J. Engine Res., 2006, 7, 51 CrossRef CAS.
- J. Lange, W. D. Graaf and R. J. Haan, ChemSusChem, 2009, 2, 437 CrossRef CAS.
- R. Gunawan, X. Li, A. Larcher, X. Hu, D. Mourant, W. Chaiwat, H. Wu and C.-Z. Li, Fuel, 2012, 95, 146 CrossRef CAS.
- (a) W. Deng, M. Liu, Q. Zhang, X. Tan and Y. Wang, Chem. Commun., 2010, 46, 2668 RSC; (b) G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2007, 107, 2411 CrossRef.
- (a) M. Chia and J. A. Dumesic, Chem. Commun., 2011, 47, 12233 RSC; (b) A. Primo, P. Concepción and A. Corma, Chem. Commun., 2011, 47, 3613 RSC.
- (a) F. S. Asghari and H. Yoshida, Ind. Eng. Chem. Res., 2007, 46, 7703 CrossRef CAS; (b) A. Chuntanapum and Y. Matsumura, Ind. Eng. Chem. Res., 2010, 49, 4055 CrossRef CAS; (c) X. Qi, M. Watanabe, T. M. Aida and R. L. Smith Jr., Green Chem., 2008, 10, 799 RSC.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental details, Tables and Figures. See DOI: 10.1039/c2ra21597g |
| This journal is © The Royal Society of Chemistry 2012 |
