Triazole: a new motif for anion recognition
V.
Haridas
*,
Srikanta
Sahu
,
P. P.
Praveen Kumar
and
Appa Rao
Sapala
Department of chemistry, Indian Institute of Technology (IIT), Hauz Khas, New Delhi, 110016. E-mail: h_haridas@hotmail.com; Tel: 01126591380
First published on 25th September 2012
Abstract
Anion receptors have attracted growing interest because of their role in chemistry, the environment, biology and medicine. The mis-regulation of anion flux causes a variety of lethal human diseases. Recently, triazole has been found to be an excellent motif for molecular recognition. This review depicts an overall picture of developments in the design and synthesis of anion receptors along with an up-to-date emphasis on the triazole unit as a motif for anion recognition. The acidic CH of triazole is involved in binding with the anions, which makes these receptors different from other classes of receptors. The chemo- and regio-selectivity of the click reaction provides further impetus for future developments in this area.
 V. Haridas | Dr V. Haridas is an Associate Professor in the Department of Chemistry at the Indian Institute of Technology Delhi (IITD), India. He finished his PhD under the guidance of Prof. D. Ranganathan (National Institute for Interdisciplinary Science and Technology, India). He did his post doctoral studies with Prof. R. M. Ghadiri (The Scripps Research Institute, USA) and with Prof. Herbert Waldmann (Max Planck Institute, Germany). His research group at IIT Delhi is involved in the design and synthesis of dendrimers, and secondary structure mimetics. In addition to that the group is also working on various aspects of synthetic and bioorganic chemistry. |
 Srikanta Sahu | Srikanta Sahu was born in Karkachia (Mayurbhanj), Orissa, India. He received his BSc from the North Orissa University, Orissa, India, in 2002 and completed his MSc in Organic Chemistry in 2004, from Ravenshaw Autonomous College (Utkal University), Orissa, India, in 2004. He qualified the National Eligibility Test (NET), jointly conducted by CSIR & UGC (New Delhi, Govt. of India), the Graduate Aptitude Test Examination conducted by IITs, India, and continued his PhD under the supervision of Professor V. Haridas at Indian Institute of Technology Delhi (IITD), India. |
 Praveen Kumar P. P | Mr. Praveen Kumar P. P was born in 1987 in Kerala, India. He received his Masters degree in synthetic organic chemistry from Christ College affiliated to Calicut University, Kerala, in 2009. In the same year he was honoured with CSIR–JRF, and GATE. Presently he is a PhD student in the department of chemistry, IIT Delhi, under the guidance of Dr V. Haridas. He is working on the design and synthesis of molecular receptors for various anions and cations. |
 Appa Rao Sapala | Appa Rao Sapala was born in Rajahmundry, Andhra Pradesh, India (1984), received his bachelor's degree (2005) from the same place and MSc degree (2007) in Chemistry from Gitam College, Andhra University, Visakhapatnam. He had 3 years experience in G. V. K Bio Pvt. ltd, Hyderabad. He qualified CSIR–JRF and is currently pursuing his PhD degree at IIT Delhi under the guidance of Dr V. Haridas. His research interests are in the area of peptide design, synthesis and their applications. |
Introduction
Molecular recognition has attracted considerable interest in recent years because it is integral in many scientific areas, such as biology, chemistry, and pharmacology.1 Molecular recognition of cations,2 anions,3 and neutral molecules4 are important in biology and chemistry. Although rigorous attention has been paid to the recognition of cations, recognition of anions has received less attention.5 Anions are everywhere in living systems and are vital in carrying out many biochemical operations for sustaining life. Anions such as chloride, phosphate, and sulfate regulate the flux of key metabolites into and out of cells while maintaining osmotic balance.6 Among all anions, anion receptors specific to chloride and fluoride have attracted growing interest because of their role in chemistry, biology and medicine.7 The mis-regulation of chloride ion flux causes severe human diseases, such as cystic fibrosis,8 myotonia,9 and epilepsy.10 Accumulation of excess fluoride ions in living organisms causes collagen breakdown, bone disorder, impact on the immune system and thyroid activity.11 Oxalate, arsenate, and nitrite can produce chronic diseases.12 Naturally occurring phosphate and sulfate can cause renal failure for patients, due to poor catabolic activity.13In recent years, increasing attention has been devoted to the synthesis of receptors for recognition of anions. Serious efforts have been paid since the beginning of anion co-ordination chemistry in the late 1960s for the development of synthetic receptors for recognition of anions. However, the major binding motifs that have been utilized until now are as follows: cationic polyammonium, quaternary ammonium, amide, urea, thiourea, guanidinium, pyrrole, imidazolium and boron containing receptors (Fig. 1).14
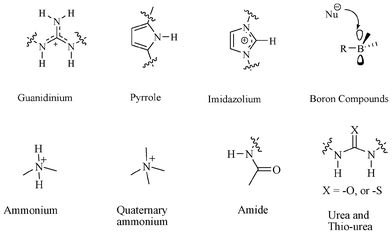 | ||
| Fig. 1 Commonly used motifs for anion receptors. | ||
These motifs have been utilized for the construction of receptors for anions with varying selectivity. A preliminary discussion about these receptors is given below. The discovery of novel motifs for the recognition of anions is a rate limiting step in the area of anion recognition research. In the latter part of this review, we provide a brief history, detailed analysis of the origin of triazole as an anion recognition motif, and the recent developments in this area using triazole as an anion recognition motif. Most of the examples of the receptors presented here are designed for use in organic solvents.
1.1 Polyammonium-based receptors
The first synthetic anion receptor (Fig. 2) was reported by Park and Simons in 1968.15 These macrocyclic compounds, consisting of two bridgehead ammonium units, showed binding behaviour towards halide ions. However, the [8.8.8] and [10.10.10]-bridged compounds 1 and 3 showed no appreciable binding behavior towards halide ions; while the analogous [9.9.9]-bridged compound 2 binds chloride ions with an affinity of 102 M−1, with modest selectivity for chloride over bromide by a factor of eight.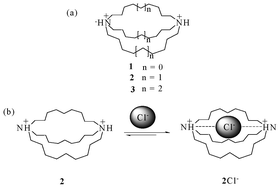 | ||
| Fig. 2 (a) Ammonium receptors 1, 2, and 3 (b) complex of receptor 2 with Cl− ion. | ||
Bowman-James et al. synthesized various bicyclic azacryptands (Fig. 3) containing ammonium units, for binding of anions.16 Crystal structure analysis of 4 showed the encapsulation of a single F− ion with a water molecule inside the cavity. Whereas the bicyclic azacryptand 5, having a bigger cavity than 4, accommodated two F−s with a water molecule inside the cavity. The water molecule acts as bridge between the two fluoride ions and thus generates an anion-based cascade complex.
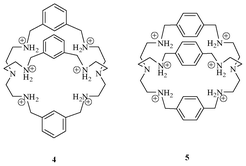 | ||
| Fig. 3 Azacryptands 4 and 5 for F−. | ||
1.2 Quaternary ammonium-based receptors
Schmidtchen et al. first reported a series of quaternary ammonium-based receptors (Fig. 4) for the recognition of anions.17 The most attractive feature in such receptors is the utilization of electrostatic interaction for the recognition of anions. Receptor 6 contains four tetra-alkylammonium-bridged centres, which are connected through (CH2)6 alkyl linkers. Crystal structure analysis of this receptor with iodide shows that it encapsulates iodide into its tetrahedral cage by utilizing the electrostatic interactions. The receptor 7 binds to p-nitrophenolate as a result of having a higher cavity size than receptor 6.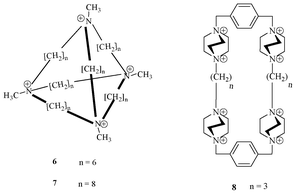 | ||
| Fig. 4 Quaternary ammonium receptors 6 and 7 that bind I− electrostatically. Quaternary ammonium receptor 8 for ATP. | ||
Menger et al. designed and synthesized a new class of quaternary ammonium-based receptors (Fig. 4) for recognition of anions.18 Receptor 8 showed binding with various anions: benzene sulphonate, naphthalene-2-sulfonate and naphthalene-2,7-disulphonate in aqueous solution. It also binds to ATP very strongly with an association constant of 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 M−1.
300 M−1.
1.3 Amide-based receptors
Pascal et al. first reported abiotic amide-based receptor (Fig. 5) for recognition of anions by utilizing amide –NHs as hydrogen-bonding donor groups.19 The crystal structure analysis of cyclophane 9 offers a cylindrical cavity of approximately 4 Å in length and 3 Å in diameter, due to the presence of two facial aromatic rings that are connected through three bridging arms. The crystal structure revealed that three amide–NHs are inclined by 47, 54, and 68° to radii drawn from the central axis through the nitrogen atoms.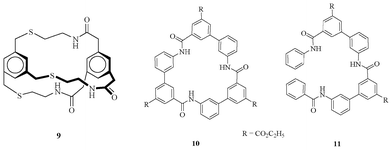 | ||
| Fig. 5 Amide receptors 9–11. | ||
Choi and Hamilton reported a series of amide-containing rigid macrocycles and acyclic compounds (Fig. 5).20 These compounds bind to various anions as follows: I−, Cl−, NO3−, pTsO−, HSO4−, and H2PO4− with varying ability. Binding studies showed that macrocycle 10 is a better binder towards anions than the acyclic 11. The receptor 10 showed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding with a tosylate anion, whereas 11 binds to I−, Cl−, and NO3− in 2
1 binding with a tosylate anion, whereas 11 binds to I−, Cl−, and NO3− in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry.
1 stoichiometry.
Bowman-James et al. designed and synthesized a polyamide-based cryptand (Fig. 6) to investigate the binding behaviour for various anions.21 The receptor 12 showed promising binding behaviour for the fluoride ion in DMSO-d6 with a log K value of 5.0 followed by Cl− (log K = 3.47), CH3COO− (log K = 3.38), H2PO4− (log K = 3.30), NO3− (log K = 1.93), HSO4− (log K = 1.83), and Br− (log K = 1.6) with the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes.
1 complexes.
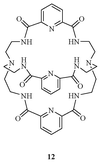 | ||
| Fig. 6 Polyamide cryptand receptor 12 for F−. | ||
1.4 Urea and thiourea-based receptors
Wilcox et al. reported urea and thiourea-based synthetic receptors for recognition of various oxo-anions.22 The binding behavior of receptor 13 (Fig. 7) with various oxo-anions a–d in chloroform was examined by UV/vis titration experiments. The thiourea-based receptor 14 is a better binder than 13 for oxo-anions a–d.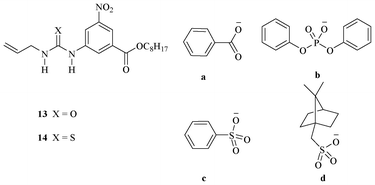 | ||
| Fig. 7 Urea and thiourea-based receptors 13 and 14. | ||
Reinhoudt et al. designed and synthesized various urea- and thiourea-based molecules (Fig. 8) for recognizing anions.23 The cleft-like acyclic urea-based receptor 15 binds to H2PO4−, in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry with an association constant of 5 × 107 M−1. It showed very poor binding affinity towards Cl−, Br−, NO3−, and HSO4−. The thiourea-based receptor 16 binds similarly to 15. The rigid macrocycles 17 and 18 offered a 1
1 stoichiometry with an association constant of 5 × 107 M−1. It showed very poor binding affinity towards Cl−, Br−, NO3−, and HSO4−. The thiourea-based receptor 16 binds similarly to 15. The rigid macrocycles 17 and 18 offered a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry toward H2PO4− with association constants of 4 × 103 and 2.5 × 103 M−1, respectively.
1 binding stoichiometry toward H2PO4− with association constants of 4 × 103 and 2.5 × 103 M−1, respectively.
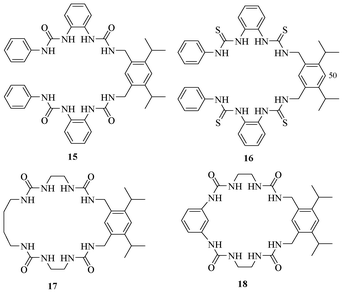 | ||
| Fig. 8 Urea and thiourea-based receptors 15–18 for recognition of H2PO4−. | ||
Gale et al. proposed an acyclic, urea-based receptor 19 (Fig. 9) to acknowledge anions.24 The bis-urea containing receptor 19 binds to the acetate ion more selectively with a binding constant of 3210 M−1 over Cl−, Br−, H2PO4−, and HSO4−. The receptor 19 binds to acetate more strongly than receptors 20 and 21.
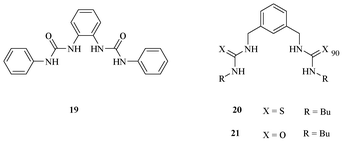 | ||
| Fig. 9 Urea receptors 19–21 for acetate. | ||
1.5 Guanidinium-based receptors
Since the beginning of anion co-ordination chemistry, the guanidinium moiety was used for design of anion receptors, as the guanidinium moiety has two key major features for the creation of abiotic anion receptors: (1) its high basic nature, which helps in sustaining a wide pH range; (2) its ability to donate two parallel hydrogens for hydrogen bond donors. Because of these unique features, guanidinium-based receptors show high affinities and selectivities for oxyanions. Taking advantage of this moiety, Lehn et al. reported a series of novel guanidinium-based cyclophanes (Fig. 10).25 The binding studies of these macrocycles 22, 23, and 24 showed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding with trianionic phosphate (PO43−) in methanol–water (9
1 binding with trianionic phosphate (PO43−) in methanol–water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution with log Ka = 3.1, 3.4, and 4.3 respectively.
1) solution with log Ka = 3.1, 3.4, and 4.3 respectively.
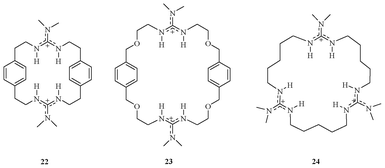 | ||
| Fig. 10 Guanidinium receptors 22–24 for PO43−. | ||
 | ||
| Fig. 11 Guanidinium receptor 25 for the selective recognition of thymidine-5′-phosphate e. 26 for the selective recognition of tartrate. | ||
Schmidtchen designed and synthesized a bis-guanidinium-based acyclic molecule (Fig. 11) for the binding of anions.26 The two-guanidinium moieties in 25 converged in the presence of tetrahedral anions, such as thymidine-5′-phosphate (e) and thus provided a suitable geometry for binding. The NMR titration experiment showed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex of 25 with e, having a binding constant of 106 M−1 in water. The compound 25 displayed no binding for simple HPO42− anions.
1 complex of 25 with e, having a binding constant of 106 M−1 in water. The compound 25 displayed no binding for simple HPO42− anions.
Lavigne and Anslyn reported on a guanidinium-based receptor (Fig. 11) for detecting tartrate anions.27 The receptor 26, containing two guanidinium moieties, provides a suitable geometry with the correct cavity size for binding of tartrate. It also responded to other analytes: ascorbate, L-malate, succinate, lactate, and sugars. It binds strongly to tartrate as compared to other analytes in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry and with a binding constant of 5.5 × 104 M−1 towards tartrate.
1 stoichiometry and with a binding constant of 5.5 × 104 M−1 towards tartrate.
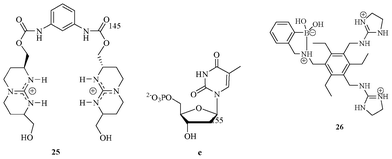
1.6 Pyrrole-based receptors
The first pentapyrrolic macrocyclic receptor (Fig. 12) was reported by Sessler et al. in the late 1990s.28 The crystal structure analysis showed the complex as a diprotonated macrocycle with fluoride residing inside the cavity. Solution phase studies showed that the diprotonated sapphyrin (expanded porphyrin containing five pyrrole units) 27 binds to fluoride 103 fold more than chloride and bromide. This finding opened up a new direction for the exploration of anion co-ordination chemistry of pyrrole-containing systems.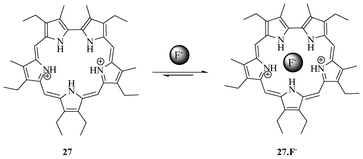 | ||
| Fig. 12 Sapphyrin receptor 27 for F−. | ||
A new type of calixpyrrole (Fig. 13) was synthesized for binding towards anions.29 The crystal structure analysis of 28 showed a wing-like architecture with a benzoate ion between the two wings. It showed a strong affinity for acetate in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry with binding constant value of 229
1 stoichiometry with binding constant value of 229![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1.
000 M−1.
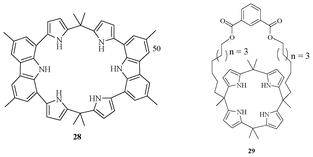 | ||
| Fig. 13 Pyrrole-based receptor 28, 29 with controlled cavity size for anions. | ||
Lee et al. designed and synthesized a new class of calix[4]pyrrole containing a flexible strap on one side of the molecule (Fig. 13) for controlling the cavity for better selectivity and affinity toward various anions.30 The receptor 29 showed significant binding behaviour for fluoride and chloride. It showed better binding ability for fluoride and chloride than the simple calix[4]pyrrole moiety. However, it does not show any appreciable binding ability with bromide, iodide, sulphate, and phosphate. The smaller cavity of these molecules disfavoured the accommodation of larger anions for binding.
1.7 Imidazolium-based receptors
Welton et al. designed and synthesized imidazolium-based (Fig. 14) compound 30.31 The 1H NMR study of 30 with halide ions (Cl−, Br−, and I−) showed significant interaction to the C2H group of imidazolium located between the two nitrogen atoms. This result demonstrated that the imidazolium –CH could be exploited for binding towards halides. Later, Sato et al. investigated the binding event of 31 with halide ions (Cl−, Br−, and I−).32 The binding constant values of 31 with chloride, bromide, and iodide ions were 78, 59, and 29 M−1, respectively. Taking the structural benefits of the imidazolium moiety, Sato et al. designed and synthesized receptors 32–34. These receptors showed better binding ability for halides than mono-imidazolium 31.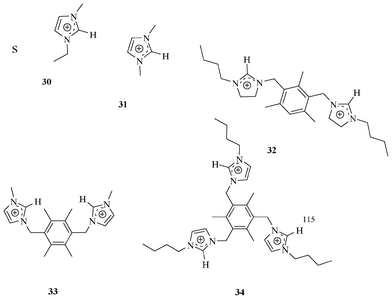 | ||
| Fig. 14 Imidazolium-based receptors 30–34. | ||
Kim et al. reported a cyclic imidazolium receptor 35 (Fig. 15) and discussed its binding behaviour toward various anions.331H NMR studies showed that receptor 35 binds to fluoride more strongly than to other anions, such as chloride, bromide, iodide, and hydrogen sulphate ions. Crystal structure analysis, as well as Job's plot, supported the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex of 35 with a fluoride ion, whereas for other anions, it was a 1
1 complex of 35 with a fluoride ion, whereas for other anions, it was a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding stoichiometry. The binding constant value of 35 with the fluoride ion was found to be 28
2 binding stoichiometry. The binding constant value of 35 with the fluoride ion was found to be 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 M−1.
900 M−1.
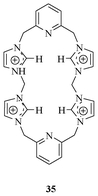 | ||
| Fig. 15 Cyclic imidazolium-based receptor 35 for the selective recognition of F−. | ||
1.8 Boron-based receptors
Boron in the trisubstituted state with sp2 hybridization has a vacant p orbital that can easily accommodate nucleophiles (Fig. 16) and therefore can act as good receptors for anions.34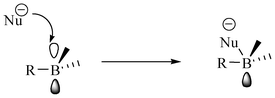 | ||
| Fig. 16 Coordination of boron with nucleophiles. | ||
In 1985, Katz studied the binding affinity of receptor 36 (Fig. 17) and utilizing 19F–1H, 19F–13C and 11B NMR, and found that the B-B distance becomes shorter after binding with F−.35a
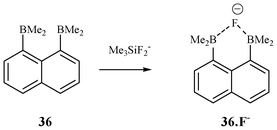 | ||
| Fig. 17 Boron-based receptor for F−. | ||
A mixed Lewis system (Fig. 18) containing boron and silicon centers on an o-phenylene backbone showed stronger binding toward F− than the monodentate boron analogues.35b
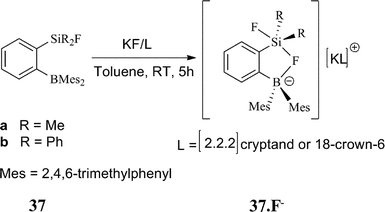 | ||
| Fig. 18 Mixed Lewis system for F− recognition. | ||
1.9 Triazole-based receptors
The examples illustrated above delineate some of the developments in the anion receptor design. The majority of anion binding motifs make use of strong hydrogen bond donor groups like NH and OH. The proton transfer and strong binding affinity are attributes of such moieties. The untapped potential of the non-conventional hydrogen bonding interaction could be exploited in the receptor design, in order to craft a truly reversible system with good binding affinity and selectivity. Therefore, novel motifs utilizing a variety of less explored non-covalent interactions for binding to the guest are much sought after. Design and synthesis of neutral receptor molecules for the anion are more difficult, because they mainly make use of the hydrogen bond, which is weaker than the coulombic interaction utilized in charged anion receptors. The maximum use of hydrogen bond donor units and the pre-organized cavity are the typical design criteria for neutral anion receptors. The triazole moiety is one of the recent examples that showed good promise for the design of various neutral receptors. The testimony to that is the fairly good number of receptors that appeared in the literature in a short period of time.The H-bonding ability of triazole CH could be modulated by substituents (R1 and R2) and thereby provide an additional benefit for making truly reversible systems. The high yield and chemoselective nature of the click reaction makes the introduction of triazole an easier task, and thus this reaction36 provides a better future for the design of receptors for neutral as well as charged guest molecules (Fig. 19).
 | ||
| Fig. 19 Synthesis of a triazole moiety and the interaction of the CH of triazole with an anion. | ||
In 2008, our group reported a neutral triazolophane that can bind to an acetonitrile molecule (Fig. 20).37 Compound 38 showed a unique type of binding with acetonitrile because of non-classical hydrogen-bonding interactions. The only available hydrogen bond donor in 38 was the CH of triazole, and the macrocycle could bind the acetonitrile molecule by the non-classical hydrogen bonds and CH⋯π interactions.
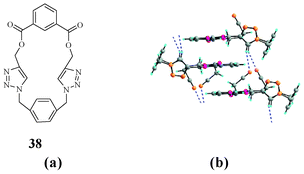 | ||
| Fig. 20 Structural representation (a) and acetonitrile mediated assembly in the solid state of triazolophane 38 (b). | ||
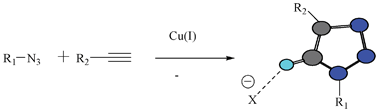
In the same year, Li and Flood designed and synthesized a series of shape-persistent preorganized triazolophanes (Fig. 21) by exploiting the click reaction for the recognition of anions.38 Macrocycle 39 binds the chloride ion with a high affinity and selectivity over all halide ions. This is due to its ideal cavity size with cumulative binding effects of all the triazole CHs, and the endocyclic benzene CHs, which are oriented inwards in the cavity. The triazolophane 39 showed a very strong binding affinity value (K = 1.1 × 107 M−1) with the chloride ion in dichloromethane.
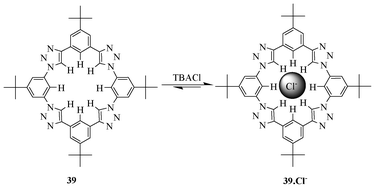 | ||
| Fig. 21 Shape-persistent preorganised triazolophane 39 binds Cl− selectively. | ||
A new class of triazolophanes (40 and 41) containing pyridine rings (Fig. 22) were synthesized.39 This generated a negative electrostatic potential due to the presence of a nitrogen lone pair in the pyridyl ring; thus, the cavity became oval, which favoured a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding towards various halide ions. The receptor 40 showed the highest binding towards I−, followed by Br− and F−. However, it shows a negative cooperative effect with the Cl− ion. The receptor 40 showed relative values of K1 and <3200 M−1 and >32
1 binding towards various halide ions. The receptor 40 showed the highest binding towards I−, followed by Br− and F−. However, it shows a negative cooperative effect with the Cl− ion. The receptor 40 showed relative values of K1 and <3200 M−1 and >32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1, respectively, with the iodide ion. These results clearly indicate that the presence of pyridyl units in the ring destabilizes the formation of 1
000 M−1, respectively, with the iodide ion. These results clearly indicate that the presence of pyridyl units in the ring destabilizes the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 triazolophane complexes due to N⋯X− electron pair repulsion; rather, it favours 2
1 triazolophane complexes due to N⋯X− electron pair repulsion; rather, it favours 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 sandwich complexes.
1 sandwich complexes.
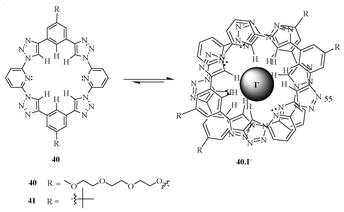 | ||
| Fig. 22 Pyridyl-containing triazolophanes 40 and 41. | ||
The receptor 42, which has two hydroxyl groups on the central phenylene ring, makes an intramolecular hydrogen bond with the N3 of the triazole ring, and thus brings about a preorganized structure (Fig. 23). Because of preorganisation, it binds the chloride ion with ∼50 fold greater affinity compared to non-preorganized pentad receptor 43.40
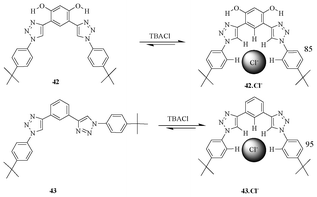 | ||
| Fig. 23 Preorganised vs. non-preorganised receptors 42 and 43. | ||
In 2008, Craig et al. demonstrated the ideal manipulation of weak CH interactions for synthesizing anion assisted foldamers (Fig. 24).41 The receptor 45, which contains four triazole moieties, shows better binding ability for the chloride ion due to the involvement of more hydrogen bond donors, compared to receptor 44. The result is the folding of 45 in the presence of the chloride ion and is confirmed by detailed 2D NOESY experiments. The titration of 45 with the chloride ion gives a binding constant of 1.7 × 104 M−1, which is higher than the receptor 44.
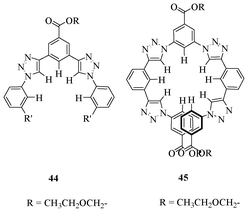 | ||
| Fig. 24 Acyclic triazole-based receptors 44 and 45. | ||
In 2008, Meudtner and Hecht demonstrated the design and synthesis of a novel class of triazole-based clickamers (Fig. 25), via the click reaction, and their folding behavior under various conditions.42 The clickamer 47, which contains two complete turns with a number of π–π stacking units, showed very insignificant folding behavior in acetonitrile. The population of the helical conformation was observed upon the addition of substantial amounts of water. The helicity with addition of water is due to the intramolecular chirality transfer from the chiral side chains to the backbone, which is evidenced from temperature dependent circular dichroism (CD) as well as dynamic light-scattering (DLS) and UV/Vis absorption spectroscopy studies. The shorter oligomer 46 also exists in a helical conformation. The foldamer 47 showed very unusual folding behavior toward various halides. The size of the halide ion plays a major role in helix inversion by transforming intramolecular chirality from the chiral side chain to the backbone, thus establishing an equilibrium between left- and right-handed helices.
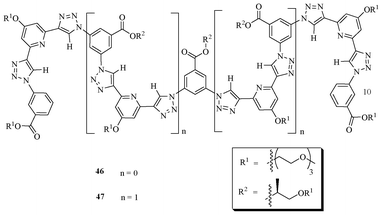 | ||
| Fig. 25 Triazole-based foldamers 46 and 47. | ||
Sanotoyo-Gonzalez et al. synthesized various calixarene based cavitands (48–50) using the click reaction. Interestingly, compound 50 showed binding affinities towards various anions (Fig. 26).43
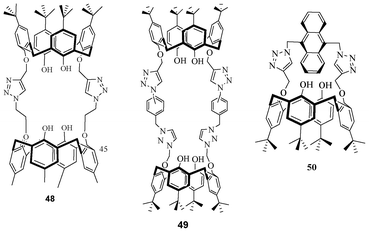 | ||
| Fig. 26 Calixarene-based cavitands. | ||
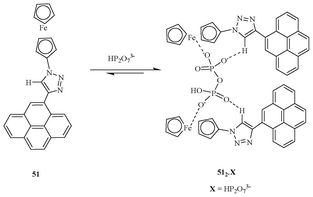
Molina et al. synthesized ferrocene-pyrene dyad 51 (Fig. 27) by coupling the terminal alkyne of pyrene with that of ferrocenyl azide via a click reaction.44 The receptor 51 displays a highly selective binding event for trianionic HP2O73− over various anions such as F−, Cl−, AcO−, NO3−, HSO4−, and H2PO4−. A fluorescence titration experiment of 51 with HP2O73− shows a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex formation.
1 complex formation.
Sessler et al. discussed a pyrrolyl-based triazolophane (Fig. 28), which displays highly selective binding affinity for the pyrophosphate anion, followed by HSO4−, H2PO4−, Cl− and Br−.45 The receptor 52 binds to trianionic pyrophosphate with a 10-fold greater affinity and selectivity as compared to hydrogen sulphate. However, the binding constant was found to be (2.30 ± 0.40) × 106 M−1 for pyrophosphate. The X-ray crystal structure analysis shows that all the pyrrole NH, triazole CH, and the endocyclic benzene CH protons are involved in stabilizing a pyrophosphate molecule in its cavity.
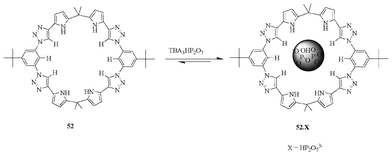 | ||
| Fig. 28 Pyrrole-based triazolophane 52 for the recognition of pyrophosphate. | ||
In 2012, Beer et al. synthesised Zn containing porphyrin-cages (Fig. 29) for the recognition of anions with the aid of click chemistry.46 The 1H NMR and UV/vis spectroscopic titration experiments showed that the receptor can bind with Cl− with a binding constant of 104 M−1 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry.
1 stoichiometry.
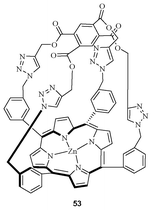 | ||
| Fig. 29 Porphyrin cages for anions. | ||
Jiang et al. demonstrated a light-induced triazole-based foldamer, containing a photoresponsive azo-benzene in between the two phenyl-triazole oligomer units (Fig. 30).47 The compound 54 adopts two conformations, 54trans and 54cis, with respect to azo-linkage. The 54cis isomer predominates upon irradiation of UV light; however, it binds anions more strongly than the 54trans conformer. This behavior is expected due to its scissor-like conformation, which results in assembling all the binding sites ideally for the recognition of ions. The 54trans conformer predominantly exists in the presence of visible light, and it binds weakly as compared to 54cis to various anions, due to the extended conformation of the azo-benzene core. The receptor 54cis binds the chloride ion strongly with a binding constant of 290 M−1, which is approximately a 4-fold excess compared to the 54cis conformer.
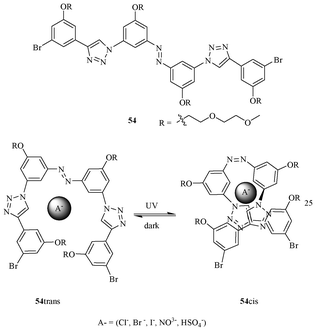 | ||
| Fig. 30 Photoswitchable receptor 54. | ||
In 2011, Kim synthesised a neutral ferrocene appended aryl triazole receptor 55 that can bind strongly with phosphate (Fig. 31).48 Ferrocene, being an electrochemical sensor, enabled detection of phosphate using cyclic voltammetry (CV) and differential pulse voltammetry (DPV). All of the triazole CHs, phenyl CH, and ferrocene CH, take part in binding with phosphate. These interactions induce a large shift in CV and DPV and thus act as an electrochemical sensor.
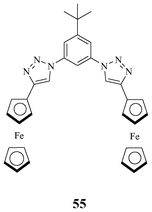 | ||
| Fig. 31 Ferrocene appended redox neutral receptor for H2PO4−. | ||
Hua and Flood addressed the photoisomerisation behavior leading to foldamer and its binding ability towards the chloride ion of a triazole-based azo-benzene molecule (Fig. 32).49 The compound 56 exists in three isomeric forms: 56trans–trans, 56cis–trans and 56cis–cis. Among them, the 56trans–trans isomer prefers the helical form under visible light (436 nm) and is more preorganised for chloride binding. Owing to its ideal arrangement of H–bonding donor sites, the 56trans–trans isomer binds chloride more strongly than 56cis–trans and 56cis–cis isomers. The binding constant value of receptor 56trans–trans with the chloride ion under dark conditions was found to be 3000 M−1.
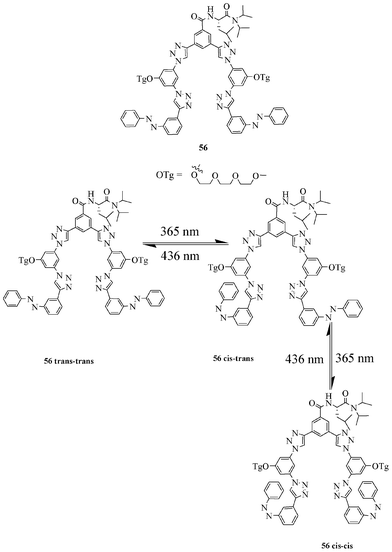 | ||
| Fig. 32 Photoswitchable triazole-based receptor. | ||
Jiang et al. investigated the anion-induced folding behavior with binding properties of novel oligo(phenyl-amide-triazoles) (Fig. 33) in great detail.50 The NMR titration experiments of chloride, bromide, and iodide ions (TBACl, TBABr and TBAI) with oligomer 57 showed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry, with association constants of 350, 80 and 15 M−1, respectively. However, the longer oligomers 58 and 59 showed 1
1 binding stoichiometry, with association constants of 350, 80 and 15 M−1, respectively. However, the longer oligomers 58 and 59 showed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes with both the chloride and bromide ions, and both of the oligomers bind to the chloride ion more strongly than to the bromide ion. Stepwise association constants for oligomer 57 with the chloride ion were found to be K1 = 4.9 × 103 M−1 and K2 = 13 M−1, indicating a negative cooperative effect for folding.
2 complexes with both the chloride and bromide ions, and both of the oligomers bind to the chloride ion more strongly than to the bromide ion. Stepwise association constants for oligomer 57 with the chloride ion were found to be K1 = 4.9 × 103 M−1 and K2 = 13 M−1, indicating a negative cooperative effect for folding.
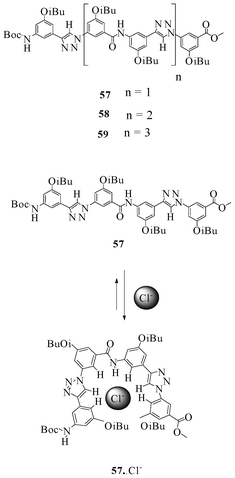 | ||
| Fig. 33 Oligo(phenyl-amide-triazoles) 57–59 and the chloride assisted folding of 57. | ||
Similar results were observed for oligomer 59 in the presence of the chloride ion, but it showed a better binding ability than oligomer 58.
Sanchez et al. described the self-assembly behavior of aryl triazole molecules (Fig. 34), with their anion binding properties leading to disruption of the molecular self-assembly due to the conformational changes in the molecules.51 Molecular self-assembly of aryl triazole 60 resulted in flat lamella-like architectures, while 61 was organized into spheres, which was confirmed by scanning electron microscopy (SEM) studies. NMR studies of 60 and 61 indicated the existence of “anti” conformations, which are switched over to “syn” conformations in the presence of bromide ions, resulting in disorder of the structural morphologies. The receptor 60 binds to bromide in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry with a binding constant of 15 M−1, which is higher than 61.
1 stoichiometry with a binding constant of 15 M−1, which is higher than 61.
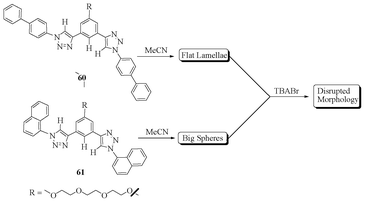 | ||
| Fig. 34 Molecular self-assembly and binding behaviour of 60 and 61. | ||
Our group successfully used triazole in conjunction with an amide unit as a excellent moiety for anion recognition (Fig. 35). Various receptor systems (63–67) were synthesised and validated for anion binding. Since the triazole moiety can mimic an amide bond, the triazole with amide could be compared with two peptide linkages.52
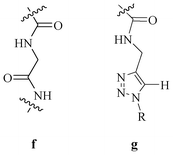 | ||
| Fig. 35 Comparison of amide-triazole and peptide linkages. | ||
Interestingly, the dialkyne precursor 62 showed less binding compared to the amide-triazole version, thus underscoring the usefulness of this moiety in anion recognition.53
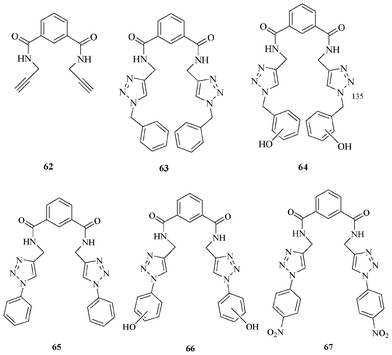 | ||
| Fig. 36 The dialkyne precursor 62 and triazole receptors 63–67 for anion binding. | ||
Increasing the acidity of triazole CH is another way to modulate the binding affinity. The introduction of phenyl substituents on the triazole rings showed higher binding ability compared to the benzyl substituents. Receptor 65 binds F− with a high binding constant (K ∼ 105 M−1). Receptor 67 showed a color change from pale yellow to orange upon adding F−.52
Various triazole based receptors (64 and 66) containing a phenolic group were designed and synthesized in order to provide extra binding sites for anions (Fig. 36). Interestingly, in most of the cases, proton exchange was observed between the F− and the phenolic –OH. All other anions showed less binding to phenolic receptors. These results further emphasize the challenge in anion receptor design.
Li et al. replaced one of the amide NH of urea with a triazole to generate various receptors (Fig. 37).54 The amide-triazole combines the characteristics of urea and triazole. The ease of synthesis, coupled with better solubility for the amide-triazole compounds compared to urea-based compounds, is a factor that enhances the utility of this moiety in the future.
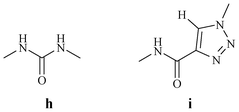 | ||
| Fig. 37 Comparison of urea and the amide-triazole moieties. | ||
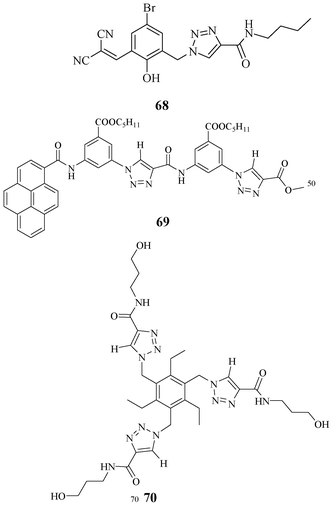
Various acyclic receptors were synthesized and they showed good binding affinities for tetrahedral oxyanions (Fig. 38). Receptor 68, containing OH, NH and CH motifs at the binding site, showed good colorimetric response in the presence of fluoride.55 Various acyclic receptors, such as 69 and 70, were synthesized, and they showed significant binding affinities for tetrahedral oxyanions.
Conclusion
The use of non-conventional hydrogen bonding makes the binding to anions weaker and more reversible and therefore useful for a variety of biological applications. The use of the CH⋯X− interaction is relatively new and is not much exploited in the anion receptor design. The moderate binding ability of CH⋯X− in isolation or in conjunction with other hydrogen bonding moieties will expand the repertoire of receptors. The last four years witnessed the utilization of the acidic –CH of triazole for non-conventional hydrogen bonding with guest molecules. Notably, many receptors were found to utilize non-conventional hydrogen bonding interactions that were unknown a few years back. The explosive developments in this area of molecular recognition using the triazole motif are expected to flourish further because of the clean and high yielding nature of this reaction, along with other attributes. A combination of conventional and non-conventional hydrogen bonding in the receptor design using this reaction will enhance the power of organic chemists in designing compounds with high binding and good release rates of guest molecules.Most of the anion receptors are designed for binding in organic solvents. The design and synthesis of receptors for binding anions in the aqueous environment is an additional challenge to chemists. The chemoselective nature of the click reaction will be a useful attribute for the synthesis of water soluble receptors.
Acknowledgements
We thank the Department of Science and Technology (DST), New Delhi, for financial assistance.References
- (a) R. E. Babine and S. L. Bender, Chem. Rev., 1997, 97, 1359–1472 CrossRef CAS; (b) K. Ariga, H. Ito, J. P. Hill and H. Tsukube, Chem. Soc. Rev., 2012, 41, 5800–5835 RSC.
- K. L. Haas and K. J. Franz, Chem. Rev., 2009, 109, 4921–4960 CrossRef CAS.
- M. Wenzel, J. R. Hiscock and P. A. Gale, Chem. Soc. Rev., 2012, 41, 480–520 RSC.
- T. H. Webb and C. S. Wilcox, Chem. Soc. Rev., 1993, 383–395 Search PubMed.
- P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed., 2001, 40, 486–516 CrossRef CAS.
- J. L. Sessler, P. A. Gale and W-S. Cho, Anion Receptor Chemistry, RSC publishing, Cambridge, UK, 2006 Search PubMed.
- P. A. Gale, Acc. Chem. Res., 2006, 39, 465–475 CrossRef CAS.
- (a) M. P. Anderson, R. J. Gregory, S. Thompson, D. W. Souza, S. Paul, R. C. Mulligan, A. E. Smith and M. J. Welsh, Science, 1991, 253, 202–205 CAS; (b) D. C. Gadsby, P. Vergani and L. Csanady, Nature, 2006, 440, 477–483 CrossRef CAS.
- (a) K. Steinmeyer, C. Ortland and T. J. Jentsch, Nature, 1991, 354, 301–304 CrossRef CAS; (b) M. C. Koch, K. Steinmeyer, C. Lorenz, K. Ricker, F. Wolf, M. Otto, B. Zoll, F. Lehmann-Horn, K-H. Grzeschik and T. J. Jentsch, Science, 1992, 257, 797–800 CAS.
- (a) M. Ito, H. Tsuda, K. Oguro, K. Mutoh, H. Shiraishi, Y. Shirasaka and H. Mikawa, Neurochem. Res., 1990, 15, 933–936 CrossRef CAS; (b) D. M. Treiman, Epilepsia, 2001, 42, 8–12 CrossRef.
- (a) M. Cametti and K. Rissanen, Chem. Commun., 2009, 2809–2829 RSC; (b) W. H. Bowen, J. Am. Dent. Assoc., 2002, 133, 1405–1407 CAS.
- (a) M. Hatch, R. W. Freel and N. D. Vaziri, J. Am. Soc. Nephrol., 1994, 5, 1339–1343 CAS; (b) Y-M. Hsueh, G-S. Cheng, M-M. Wu, H-U. Yu, T-L. Kuo and C-J. Chen, Br. J. Cancer, 1995, 71, 109–114 CrossRef CAS; (c) J. Liu, A. Sandrini, M. C. Thurston and D. H. Yates, Respiration, 2007, 74, 617–623 CrossRef CAS.
- (a) I. B. Salusky, Kidney Int., 2006, 70, S10–S15 CrossRef; (b) I. Fernandes, D. Laouari, P. Tutt, G. Hampson, G. Friedlander and C. Silve, Kidney Int., 2001, 59, 210–221 CrossRef CAS.
- (a) F. P. Schmidtchen and M. Berger, Chem. Rev., 1997, 97, 1609–1646 CrossRef CAS; (b) K. Bowman-James, Acc. Chem. Res., 2005, 38, 671–678 CrossRef CAS; (c) V. Amendola, M. Bonizzoni, D. Esteban-Gomez, L. Fabbrizzi, M. Licchelli, F. Sancenon and A. Taglietti, Coord. Chem. Rev., 2006, 250, 1451–1470 CrossRef CAS; (d) S. O. Kang, R.A. Begum and K. Bowman-James, Angew. Chem., Int. Ed., 2006, 45, 7882–7894 CrossRef CAS; (e) C-H. Lee, H. Miyaji, D. W. Yoon and J. L. Sessler, Chem. Commun., 2008, 24–34 RSC; (f) P. A. Gale, Chem. Commun., 2008, 4525–4540 RSC; (g) P. A. Gale, S. E. Garcıa-Garrido and J. Garric, Chem. Soc. Rev., 2008, 37, 151–190 RSC; (h) X. Le, Y-D. Wu and D. Yang, Acc. Chem. Res., 2008, 41, 1428–1438 CrossRef; (i) C. Caltagirone and P. A. Gale, Chem. Soc. Rev., 2009, 38, 520–563 RSC; (j) S. Kubik, Chem. Soc. Rev., 2009, 38, 585–605 RSC; (k) A. E. Hargrove, S. Nieto, T. Zhang, J. L. Sessler and E. V. Anslyn, Chem. Rev., 2011, 111, 6603–6782 CrossRef CAS.
- C. H. Park and H. E. Simmons, J. Am. Chem. Soc., 1968, 90, 2431–2432 CrossRef CAS.
- M. A. Hossain, J. M. Llinares, S. Mason, P. Morehouse, D. Powell and K. Bowman-James, Angew. Chem., Int. Ed., 2002, 41, 2335–2338 CrossRef.
- F. P. Schmidtchen, Angew. Chem., Int. Ed. Engl., 1977, 16, 720–721 CrossRef.
- F. M. Menger and K. K. Catlin, Angew. Chem., Int. Ed. Engl., 1995, 34, 2147–2150 CrossRef CAS.
- R. A. Pascal, J. Spergel and D. V. Engbersen, Tetrahedron Lett., 1986, 27, 4099–4102 CrossRef CAS.
- K. Choi and A. D. Hamilton, J. Am. Chem. Soc., 2001, 123, 2456–2457 CrossRef CAS.
- S. O. Kang, J. M. Llinares, D. Powell, D. V. Velde and K. Bowman-James, J. Am. Chem. Soc., 2003, 125, 10152–10153 CrossRef CAS.
- P. J. Smith, M. V. Reddington and C. S. Wilcox, Tetrahedron Lett., 1992, 33, 6085–6088 CrossRef CAS.
- B. H. M. Snellink-Ruel, M. M. G. Antonisse, J. F. J. Engbersen, P. Timmerman and D. N. Reinhoudt, Eur. J. Org. Chem., 2000, 165–170 CrossRef CAS.
- S. J. Brooks, P. A. Gale and M. E. Light, Chem. Commun., 2005, 4696–4698 RSC.
- B. Dietrich, T. M. Fyles, J. M. Lehn, L. G. Pease and D. L. Fyles, J. Chem. Soc., Chem. Commun., 1978, 934–936 RSC.
- F. P. Schmidtchen, Tetrahedron Lett., 1989, 30, 4493–4496 CrossRef CAS.
- J. J. Lavigne and E. V. Anslyn, Angew. Chem., Int. Ed., 1999, 38, 3666–3669 CrossRef CAS.
- J. L. Sessler, M. J. Cyr, V. Lynch, E. McGhee and J. A. Ibers, J. Am. Chem. Soc., 1990, 112, 2810–2813 CrossRef CAS.
- P. Piatek, V. M. Lynch and J. L. Sessler, J. Am. Chem. Soc., 2004, 126, 16073–16076 CrossRef CAS.
- D-W. Yoon, H. Hwang and C-H. Lee, Angew. Chem., Int. Ed., 2002, 41, 1757–1759 CrossRef CAS.
- A. G. Avent, P. A. Chaloner, M. P. Day, K. R. Seddon and T. Welton, J. Chem. Soc., Dalton Trans., 1994, 3405–3413 RSC.
- K. Sato, S. Arai and T. Yamagishi, Tetrahedron Lett., 1999, 40, 5219–5222 CrossRef CAS.
- K. Chellappan, N. J. Singh, I-C. Hwang, J. W. Lee and K. S. Kim, Angew. Chem., Int. Ed., 2005, 44, 2899–2903 CrossRef CAS.
- E. Galbraith and T. D. James, Chem. Soc. Rev., 2010, 39, 3831–3842 RSC.
- (a) H. E. Katz, J. Am. Chem. Soc., 1986, 108, 7640–7645 CrossRef CAS; (b) A. Kawachi, A Tani, J. P. Shimada and Y. Yamamota, J. Am. Chem. Soc., 2008, 130, 4222–4223 CrossRef CAS.
- (a) V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596–2599 CrossRef CAS; (b) C. W. Tornoe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057–3064 CrossRef CAS.
- V. Haridas, K. Lal, Y. K. Sharma and S. Upreti, Org. Lett., 2008, 10, 1645–1647 CrossRef CAS.
- Y. Li and A. H. Flood, Angew. Chem., Int. Ed., 2008, 47, 2649–2652 CrossRef CAS.
- Y. Li, M. Pink, J. A. Karty and A. H. Flood, J. Am. Chem. Soc., 2008, 130, 17293–17295 CrossRef CAS.
- S. Lee, Y. Hua, H. Park and A. H. Flood, Org. Lett., 2010, 12, 2100–2102 CrossRef CAS.
- H. Juwarker, J. M. Lenhardt, D. M. Pham and S. L. Craig, Angew. Chem., Int. Ed., 2008, 47, 3740–3743 CrossRef CAS.
- R. M. Meudtner and S. Hecht, Angew. Chem., Int. Ed., 2008, 47, 4926–4930 CrossRef CAS.
- J. Morales-Sanfrutos, M. Ortega-Munoz, J. Lopez-Jaramillo, F. Hernandez-Mateo and F. Santoyo-Gonzalez, J. Org. Chem., 2008, 73, 7768–7771 CrossRef CAS.
- T. Romero, A. Caballero, A. Tarraga and P. Molina, Org. Lett., 2009, 11, 3466–3469 CrossRef CAS.
- J. L. Sessler, J. Cai, H-Y. Gong, X. Yang, J. F. Arambula and B. P. Hay, J. Am. Chem. Soc., 2010, 132, 14058–14060 CrossRef CAS.
- L. C. Gilday, N. G. White and P. D. Beer, Dalton Trans., 2012, 41, 7092–7097 RSC.
- Y. Wang, F. Bie and H. Jiang, Org. Lett., 2010, 12, 3630–3633 CrossRef CAS.
- Q-Y. Cao, T. Pradhan, S. Kim and J. S. Kim, Org. Lett., 2011, 13, 4386–4389 CrossRef CAS.
- Y. Hua and A. H. Flood, J. Am. Chem. Soc., 2010, 132, 12838–12840 CrossRef CAS.
- Y. Wang, J. Xiang and H. Jiang, Chem.–Eur. J., 2011, 17, 613–619 CrossRef CAS.
- F. Garcia, M. R. Torres, E. Matesanz and L. Sanchez, Chem. Commun., 2011, 47, 5016–5018 RSC.
- V. Haridas, S. Sahu and P. P. Praveen Kumar, Tetrahedron Lett., 2011, 52, 6930–6934 CrossRef CAS.
- V. Haridas, S. Sahu and P. Venugopalan, Tetrahedron, 2011, 67, 727–733 CrossRef CAS.
- Y-J. Li, L. Xu, W-L. Yang, H-B. Liu, S-W. Lai, C-M. Che and Y-L. Li, Chem.–Eur. J., 2012, 18, 4782–4790 CrossRef CAS.
- L. Xu, Y. Li, Y. Yu, T. Liu, S. Cheng, H. Liu and Y. Li, Org. Biomol. Chem., 2012, 10, 4375–4380 CAS.
| This journal is © The Royal Society of Chemistry 2012 |