Facile synthesis of covalent organic frameworks COF-1 and COF-5 by sonochemical method†
Seung-Tae
Yang
,
Jun
Kim
,
Hye-Young
Cho
,
Sangho
Kim
and
Wha-Seung
Ahn
*
Department of Chemical Engineering, Inha University, Incheon, 402-751, Republic of Korea. E-mail: whasahn@inha.ac.kr; Fax: +82 32 872 0959; Tel: +82 32 860 7466
First published on 4th September 2012
Abstract
Covalent organic frameworks COF-1 and COF-5 crystals with excellent textural properties were obtained for the first time in a 0.5 L synthesis vessel within 1–2 h by a sonochemical route.
Covalent organic frameworks (COFs) are structures composed entirely of light elements (H, B, C, N, and O) through covalent bonding. These materials achieve atomically precise integration of their building blocks into a porous structure throughout their extended periodic networks in two or three dimensions.1–6 In addition, they exhibit many desirable properties including outstanding thermal stability, high specific surface area, and the lowest densities of any organic material7 with potential applications in gas storage,8 catalysis9 and optoelectronic devices.10
COFs are typically synthesized by a solvothermal reaction in a sealed Pyrex tube using an organic solvent (mixture of 1,4-dioxane and mesitylene) under vacuum conditions (0.15 mtorr). The sparing solubility of the starting material in this solvent system was reported to control the diffusion of the building blocks into solution and facilitate the nucleation of a crystalline material, whereas the use of a closed reaction system sustains the availability of H2O to maintain reversible conditions favourable for crystallite growth.11 Typically, crystallisation takes place slowly over a period of several days. An easier synthesis method is needed because the reported synthesis of COFs is cumbersome and limited to obtaining only small amounts of product. Recently, the microwave synthesis of COF-5 and COF-102 was reported.12 This method is faster than the solvothermal method with the COF crystals produced exhibiting comparable physical properties to materials prepared using the solvothermal route.
In the present study, a sonochemical method was applied to the synthesis of COF structures. Acceleration of the crystallisation rate in the sonochemical synthesis protocol stems from the formation and collapse of bubbles in solution, termed acoustic cavitation, which produces exceedingly high local temperatures (>5000 K) and pressures (>1000 bar), resulting in extremely fast heating and cooling rates.13 Sonochemical synthesis is economical and power consumption is quite low because no induction period is needed and the synthesis time is very short.14 COF-5, which is a mesoporous 2D framework of 1,4-benzene diboronic acid (BDBA) and 2,3,6,7,10,11-hexahydroxytriphenylene (HHTP), and COF-1, which is a 2D framework of planar six-membered B3O3 (boroxine) rings by molecular dehydration reaction of BDBA alone, were chosen to demonstrate the feasibility of the sonochemical synthesis of COF structures (see Scheme 1). The effects of various sonochemical synthesis parameters on their textural properties were examined systematically.
 | ||
| Scheme 1 COF-1 and COF-5 synthesis via sonochemical routes. | ||
COF-1 was prepared using BDBA in 40 mL of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v
1 v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v solution mixture of mesitylene and 1,4-dioxane. The substrate mixture was introduced to a custom-made horn-type Pyrex reactor fitted to a sonicator unit (VCX500, SONICS, USA) and reacted for 1 h (see ESI† for experimental details). As shown in Fig. S1 (see ESI†), sonication was quite effective in promoting the homogeneous mixing of sparingly soluble BDBA in the solvent mixture. According to the results listed in Table 1, the BDBA concentration and sonication power affected the textural properties of COF-1 significantly; the former due to the low solubility of BDBA and the latter affecting the synthesis temperature moderately (387 K and 392 K, respectively, under a 50 and 100% power level). The COF-1 sample of entry 1 in Table 1 was not optimized (see also Fig. 1(b) and Fig. S2(f)†) and showed impurities due to BDBA (Fig. 1(a) and Fig. S2(g)†). The optimized sample (entry 4), on the other hand, exhibited a pure COF-1 structure (see Fig. S2, ESI†) and demonstrated stable thermal behaviour up to 823 K (see Fig. S3, ESI†). As shown in Fig. 1(c), the COF-1 particle size was ca. 50 nm, which is approximately 400 times smaller than that of the conventionally prepared COF-1, with a BET surface area of 719 m2 g−1 (see Fig. S4, ESI†) that was comparable to the 711 m2 g−1 reported elsewhere.11
v solution mixture of mesitylene and 1,4-dioxane. The substrate mixture was introduced to a custom-made horn-type Pyrex reactor fitted to a sonicator unit (VCX500, SONICS, USA) and reacted for 1 h (see ESI† for experimental details). As shown in Fig. S1 (see ESI†), sonication was quite effective in promoting the homogeneous mixing of sparingly soluble BDBA in the solvent mixture. According to the results listed in Table 1, the BDBA concentration and sonication power affected the textural properties of COF-1 significantly; the former due to the low solubility of BDBA and the latter affecting the synthesis temperature moderately (387 K and 392 K, respectively, under a 50 and 100% power level). The COF-1 sample of entry 1 in Table 1 was not optimized (see also Fig. 1(b) and Fig. S2(f)†) and showed impurities due to BDBA (Fig. 1(a) and Fig. S2(g)†). The optimized sample (entry 4), on the other hand, exhibited a pure COF-1 structure (see Fig. S2, ESI†) and demonstrated stable thermal behaviour up to 823 K (see Fig. S3, ESI†). As shown in Fig. 1(c), the COF-1 particle size was ca. 50 nm, which is approximately 400 times smaller than that of the conventionally prepared COF-1, with a BET surface area of 719 m2 g−1 (see Fig. S4, ESI†) that was comparable to the 711 m2 g−1 reported elsewhere.11
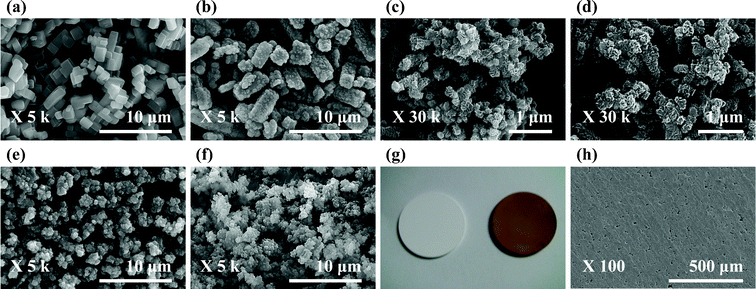 | ||
| Fig. 1 SEM and digital image of COF-1 and COF-5: (a) BDBA, (b) entry 1, (c) entry 4, (d) entry 5, (e) entry 8, (f) entry 14, (g) digital image of a COF-5 film (left: original alumina disc, right: COF-5-coated alumina disc), and (h) COF-5 film (top view). | ||
| Sample | Entry | BDBA (g) | HHTP (g) | BDBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HTTPa HTTPa |
Sonication power (%) | Time (h) | S BET (m2 g−1) | V total (cm3 g−1) |
|---|---|---|---|---|---|---|---|---|
| a Mole ratio of BDBA : HTTP used in the synthesis; b 400 mL batch (scale-up factor of 10); c 2 times the standard substrate amounts in weight. | ||||||||
| COF-1 | 1 | 1.00 | — | — | 50 | 1 | 391 | 0.27 |
| 2 | 1.00 | — | — | 100 | 1 | 439 | 0.26 | |
| 3 | 0.25 | — | — | 50 | 1 | 569 | 0.36 | |
| 4 | 0.25 | — | — | 100 | 1 | 719 | 0.50 | |
| 5b | 2.5 | — | — | 100 | 1 | 732 | 0.55 | |
| COF-5 | 6 | 0.177 | 0.242 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
40 | 1 | 1143 | 0.58 |
| 7 | 0.177 | 0.242 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
50 | 0.5 | 1098 | 0.59 | |
| 8 | 0.177 | 0.242 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
50 | 1 | 2122 | 1.38 | |
| 9 | 0.177 | 0.242 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
50 | 2 | 2092 | 1.09 | |
| 10c | 0.354 | 0.484 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
50 | 1 | 1268 | 0.54 | |
| 11 | 0.177 | 0.121 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50 | 1 | 842 | 0.39 | |
| 12 | 0.177 | 0.242 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
70 | 1 | 1145 | 0.55 | |
| 13b | 1.77 | 2.42 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
50 | 1 | 1427 | 0.88 | |
| 14b | 1.77 | 2.42 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
50 | 2 | 1948 | 1.32 | |
COF-5 was similarly prepared using BDBA and HHTP in 40 mL of the same solvent mixture. The sonication-promoted mixing of the substrates was clearly visible (Fig. S5†). As shown in Fig. S6 (see ESI†), all COF-5 samples obtained at different power levels (the reaction temperature remained practically constant at ca. 353 K) after 1 h sonication showed a good match with the XRD patterns of COF-5 reported previously,11 and there was no XRD peak dependence on the sonication power. Table 1 lists the corresponding surface area and pore volume data. The N2 adsorption–desorption isotherms in Fig. S7 (see ESI†) were of type IV and reversible, which showed similar isotherm shapes to that reported previously for COF-5, including the characteristic “double step” in the relative pressure range of P/P0 = 0–0.2. On the other hand, the BET surface areas were affected by the power level, and exhibited a maximum of 2122 m2 g−1 at 50% (entry 8). This is significantly higher than the value reported for the solvothermally prepared sample (1590 m2 g−1)11 and closer to the value reported recently using microwave heating (2019 m2 g−1).12 As shown in Fig. 1(e), the mean crystal size of COF-5 was ca. 250 nm, which was approximately 100 times smaller than the solvothermally-prepared sample.11 TGA confirmed the high thermal stability to ca. 893 K (see Fig. S8, ESI†). Both 40 and 70% power produced samples with a lower surface area of ca. 1145 m2 g−1. A close examination of the SEM images of these samples (not shown) did not indicate any trace of un-reacted starting material (BDBA) and exhibited disc-shaped particles in agglomeration. After fixing the sonication power level to 50%, the duration of the sonic treatment was varied from 0.5 to 2 h. Although the XRD patterns, SEM images and TGA data were similar, the BET surface areas increased with increasing sonication time; 1098 m2 g−1 and 2122 m2 g−1 after 30 and 60 min sonication, respectively, then levelled off at longer times (entries 7 and 9).
The concentration of BDBA and HHTP and their mole ratio in the substrate mixture is critical to obtaining a phase pure COF-5 sample. When the substrate concentrations were increased twice at the same BDBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HHTP molar ratio of 3
HHTP molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (entry 10), the surface area of the sample decreased to 1268 m2 g−1 and SEM revealed un-reacted rectangular BDBA (see Fig. S9, ESI†). Increases in either the synthesis time or temperature did not improve the phase purity of the COF-5. At BDBA
2 (entry 10), the surface area of the sample decreased to 1268 m2 g−1 and SEM revealed un-reacted rectangular BDBA (see Fig. S9, ESI†). Increases in either the synthesis time or temperature did not improve the phase purity of the COF-5. At BDBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HHTP = 3
HHTP = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the BET surface area of COF-5 was even lower (842 m2 g−1, entry 11). In the accelerated crystallisation scheme using sonochemistry, some fraction of BDBA can remain un-dissolved due to the short synthesis time. Furthermore, if the BDBA to HHTP mole ratio is changed from 3
1, the BET surface area of COF-5 was even lower (842 m2 g−1, entry 11). In the accelerated crystallisation scheme using sonochemistry, some fraction of BDBA can remain un-dissolved due to the short synthesis time. Furthermore, if the BDBA to HHTP mole ratio is changed from 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 3
2 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the BDBA actively involved in condensation with HHTP at one time would decrease due to the reduced number of HHTP molecules acting as nucleation cores (see Scheme 1).
1, the BDBA actively involved in condensation with HHTP at one time would decrease due to the reduced number of HHTP molecules acting as nucleation cores (see Scheme 1).
Ultrasonication has been used successfully to deposit a range of nanomaterials (metals, metal oxides, semiconductors)15 on the surfaces of fabrics,16 ceramics,17 and polymeric fibers.18 Interestingly, it was also possible to produce a COF-5 film onto an alumina surface using the sonochemical method (see ESI† for experimental details). According to Fig. 1(g) and 1(h), a dense and homogeneous COF-5 layer was deposited on the external surface of an alumina disc. COF-5 particles formed in the reaction vessel must be deposited onto the alumina surface via the microjets of the fluid formed after the collapse of the acoustic bubble near the solid surface, leading to the formation of a homogeneous layer composed of nanoparticles.15
The far smaller COF-5 crystals obtained by the sonochemical method would apparently have been useful for film formation as well.
The synthesis scale of COF-5 reported thus far has been limited to 1 to 40 mL batches,11,12 which severely limits the availability of the material for testing potential applications. Consequently, COF-5 and COF-1 synthesis was attempted in a 0.5 L-capacity beaker with a sonicator immersed in the middle of the substrate mixture solution and the top covered with aluminium foil as shown in Fig. S10† (scale-up by a factor of 10). Both COF-1 (within 1 h) and COF-5 (2 h) products could be prepared successfully with excellent structural and textural properties (entry 5 in Table 1, Fig. 1(d), Fig. S2(b),†and Fig. S4† for COF-1, and entry 14, Fig. 1(f), Fig. S6(a)†, and S7† for COF-5). Furthermore, the space-time yield (STY; kilograms of product per cubic meter of reaction mixture per day, kg m−3 day−1)19 of the COF-5 in entry 14 was 45 kg m−3 day−1, which was ca. 9 times higher than the conventional solvothermal synthesis.14
In summary, COF-1 and COF-5 in both powder and film form were prepared using a sonochemical method under atmospheric pressure conditions. The synthesis time for COF-1 and COF-5 was reduced substantially from 3 days to 1 h, and significantly smaller crystals (50–250 nm) were obtained. Their physicochemical properties were similar or somewhat superior to those of the COF samples prepared by conventional solvothermal heating in a vacuum.
Acknowledgements
This work was supported by the NRF grant funded by MEST (No. 2010-0012257) in Korea.References
- F. J. Uribe-Romo, J. R. Hunt, H. Furukawa, C. Klöck, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 4570 CrossRef CAS.
- (a) A. P. Côté, H. M. El-Kaderi, H. Furukawa, J. R. Hunt and O. M. Yaghi, J. Am. Chem. Soc., 2007, 129, 12914 CrossRef; (b) J. R. Hunt, C. J. Doonan, J. D. LeVangie, A. P. Côté and O. M. Yaghi, J. Am. Chem. Soc., 2008, 130, 11872 CrossRef CAS.
- S. Wan, J. Guo, J. Kim, H. Ihee and D. Jiang, Angew. Chem., Int. Ed., 2008, 47, 8826 CrossRef CAS.
- S. Wan, J. Guo, J. Kim, H. Ihee and D. Jiang, Angew. Chem., Int. Ed., 2009, 48, 5439 CrossRef CAS.
- X. Ding, J. Guo, X. Feng, Y. Honsho, J. Guo, S. Seki, P. Maitarad, A. Saeki, S. Nagase and D. Jiang, Angew. Chem., Int. Ed., 2011, 50, 1289 CrossRef CAS.
- S. Wan, F. Gándara, A. Asano, H. Furukawa, A. Saeki, S. K. Dey, L. Liao, M. W. Ambrogio, Y. Y. Botros, X. Duan, S. Seki, J. F. Stoddart and O. M. Yaghi, Chem. Mater., 2011, 23, 4094 CrossRef CAS.
- H. M. El-Kaderi, J. R. Hunt, J. L. Mendoza-Cortés, A. P. Côté, R. E. Taylor, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2007, 316, 268 CrossRef CAS.
- H. Furukawa and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 8875 CrossRef CAS.
- S. Y. Ding, J. Gao, Q. Wang, Y. Zhang, W. G. Song, C. Y. Su and W. Wang, J. Am. Chem. Soc., 2011, 133, 19816 CrossRef CAS.
- J. W. Colson, A. R. Woll, A. Mukherjee, M. P. Levendorf, E. L. Spitler, V. B. Shields, M. G. Spencer, J. Park and W. R. Dichtel, Science, 2011, 332, 228 CrossRef CAS.
- A. P. Côté, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166 CrossRef.
- (a) N. L. Campbell, R. Clowes, L. K. Ritchie and A. I. Cooper, Chem. Mater., 2009, 21, 204 CrossRef CAS; (b) L. K. Ritchie, A. Trewin, A. Reguera-Galan, T. Hasell and A. I. Cooper, Micropor. Mesopor. Mater., 2010, 132, 132 CrossRef CAS.
- (a) K. S. Suslick, D. A. Hammerton and R. E. Cline, J. Am. Chem. Soc., 1986, 108, 5641 CrossRef CAS; (b) S. T. Meek, J. A. Greathouse and M. D. Allendorf, Adv. Mater., 2011, 23, 249 CrossRef CAS; (c) N. Stock and S. Biswas, Chem. Rev., 2012, 112, 933 CrossRef CAS.
- W. J. Son, J. Kim, J. Kim and W. S. Ahn, Chem. Commun., 2008, 6336 RSC.
- (a) A. Gedanken, Ultrason. Sonochem., 2004, 11, 47 CrossRef CAS; (b) R. Gottesman, S. Shukla, N. Perkas, L. A. Solovyov, Y. Nitzan and A. Gedanken, Langmuir, 2011, 27, 720 CrossRef CAS.
- I. Perelshtein, G. Applerot, N. Perkas, E. Wehrschetz-Sigl, A. Hasmann, G. M. Guebitz and A. Gedanken, ACS Appl. Mater. Interfaces, 2009, 1, 361 CAS.
- Z. Zhong, Y. Mastai, Y. Koltypin, Y. Zhao and A. Gedanken, Chem. Mater., 1999, 11, 2350 CrossRef CAS.
- I. Perelshtein, G. Applerot, N. Perkas, G. Guibert, S. Mikhailov and A. Geddanken, Nanotechnology, 2008, 19, 245705 CrossRef.
- D. Farrusseng, Metal–Organic Frameworks – Applications from catalysis to gas storage, Wiley-VCH Verlag GmbH & Co. KGaA, 1st edn, 2011, ch. 14, pp. 344–345 Search PubMed.
Footnote |
| † Electronic Supplementary Information (ESI) available: Synthesis and characterization details. See DOI: 10.1039/c2ra21531d |
| This journal is © The Royal Society of Chemistry 2012 |
