Uptake and translocation of polymeric nanoparticulate drug delivery systems into ryegrass†
Ming
Zhang
a,
E. Ann
Ellis
b,
Luis
Cisneros-Zevallos
c and
Mustafa
Akbulut
*a
aArtie McFerrin Department of Chemical Engineering, Texas A&M University, College Station, TX 77843-3122. E-mail: makbulut@tamu.edu; Fax: 1 (979) 845-6446; Tel: 1 (979) 847-8766
bMicroscopy & Imaging Center, Texas A&M University, College Station, TX 77843-2257
cDepartment of Horticultural Sciences, Texas A&M University, College Station, TX 77843-3244
First published on 15th August 2012
Abstract
This study focuses on the transport behavior of model polymeric nanoparticulate drug delivery systems (PNDDSs) across ryegrass roots to determine whether uncontrolled and accidental releases of PNDDSs may enter into the food chain. It was shown that uptake of PNDDS ranging from 46 nm to 271 nm into ryegrass roots could take place. Upon exposing ryegrass to an aqueous PNDDS dispersion for 312 h, 91 ± 6%, 64 ± 3%, and 26 ± 8% of PNDDSs were localized in and on the ryegrass for 46 nm, 159 nm, and 271 nm PNDDS, respectively. The overall transport of PNDDSs from the solution to the ryegrass could be modeled well as a first-order adsorption process, which was followed by a first-order uptake process. The adsorption of PNDDSs onto the roots was found to be much faster than the uptake of PNDDSs into the roots.
Introduction
In recent years, rapid advances in science and technology have opened up the burgeoning new field of nanotechnology, bringing a myriad of opportunities and possibilities for the development and fabrication of novel materials and nanodevices. Nanotechnology is expected to have an impact on $2.9 trillion worth of products across the value chain by 2014.1 The area of nanotechnology has been especially beneficial in the field of medicine. Recent studies have estimated that more than 130 nanotechnology-based drugs and delivery systems (nanomedicine) have entered pre-clinical, clinical, or commercial development.2 With the increasing production and consumption of nanotherapeutics, their occurrence, fate, and impact on the environment have been increasingly recognized as issues warranting consideration.3–9Currently, among the various types of nanomedicine, polymeric nanoparticulate drug delivery systems (PNDDSs) filled with therapeutic agents represent one of the most commonly used forms of nanomedicine due to their ability to solubilize hydrophobic molecules as well as their increased bioavailability, higher payload capacity, excellent stability in aqueous environments and in blood, and prolonged blood circulation time.10–13 While these properties of PNDDSs are very beneficial from a pharmaceutical science perspective, they can be detrimental from an environmental science perspective because these features can cause them to persist for a prolonged period of time in the environment, and thus have a larger impact upon uncontrolled releases and accidental spills.
Because most PNDDSs are loaded with therapeutic materials that are ecotoxic, nanomedicine is an environmental concern. This concern is exacerbated by recent in vivo studies indicating that some of the intravenously administered nanomedicine can be excreted from the body intact through the kidneys or as a metabolite through the liver/bile duct.14–17 Engineered nanoparticles released into wastewater and wastewater sludge may find their way into the food chain.18–20 Thus, transport of PNDDSs in the environment needs to be studied to predict the fate and potential environmental destination of PNDDSs in case of uncontrolled releases and accidental spillage.
In this study, we investigate the transport behavior of a model PNDDS in the vicinity of the roots of ryegrass (Lolium perenne), which is the first level in the food chain and a common model plant used in environmental science studies.21,22 Ibuprofen loaded poly(ethyleneglycol-b-ε-caprolactone) (PEG-b-PCL) encapsulated nanoparticles of various sizes were selected as a model PNDDS. PEG-b-PCL was selected as the carrier because it is one of the most popular, FDA approved amphiphilic copolymers used in formulations of current polymeric drug delivery systems.23,24 Ibuprofen is chosen as a therapeutic building block due to its low water solubility, which is a shared feature of most therapeutics used in PNDDS formulations.25,26
Materials and methods
Materials
Poly(ethylene glycol-b-ε-caprolactone) (PEO-b-PCL, 5000-b-6500 g mol−1, Polymer Source Inc.), polystyrene (PS, 125![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, Alfa Aesar), ibuprofen (≥98%, Sigma-Aldrich), Nile Red (Tokyo Kasei Kogyo co. LTD), and tetrahydrofuran (THF, 99.9%, Sigma-Aldrich) were used as received.
000 g mol−1, Alfa Aesar), ibuprofen (≥98%, Sigma-Aldrich), Nile Red (Tokyo Kasei Kogyo co. LTD), and tetrahydrofuran (THF, 99.9%, Sigma-Aldrich) were used as received.
Preparation and characterization of PNDDS
The PNDDS were prepared using a solution precipitation method.27,28 In this method, a hydrophobic therapeutic agent (ibuprofen) and an amphiphilic diblock copolymer (PEG-b-PCL) were molecularly dissolved in tetrahydrofuran (THF). The mixture was then very rapidly mixed against a Milli-Q water stream to produce polymer encapsulated ibuprofen nanoparticles. The flow rates of the organic and water streams were 5 ml min−1 and 50 ml min−1, respectively. The resultant dispersion was dialyzed overnight to completely remove THF. The sizes of these nanoparticles were adjusted by varying the concentration of ibuprofen and/or adding an extra filler agent (PS) in the organic stream. In addition, a trace amount (0.001% wt.) of Nile red was introduced into the THF stream to produce fluorescent PNDDS so that the fluorophore was imbibed into the PNDDS, not onto its surface, to ensure that the surface chemistry was not altered.The size distributions and zeta potentials of the PNDDS were measured using dynamic light scattering (DLS) (Zetasizer Nano ZS90, Malvern). The morphology of the PNDDS was characterized using transmission electron microscopy (TEM) (JEM-2010, Jeol).
Plant germination and exposure of PNDDS to ryegrass
1 g perennial ryegrass seeds (Pennington Seed Inc., GA) was germinated in pots with 200 g soil (Miracle-Gro® Organic Choice®, ScottsMiracle-Gro, OH). After germination and growing for about one week at room temperature, the leaves were typically about 10 cm while the root was approximately 8 cm. At this stage, after gentle washing and removing residual soil from the root, the ryegrass was transferred into vials filled with tap water and kept there for one day. Then the ryegrass was transferred into vials filled with PNDDS of a given size and concentration of 0.133 mg ml−1 and kept in the vials for a predefined amount of time. Here it is important to emphasize that only the roots of the ryegrass were exposed to the PNDDS. Each experiment was replicated at least three times for statistical reliability. A separate solution of the PNDDS without the ryegrass was used as a control experiment to keep track of the fluorescence level of the PNDDS solution. The other control experiments involved the exposure of the ryegrass to molecular Nile Red in water or just water instead of Nile Red loaded PNDDS in water.Spectrofluorometry (SFM)
To determine the transport of the PNDDS from the aqueous media onto and into the ryegrass as a function of time, we immersed the ryegrass roots in 6 ml dispersions of the PNDDS (0.133 mg ml−1), loaded with a trace amount of fluorescent Nile Red for 0 h, 1 h, 3 h, 9 h, 27 h, 81 h, and 312 h. Then, we measured the fluorescence intensity of the PNDDS solution using SFM at these predefined intervals. Similarly, a control experiment measuring the fluorescence intensities in the absence of ryegrass root was conducted to determine the variation in fluorescence level with respect to time. All fluorescence measurements were made by a PTI QuantaMaster series spectrofluorometer (Photon Technology International, Inc., NJ, USA) equipped with a PTI LPS-220B lamp, using 1.00 cm disposable cells. The excitation wavelength was 549 nm, and the corresponding emission spectra ranged from 559 nm to 700 nm.Confocal microscopy
A confocal microscope (Leica TCS SP5) was used to determine the distribution of PNDDS on and in the ryegrass by focusing on different planes of ryegrass. The excitation laser wavelength was 543 nm, and the observed wavelength ranged from 560 nm to 650 nm. This range was selected to distinguish the fluorescence signal of chlorophylls (650 nm to 800 nm) from that of Nile Red. Since each PNDDS size had a slightly different fluorescence spectrum, their confocal micrographs were normalized with respect to the corresponding fluorescence intensities using ImageJ. PNDDS exposed to molecular Nile Red in water and plain water were the control groups in the confocal microscopy studies.Scanning electron microscopy (SEM)
The grass roots were observed by SEM (JEOL JSM-7500F) to determine the local distribution of the PNDDS on the root surface after immersing the roots in the PNDDS dispersion and rinsing with an excess amount of water. PNDDS exposed to only water worked as a control group. For both cases, the root was coated with a 4 nm Au layer to fulfill the conductivity requirements of SEM.Cross sectional TEM
Sections from roots, stems and leaves of the treated and control plants were fixed in 2.5% (v/v) glutaraldehyde–1.0% (v/v) acrolein in 0.1 M HEPES buffer, pH 7.4 for 30 min with intermittent vacuum at room temperature. Fixation was terminated by a 6 min cycle (2 min ON; 2 min OFF; 2 min ON) at 250 watts with intermittent vacuum in a Ted Pella Biowave laboratory microwave (Ted Pella, Inc, Redding, CA) at 20 °C. Specimens were then washed three times for 1 min at 250 watts in 0.1 M HEPES buffer pH 7.4 followed by post fixation overnight at 4 °C in 1% (wt/v) osmium tetroxide in the same buffer. Specimens were then dehydrated in a graded methanol series [5% (v/v) steps from 5% to 3 × 100%]. Each dehydration step was done for 1 min at 250 watts with intermittent vacuum. Infiltration and embedding were done in a low viscosity epoxy resin.29 Ultrathin sections (90–100 nm) were cut on a Reichert Ultracut E ultramicrotome (Leica Microsystems, Inc., Buffalo Grove, IL). These thin sections were imaged in a JEOL 1200EX transmission electron microscope at an accelerating voltage of 100 kV. Samples of the nanoparticles were enrobed in 2% (wt/v) agarose and then fixed and processed in the same manner in which the plant material was handled to determine the influence of the fixation method on the nanoparticles.Results
Characterization of PNDDS
Table 1 summarizes the formulations used in the preparation of PNDDSs with varying size and the resultant mean intensity-averaged PNDDS sizes. These formulations give rise to particle mean intensity-averaged sizes ranging from 46 nm to 271 nm, enabling us to systematically study the effect of PNDDS size on its transport behavior across ryegrass. All of the size distributions were unimodal and fairly narrow (Fig. 1a). Transmission electron microscopy (TEM) micrographs indicated that the PNDDSs were mostly spherical (Fig. 1b). Stability studies revealed that there was no significant change in the particle size (hydrodynamic radius) after a period of over one month or after diluting 10 times, suggesting an excellent stability in water (Fig. 1c). The superior stability is ascribed to the combination of steric and electrostatic effects because of the presence of long PEG chains and the fact that the zeta potential of particles ranges from −25 mV to −30 mV. However, it is important to underline that the stability of nanoparticles with respect to the hydrodynamic size does not necessarily mean that there is no release of the drug out of the PNDDS. The release of the drug from the core of the PNDDS can lead to a more porous PNDDS, while maintaining the same hydrodynamic radius. Such a structural change is not usually detected by DLS. | ||
| Fig. 1 (a) Intensity weighted particle size distributions for PNDDS. The mean intensity-averaged sizes of three different batches of PNDDS were 46 ± 1 nm (black, A), 117 ± 4 nm (red, B), 159 ± 1 nm (cyan, C), 197 ± 4 nm (magenta, D), 238 ± 7 nm (dark green, E), and 271 ± 2 nm (orange, F). (b) TEM images of a PNDDS of 46 nm. (c) DLS results of the initial 197 nm PNDDS (black), 197 nm PNDDS after diluting 10 times (red), and 197 nm PNDDS after being kept for one month (blue). (Letters A–F shown in (a) indicate the formulation used (Table 1) in their preparation.) | ||
| Formulation | Ibuprofen | PEO-b-PCL | PS | Mean size (nm) |
|---|---|---|---|---|
a The concentrations were given in weight % (weight agent/weight THF) in the THF stream before mixing with Milli-Q water at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 THF/H2O (v/v) ratio. To enable fluorescence tracking, all formulations also contained 0.001 wt% Nile Red as a fluorescent building block that is imbibed in the PNDDS. 10 THF/H2O (v/v) ratio. To enable fluorescence tracking, all formulations also contained 0.001 wt% Nile Red as a fluorescent building block that is imbibed in the PNDDS.
|
||||
| A | 1.0% | 2.0% | — | 46 ± 1 |
| B | 0.1% | 0.1% | — | 117 ± 4 |
| C | 0.1% | 0.1% | 0.02% | 159 ± 1 |
| D | 0.1% | 0.1% | 0.05% | 197 ± 4 |
| E | 0.1% | 0.1% | 0.10% | 238 ± 7 |
| F | 0.1% | 0.1% | 0.20% | 271 ± 2 |
Spectrofluorometry (SFM) studies
The fluorescence emission spectra have peaks ranging from 610 nm to 654 nm depending on the PNDDS size (see Fig. S1, ESI†). Given that the peak fluorescence intensity was proportional to the concentration of the PNDDS (see Fig. S2, ESI†), the analysis of the fluorescence intensity data allowed us to calculate the concentration of PNDDS in the solution i.e. PNDDS that is not adsorbed or not taken up by the roots (Fig. 2). It can be clearly seen that the roots consume (adsorb and uptake) the smaller PNDDS much faster. For instance, at t = 312 h, the relative concentrations of 46 nm, 159 nm, and 271 nm PNDDS in the solution were 0.09 ± 0.06, 0.36 ± 0.03 and 0.74 ± 0.08, respectively. This means that 91 ± 6%, 64 ± 3%, and 26 ± 8% of PNDDS were localized in and on the ryegrass for 46 nm, 159 nm, and 271 nm PNDDS, respectively. | ||
| Fig. 2 The relative concentration, ms/ms,0, (the concentration at any time over the initial concentration) of the PNDDS in the solution (i.e. PNDDS that is not adsorbed or not taken up by the roots) as a function of exposure times for various PNDDS sizes. The data is normalized with respect to the fluorescence intensities of the control experiments to account for the variation in fluorescence level with time in the absence of ryegrass roots. | ||
Confocal microscopy
The ryegrass roots exposed to PNDDS dispersions were also characterized by confocal microscopy to determine the distribution of the PNDDS across the ryegrass (Fig. 3 and 4). Fig. 3 displays the confocal microscopy images of roots and leaves upon exposure to 271 nm PNDDS dispersion in water, molecular Nile Red in water, and plain water, respectively, for 81 h. These images revealed that while ryegrass can take up both molecular Nile Red and a PNDDS, molecular Nile Red yielded a much weaker fluorescence signal, especially in the roots. For the case of PNDDS exposure, the fluorescence intensity in the roots was much higher than that in the leaves. No fluorescence response was observed for the case of exposure to water only, indicating that there was no chlorophyll-induced interference in the fluorescence response for the selected wavelength range. | ||
| Fig. 3 Confocal microscopy images of ryegrass after exposure to aqueous PNDDS dispersion, molecular Nile Red at maximum solubility in water (saturated solution), and water. The ryegrass exposed to PNDDS shows a strong fluorescence signal in the root but a very weak signal in the leaves; the ryegrass exposed to free Nile red shows a very weak fluorescence signal in both the roots and leaves; and the ryegrass exposed to water shows no fluorescence signal. | ||
 | ||
| Fig. 4 Confocal microscopy images of ryegrass after exposure to the PNDDS (0.133 mg ml−1) for 1 h, 27 h, and 81 h. For all times, PNDDS is found in the cap of the root, the middle of root and the stem, but not in the leaf. The clear effect of PNDDS size on the uptake of PNDDSs into ryegrass can be observed at longer times (81 h). During the acquisition stage, the optical filter is applied to eliminate all wavelengths except in the range 560 nm to 650 nm, to distinguish the fluorescence signal of chlorophylls from that of the Nile Red tagged PNDDS. | ||
The difference between the fluorescence behavior of the PNDDS and that of molecular Nile Red in the roots can be attributed to the following: first, for a given concentration, the fluorescence intensity of molecular Nile Red in water is 40 times less than that in a lipophilic environment.30,31 Second, the solubility of Nile Red in water is very poor (less than 1 μg mL−1).32 Therefore, PNDDS prepared by the formulations described in Table 1 can carry higher concentrations of Nile Red to the roots compared to the solubility of Nile Red in water. Considering these points, we can conclude that Nile Red-loaded PNDDS, not molecular Nile Red, is responsible for the strong fluorescence response observed in Fig. 3. Similarities between the fluorescence behavior of leaves that are exposed to PNDDS and molecular Nile Red and that of roots that are exposed to molecular Nile Red may suggest that, for the PNDDS case, only Nile Red molecules that are released from the PNDDS core can reach the leaves. The fact that the fluorescence intensity of leaves that are exposed to the PNDDS did not show any significant variation as a function of PNDDS size also supports this argument.
Fig. 4 displays the fluorescence images of different parts of ryegrass after exposure to PNDDSs of different sizes for 1 h, 27 h and 81 h. These experiments revealed that most of the PNDDS was localized at the root and stem and almost none of the PNDDS was found at the leaves (Fig. 4). Although we only exposed the root of the plant to PNDDS dispersion, the presence of PNDDS in the stem suggests that the PNDDS was taken up by the roots and then transferred into the stem. The PNDDS uptake was also confirmed by the confocal microscopy images focusing on the different planes of the roots (see Movie S1, ESI†).
The comparison of the local and overall image intensities of the ryegrass revealed that the confocal microscopy results were also self-consistent with the SFM data: at 1 h, the fluorescence intensities of the roots and stems that had been exposed to the PNDDSs of varying sizes were not significantly different from each other (Fig. 4). On the other hand, at 81 h, while the stem and root of the ryegrass that was exposed to 46 nm PNDDS had the highest overall fluorescence intensity, the stem and root of the ryegrass that was exposed to 271 nm PNDDS had a very weak fluorescence emission intensity for a given excitation intensity.
The comparison of fluorescence intensities at the root, stem and leaf revealed that at the beginning, the fluorescence intensities in the stems were higher than those in roots. This finding suggests that the rate of PNDDS transport from the root to the stem was initially much faster than the rate of PNDDS uptake from the root surface to the root interior. It was also found that as the PNDDS accumulated in the stems over time, the rate of PNDDS transport from the root to the stem decreased (i.e. the stem was saturated with the PNDDS); thereby the PNDDS started to accumulate in the roots more.
Scanning electron microscopy (SEM) studies
To better understand the uptake mechanism of PNDDS into the plant, we characterized the root surfaces using SEM after exposing them to PNDDS and rinsing them with excess water (Fig. 5). First, it was found that PNDDS had a strong interaction with the plant root and was not washed away even with the excess water, while no nanoparticles were found in the control group. This suggests that the adsorption of PNDDS onto the root surface may be an irreversible process. Similar irreversible and partially reversible adsorption behavior on the roots has previously been observed in other systems such as bacteria,33 mineral ions,34 and pesticides.35 The adsorption of PNDDS may also be irreversible. Second, SEM images suggest that there is some intercellular space that exists between cells (Fig. 5b, highlighted in blue). These openings are known to be responsible for the transport of water and solutes into the root through the apoplast route,36,37 and presumably enable entry of PNDDSs into the ryegrass roots as well. | ||
Fig. 5 (a) Low (×5000) and (b) high (×20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) magnification SEM micrographs of the ryegrass root upon exposure to a PNDDS of 159 nm solution (0.133 mg ml−1). (c) and (d) SEM micrographs of a ryegrass root without exposure to the PNDDS. There were many PNDDSs (red circles) adsorbed on the root even after excess water rinsing. The blue circle indicates a possible PNDDS entry site into the plant. The samples were coated with a 4 nm Au film to enable SEM imaging. 000) magnification SEM micrographs of the ryegrass root upon exposure to a PNDDS of 159 nm solution (0.133 mg ml−1). (c) and (d) SEM micrographs of a ryegrass root without exposure to the PNDDS. There were many PNDDSs (red circles) adsorbed on the root even after excess water rinsing. The blue circle indicates a possible PNDDS entry site into the plant. The samples were coated with a 4 nm Au film to enable SEM imaging. | ||
Cross sectional TEM
Cross sectional TEM was used to independently confirm the uptake of the PNDDS into ryegrass roots and further study the distribution of PNDDSs in the roots. Comparison of the cross sectional TEM images of the roots with and without PNDDS (197 nm) treatment revealed that while the PNDDS treated samples contain some particles (red and green arrows in Fig. 6a and b), the control sample had no particles (Fig. 6c). The micrographs of PNDDS that underwent the same fixation procedure used for the preparation of roots for cross-sectional TEM indicate that the PNDDSs were fairly stable against such a procedure (Fig. 6d and e). Further analysis of the micrographs indicates that the average size of the particles marked with red arrows were 190 ± 30 nm (n > 10), very close to the size of the PNDDS. These findings suggest that it is indeed possible that ryegrass roots can take up PNDDSs and that PNDDSs are localized both in the root cells and in the intercellular space. | ||
| Fig. 6 Cross sectional TEM images of (a and b) the ryegrass root upon exposure to the PNDDS of 197 nm; (c) the ryegrass root in the absence of PNDDS exposure; and (d and e) PNDDS treated with the agarose gel and microtoming procedure used for the preparation of the roots for the cross sectional TEM. | ||
It is important to note that in addition to the 190 nm particles, there were other particles of 50–100 nm (shown with green arrows). The smaller PNDDSs were observed both in the PNDDS treated root and in the PNDDS treated by the agarose gel. We believe that the smaller particles are presumably lightly loaded micelles obtained during the preparation of the PNDDS. The existence of smaller particles can be seen from the particle size distributions in Fig. 1. Alternatively, the microtoming procedure may also break the PNDDS into smaller pieces. Therefore, both small and large particles appear in the micrographs.
Kinetics of PNDDS uptake
Overall transport of a PNDDS from solution to a ryegrass can be considered in two processes: (i) adsorption of the PNDDS onto the root surface, and (ii) uptake of the PNDDS from the root surface to the root interior. Considering that the adsorption of the PNDDS onto the cellulose surface is mostly an irreversible process,38 and the SEM images (Fig. 5) show that the PNDDS was not washed away from the root surface, we assume the adsorption of the PNDDS onto the ryegrass root is irreversible. And the overall process can be expressed as: | (1) |
 | (2) |
 | (3) |
 | (4) |
The initial conditions for this differential equation, ms(t = 0) is known experimentally and dms(t = 0)/dt = −kams(t = 0) × S/V. Using a numerical integration technique (Euler method) and the least squares method for experimental data within 48 h, we could calculate ka, kup and Γ*. However, in this problem, a large number of degrees of freedom exist, creating multiple solutions. Further systematic numerical calculations revealed that only when Γ* is between 0.4 g m−2 and 0.8 g m−2, fits for both ka and kup yield positive values. Table 2 shows the fit results for ka and kup when Γ* = 0.6 g m−2. Similarly, ka and kup fit results obtained using Γ* = 0.4 g m−2 and 0.8 g m−2 can be found in Table S1 and S2, ESI.† Overall, regardless of the choice of Γ* value (in the range 0.4 to 0.8 g m−2), ka and kup gave rise to similar orders of magnitude; and kup increased with decreasing PNDDS size in an exponential manner. While there is mostly an inverse correlation between adsorption rate, ka, and PNDDS size; ka for 238 nm and 271 nm PNDDS deviated from this trend presumably due to the increased effect of gravitational forces on larger particles. The relative magnitudes of adsorption flux to uptake flux increased with increasing PNDDS size, indicating that more and more bottlenecking at the uptake step will take place as the PNDDS size increases.
| Diameter (nm) | k a (m h−1) | k up (h−1) | k a m s,0 (g m−2 h−1) | Γ*kup (g m−2 h−1) |
|---|---|---|---|---|
| 46 | 0.0041 ± 0.0006 | 0.169 ± 0.058 | 0.55 ± 0.08 | 0.101 ± 0.034 |
| 117 | 0.0030 ± 0.0020 | 0.099 ± 0.027 | 0.40 ± 0.24 | 0.060 ± 0.016 |
| 159 | 0.0020 ± 0.0007 | 0.051 ± 0.001 | 0.27 ± 0.09 | 0.031 ± 0.001 |
| 197 | 0.0013 ± 0.0008 | 0.039 ± 0.007 | 0.17 ± 0.10 | 0.023 ± 0.004 |
| 238 | 0.0027 ± 0.0015 | 0.031 ± 0.013 | 0.36 ± 0.19 | 0.019 ± 0.008 |
| 271 | 0.0022 ± 0.0005 | 0.014 ± 0.010 | 0.29 ± 0.06 | 0.008 ± 0.006 |
Discussion
Regarding the possible route/mechanism of entry for PNDDS into the ryegrass: lateral roots commonly originate from the pericycle and grow through the cortex of the parent root. Once the lateral root breaks through the outer epidermal layer to the outside, PNDDS may gain entrance through the resulting crevice (Fig. 7).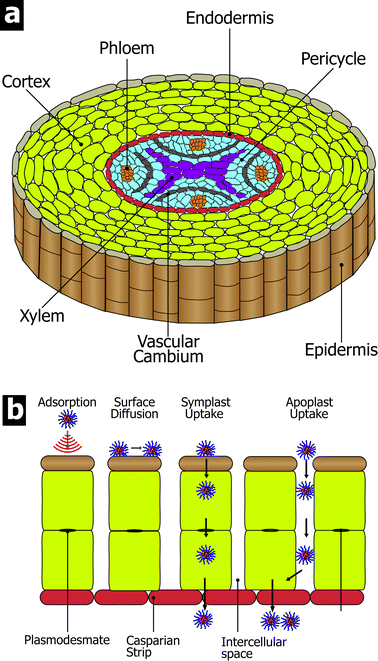 | ||
| Fig. 7 (a) Root structure and (b) possible routes for PNDDS uptake into roots. | ||
For nanoparticle transport through the root tissue to the xylem system observed in our experiments, there may be two main potential routes: apoplast and symplast routes (Fig. 7). The apoplast route involves the space between the cells and the cell walls themselves along the radial length corresponding to the root surface (openings at the surface) and the stele (containing the xylem and phloem), whereas the symplast route involves the active uptake or passive uptake of water molecules and ions through the plasma membrane and transfer from cell to cell through the plasmodesmata, which are pores between cells that connect the protoplasms and allow transfer of molecules.46–48 Plasmodesmata have been shown to transport proteins (including transcription factors), short interfering RNA, messenger RNA, and viral genomes from cell to cell.49 As such, it may also allow the transport of the PNDDS. The presence of PNDDSs in root cells and intercellular space (Fig. 6) reveals that both apoplast and symplast routes may exist in the transport of PNDDS in ryegrass.
The uptake and accumulation of nanoparticles by plants is increasingly recognized as an important environmental issue by researchers.50–55 The presence of nanoparticles in stems and roots and their absence in leaves were also observed by Lin et al.,52 when they exposed ryegrass (L. perenne) to ZnO nanoparticles of size 9–37 nm. The ZnO nanoparticles primarily adhered to the root surface and individual nanoparticles were observed in the apoplast and protoplast spaces in the root endodermis and stele. Zhu et al.53 showed that the exposure of pumpkin (Cucurbita maxima) roots to 20 nm iron oxide nanoparticles leads to their major accumulation (45.5% of fed nanoparticles) in the roots and their negligible translocation (0.6% of fed nanoparticles) to the leaves. Lin et al.54 investigated the uptake and translocation of carbon nanomaterials by rice plants (Oryza sativa) and found that fullerene C70 of 1.17 nm could be easily taken up by the roots and transported to the leaves. The differences in the concentration of PNDDS in leaves and roots are presumably due to the selective transport of the membranous ligule, which is located at the inner base of the leaf between where the leaf attaches to the main stem and the stem itself.56–58
The trend of decreasing uptake rate, kup, with increasing PNDDS size can be explained through the consideration of the mass flux of particles or molecules across a porous membrane when the particle size is comparable to the pore size.59 Similar trends were also observed in other systems: for instance, Nikido and Rosenberg60 showed that the permeability of the outer membrane of the Escherichia coli cell to sugars decreases with increasing molecule size. Likewise, the rate of internalization of hydrogel particles into HeLa cells was shown to be strongly dependent on size.61
From the standpoint of environmental impact, emerging PNDDSs are predominantly loaded with ecotoxic, hydrophobic therapeutics such as cisplatin,62,63 fluorouracil,64,65 estradiol,66,67 paclitaxel,68,69 docetaxel,70,71 and etoposide.69 Recent ecotoxicity studies on Daphnia magna have revealed that cisplatin, fluorouracil, and estradiol belong among the compounds that are highly toxic for aquatic organisms (EC50 < 1 mg L−1), paclitaxel and docetaxel are considered toxic (EC50 ranging 1–10 mg L−1), and etoposide is harmful to aquatic organisms (10–100 mg L−1).72–74 Other ecotoxicity studies on Pseudomonas putida have indicated similar trends of acute ecotoxicity associated with cisplatin and fluorouracil.74 This study suggests that if spillage or uncontrolled release of a PNDDS is to occur and to expose ryegrass roots to the PNDDS, ryegrass can adsorb and uptake it. This is very important considering that PNDDSs on and in the plants may later be consumed by animals and bacteria and accumulate in their bodies, and can adversely influence environmental health.
Conclusions
In summary, this study shows that ryegrass can uptake PNDDSs of size ranging from 46 nm to 271 nm through its roots. The rate and fraction of PNDDS uptake by ryegrass roots increases with decreasing size. It is also shown that very small amount of a PNDDS, if any, transport from stem to leaf, which is presumably due to the selectivity of the membranous ligule. Furthermore, the adsorption step was faster than the uptake step, making the uptake step the overall rate determining step for the transport of PNDDS from the aqueous media into the ryegrass root.Acknowledgements
Acknowledgement is made to the donors of The American Chemical Society Petroleum Research Fund for the partial support of this research. This project was also partially supported by Agriculture and Food Research Initiative Competitive Grant no. 2011-67017-30028 from the USDA National Institute of Food and Agriculture. Part of this material is based upon work supported the National Science Foundation under Grant No. 1236532.References
- E. Fisher and C. Selin, The yearbook of nanotechnology in society, Springer, London, 2008 Search PubMed.
- N. Moran, Nature Biotechnology, 2006, 24, 121–121 CrossRef CAS.
- A. Maynard, D. Bowman and G. Hodge, Nat Mater, 2011, 10, 554–557 CrossRef CAS.
- V. L. Colvin, Nature Biotechnology, 2003, 21, 1166–1170 CrossRef CAS.
- S. J. Klaine, P. J. J. Alvarez, G. E. Batley, T. F. Fernandes, R. D. Handy, D. Y. Lyon, S. Mahendra, M. J. McLaughlin and J. R. Lead, Environ Toxicol Chem, 2008, 27, 1825–1851 CrossRef CAS.
- M. R. Wiesner, G. V. Lowry, K. L. Jones, M. F. Hochella, R. T. Di Giulio, E. Casman and E. S. Bernhardt, Environ Sci Technol, 2009, 43, 6458–6462 CrossRef CAS.
- P. J. A. Borm and D. Muller-Schulte, Nanomedicine-UK, 2006, 1, 235–249 CrossRef CAS.
- V. S. W. Chan, Regulatory Toxicology and Pharmacology, 2006, 46, 218–224 CrossRef CAS.
- X. M. Ma, J. Geiser-Lee, Y. Deng and A. Kolmakov, Sci Total Environ, 2010, 408, 3053–3061 CrossRef CAS.
- T. M. Allen and P. R. Cullis, Science, 2004, 303, 1818–1822 CrossRef CAS.
- O. C. Farokhzad and R. Langer, ACS Nano, 2009, 3, 16–20 CrossRef CAS.
- M. E. Davis, Z. Chen and D. M. Shin, Nat Rev Drug Discov, 2008, 7, 771–782 CrossRef CAS.
- J. H. Park, S. Lee, J.-H. Kim, K. Park, K. Kim and I. C. Kwon, Progress in Polymer Science, 2008, 33, 113–137 CrossRef.
- J. Jeong, W.-S. Cho, S. H. Kim and M.-H. Cho, In Vitro and In Vivo Toxicity Study of Nanoparticles, John Wiley & Sons, Ltd, 2009 Search PubMed.
- H. L. Chen and R. S. Juang, J Membrane Sci, 2008, 325, 599–604 CrossRef CAS.
- R. Minchin, Nat Nano, 2008, 3, 12–13 CrossRef CAS.
- H. K. de Wolf, C. J. Snel, F. J. Verbaan, R. M. Schiffelers, W. E. Hennink and G. Storm, International Journal of Pharmaceutics, 2007, 331, 167–175 CrossRef CAS.
- S. K. Brar, M. Verma, R. D. Tyagi and R. Y. Surampalli, Waste Manage, 2010, 30, 504–520 CrossRef CAS.
- S. R. Smith and C.A.B. International, Agricultural recycling of sewage sludge and the environment, CAB International, Wallingford, 1996 Search PubMed.
- B. Nowack, N. C. Mueller, F. Gottschalk, T. Sonderer and R. W. Scholz, Abstr Pap Am Chem S, 2009, 237 Search PubMed.
- C. M. Rico, S. Majumdar, M. Duarte-Gardea, J. R. Peralta-Videa and J. L. Gardea-Torresdey, J Agr Food Chem, 2011, 59, 3485–3498 CrossRef CAS.
- D. H. Lin and B. S. Xing, Abstr Pap Am Chem S, 2008, 235, 1053 Search PubMed.
- W. L. Zhang, Y. L. Li, L. X. Liu, Q. Q. Sun, X. T. Shuai, W. Zhu and Y. M. Chen, Biomacromolecules, 2010, 11, 1331–1338 CrossRef CAS.
- A. Richter, C. Olbrich, M. Krause and T. Kissel, Int J Pharm, 2010, 389, 244–253 CrossRef CAS.
- J. Suksiriworapong, K. Sripha, J. Kreuter and V. B. Junyaprasert, Bioconjugate Chem, 2011, 22, 582–594 CrossRef CAS.
- W. K. Oh, H. Yoon and J. Jang, Biomaterials, 2010, 31, 1342–1348 CrossRef CAS.
- M. Akbulut, P. Ginart, M. E. Gindy, C. Theriault, K. H. Chin, W. Soboyejo and R. K. Prud'homme, Advanced Functional Materials, 2009, 19, 718–725 CrossRef CAS.
- M. Akbulut, S. M. D'Addio, M. E. Gindy and R. K. Prud'homme, Expert Review of Clinical Pharmacology, 2009, 2, 265–282 CrossRef CAS.
- E. A. Ellis, Microscopy Today, 2006, 14, 32–33 CAS.
- D. L. Sackett and J. Wolff, Anal Biochem, 1987, 167, 228–234 CrossRef CAS.
- P. Greenspan and S. D. Fowler, J Lipid Res, 1985, 26, 781–789 CAS.
- A. Hawe, M. Sutter and W. Jiskoot, Pharmaceutical Research, 2008, 25, 1487–1499 CrossRef CAS.
- P. deTroch and J. Vanderleyden, Microbial Ecol, 1996, 32, 149–169 CAS.
- K. G. Tiller, Jl. Honeyset and M. P. C. Devries, Aust. J. Soil Res., 1972, 10, 151–164 CrossRef CAS.
- G. W. Bailey and J. L. White, J. Agric. Food Chem., 1964, 12, 324 CrossRef CAS.
- F. M. W. Grundler, M. Sobczak and W. Golinowski, Eur. J. Plant Pathol., 1998, 104, 545–551 CrossRef.
- H. Wakabayashi, S. Morita, K. Koba, H. Tachibana and K. Matsumoto, J. Endod., 1995, 21, 543–545 CrossRef CAS.
- M. Zhang and M. Akbulut, Langmuir, 2011, 27, 12550–12559 CrossRef CAS.
- P. R. VanTassel, P. Viot and G. Tarjus, J. Chem. Phys., 1997, 106, 761–770 CrossRef CAS.
- A. K. Hunter and G. Carta, J. Chromatogr., A, 2002, 971, 105–116 CrossRef CAS.
- M. R. Aboudzadeh, J. W. Zhu and W. Bin, Korean J. Chem. Eng., 2006, 23, 997–1002 CrossRef CAS.
- S. H. Chen, H. Yao and K. Kimura, Langmuir, 2001, 17, 733–739 CrossRef CAS.
- Y. M. Xu and C. H. Langford, Langmuir, 2001, 17, 897–902 CrossRef CAS.
- J. A. Shar, T. Cosgrove, T. M. Obey, M. R. Warne and D. J. Wedlock, Langmuir, 1999, 15, 7688–7694 CrossRef CAS.
- M. Paul, A. Laatiris, H. Fessi, B. Dufeu, R. Durand, M. Deniau and A. Astier, Drug Dev. Res., 1998, 43, 98–104 CrossRef CAS.
- P. J. Lea and R. C. Leegood, Plant biochemistry and molecular biology, Wiley, Chichester England; New York, 1993 Search PubMed.
- K. J. Oparka, Plasmodesmata, Blackwell, Oxford, 2005 Search PubMed.
- A. W. Robards, Annu. Rev. Plant Physiol., 1975, 26, 13–29 Search PubMed.
- A. G. Roberts and K. J. Oparka, Plant, Cell Environ., 2003, 26, 103–124 CrossRef.
- W. M. Lee, Y. J. An, H. Yoon and H. S. Kweon, Environ. Toxicol. Chem., 2008, 27, 1915–1921 CrossRef CAS.
- A. Hischemoller, J. Nordmann, P. Ptacek, K. Mummenhoff and M. Haase, J. Biomed. Nanotechnol., 2009, 5, 278–284 CrossRef.
- D. H. Lin and B. S. Xing, Environ. Pollut., 2007, 150, 243–250 CrossRef CAS.
- H. Zhu, J. Han, J. Q. Xiao and Y. Jin, J. Environ. Monit., 2008, 10, 713–717 RSC.
- S. J. Lin, J. Reppert, Q. Hu, J. S. Hudson, M. L. Reid, T. A. Ratnikova, A. M. Rao, H. Luo and P. C. Ke, Small, 2009, 5, 1128–1132 CrossRef CAS.
- D. A. Navarro, M. Bisson and B. D. Aga, J. Hazard. Mater., 2012, 211–212, 427–435 CrossRef CAS.
- M. J. Spiering, G. A. Lane, M. J. Christensen and J. Schmid, Phytochemistry, 2005, 66, 195–202 CrossRef CAS.
- V. Amiard, A. Morvan-Bertrand, J. P. Billard, C. Huault and M. P. Prud'homme, J. Exp. Bot., 2003, 54, 1231–1243 CrossRef CAS.
- M. A. Moreno, L. C. Harper, R. W. Krueger, S. L. Dellaporta and M. Freeling, Genes Dev., 1997, 11, 616–628 CrossRef CAS.
- N. Endrein Basic Equations of the Mass Transport through a Membrane Layer, Elsevier, Oxford, 2012, pp. 1–34 Search PubMed.
- H. Nikaido and E. Y. Rosenberg, J. Gen. Physiol., 1981, 77, 121–135 CrossRef CAS.
- S. E. A. Gratton, P. A. Ropp, P. D. Pohlhaus, J. C. Luft, V. J. Madden, M. E. Napier and J. M. DeSimone, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 11613–11618 CrossRef CAS.
- V. Y. Alakhov, E. Y. Moskaleva, E. V. Batrakova and A. V. Kabanov, Bioconjugate Chem., 1996, 7, 209–216 CrossRef CAS.
- A. A. Bogdanov, C. Martin, A. V. Bogdanova, T. J. Brady and R. Weissleder, Bioconjugate Chem., 1996, 7, 144–149 CrossRef CAS.
- D. Bhadra, S. Bhadra, S. Jain and N. K. Jain, Int. J. Pharm., 2003, 257, 111–124 CrossRef CAS.
- M. Simeonova, R. Velichkova, G. Ivanova, V. Enchev and I. Abrahams, Int. J. Pharm., 2003, 263, 133–140 CrossRef CAS.
- J. A. Simon, Menopause, 2006, 13, 222–231 CrossRef.
- P. Lim Soo, J. Lovric, P. Davidson, D. Maysinger and A. Eisenberg, Mol. Pharmaceutics, 2005, 2, 519–527 CrossRef.
- H. M. Burt, X. Zhang, P. Toleikis, L. Embree and W. L. Hunter, Colloids Surf., B, 1999, 16, 161–171 CrossRef CAS.
- E. Batrakova, S. Li, D. Miller and A. Kabanov, Pharm. Res., 1999, 16, 1366–1372 CrossRef CAS.
- O. C. Farokhzad, J. Cheng, B. A. Teply, I. Sherifi, S. Jon, P. W. Kantoff, J. P. Richie and R. Langer, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 6315–6320 CrossRef CAS.
- J. Cheng, B. A. Teply, I. Sherifi, J. Sung, G. Luther, F. X. Gu, E. Levy-Nissenbaum, A. F. Radovic-Moreno, R. Langer and O. C. Farokhzad, Biomaterials, 2007, 28, 869–876 CrossRef CAS.
- M. Cleuvers, Toxicol. Lett., 2003, 142, 185–194 CrossRef CAS.
- D. R. Dietrich, B. C. Hitzfeld and E. O'Brien, Toxicology and Risk Assessment of Pharmaceuticals, Wiley-VCH Verlag GmbH & Co. KGaA, 2006 Search PubMed.
- R. Zounková, P. Odráška, L. Doležalová, K. Hilscherová, B. Maršálek and L. Bláha, Environ. Toxicol. Chem., 2007, 26, 2208–2214 CrossRef.
Footnote |
| † Electronic Supplementary Information (ESI) available: Emission fluorescence spectra of PNDDS of different sizes; linear fit of the SFM maximum intensity versus concentration of PNDDS; confocal microscopy images focusing on the different planes of a root after dipping it in 159-nm PNDDS solution for 81 h. See DOI: 10.1039/c2ra21469e |
| This journal is © The Royal Society of Chemistry 2012 |
