Preparation and characterization of cellulose/curcumin composite films
Nan
Luo
a,
K.
Varaprasad
b,
G. Venkata Subba
Reddy
c,
A. Varada
Rajulu
b and
Jun
Zhang
*a
aCAS Key Lab of Engineering Plastics, Institute of Chemistry, Chinese Academy of Sciences (CAS), Beijing 100190, China. E-mail: jzhang@iccas.ac.cn
bDepartment of Polymer Science and Technology, Sri Krishnadevaraya University, Anantapur 515 055, India
cDepartment of Microbiology, Sr Krishnadevaraya University, Anantgapur 515 055, India
First published on 17th July 2012
Abstract
Water-insoluble curcumin was found to be well dissolved in an ionic liquid 1-allyl-3-methylimidazolium chloride (AmimCl), which is also an effective solvent for cellulose. Using AmimCl as the solvent, cellulose/curcumin composite films with various curcumin contents ranging from 0 to 5 wt% were prepared by solution-mixing and casting. The obtained films containing curcumin were highly transparent with a bright yellow color. These composite films possessed good mechanical properties and thermal stability, both of which are comparable to the pure cellulose films. The SEM observation of the fracture surface of the cellulose/curcumin composite films indicated the uniform distribution of curcumin in the matrix. The antibacterial activity of the composite films was examined by a zone method against E. coli. The results showed that the cellulose/curcumin composite films exhibited obvious antibacterial activity, and the inhibition zone diameter against the bacterium was proportional to the curcumin content in the composite films. Hence, these cellulose/curcumin composite films prepared entirely from natural resources can be considered as novel kinds of functional films and could find applications in food packaging and medical fields.
Introduction
Owing to their outstanding properties, like light weight, high strength-to-weight ratio, ease of manufacturing, low cost, etc., synthetic polymers in general and polymer composites in particular are finding innumerable applications in all fields. Unfortunately, as most synthetic polymers are nondegradable, the environmental problems posed by them are also simultaneously increasing.1 In order to resolve these problems, the trend is now shifting towards developing environmentally friendly green composites using natural materials. In this direction, many natural materials like wood,2,3 corn stover,4,5 starch,6,7 chitosan8,9etc. have been used for making green composites, which meet a need for exploring low cost, biodegradable and renewable materials.Cellulose is the most abundant natural, renewable, biodegradable and non-edible low cost source, which has been exploited for thousands of years in the manufacture of fibers, textiles, paper, films etc. However, cellulose processing is extremely difficult in general, because this natural polymer is neither meltable nor soluble in conventional solvents due to its hydrogen bonded and partially crystalline structure. Therefore, the present industrial production of regenerated cellulose is, and has been for a long time, dominated by the polluting viscose process.10 With increasing governmental regulations for industries, the development of “green” technology for cellulose processing with more simple and effective reagents and more limited steps is becoming increasingly important.
Room-temperature ionic liquids (RTILs), defined as a class of low-melting-point organic salts, are considered as desirable ‘green’, recyclable alternatives to the traditional volatile organic solvents on account of their unique physiochemical properties such as negligible vapor pressure, high thermal stability, wide liquid range and tunable solvation properties.11,12 During the past several years, it has been reported that some ionic liquids, such as 1-butyl-3-methylimidazolium chloride (BMIMCl), 1-allyl-3-methylimidazolium chloride (AMIMCl) and 1-ethyl-3-methylimidazolium acetate (EMIMAc), exhibit an outstanding capability for dissolving cellulose.13–15 RTILs are providing a new and versatile platform for the wide utilization of cellulose resources and preparation of novel cellulose-based materials with special properties.16–20
Curcumin is a natural yellow-orange compound derived from the root of Curcuma Longa21 that is widely used for medicinal as well as food purposes.22 Curcumin exhibits potent antioxidant, antitumor, antibacterial and anticancer properties.23,24 Clinical trials have shown curcumin to be safe, even when consumed at a daily dosage of 12 g for 3 months.25 Recently, studies on the incorporation of curcumin into biocompatible or hydrophilic polymers to produce polymer composites that are bioactive have received extensive attention. Suwantong et al.26,27 prepared ultra-fine cellulose acetate (CA) fiber mats containing curcumin by electrospinning. The curcumin-loaded CA fiber mats have a potential use as carriers for topical/transdermal patches or wound dressings. Chen et al.28 prepared PLA/curcumin composite membranes by electrospinning and the obtained films were found to possess good anticoagulation behavior. Varaprasad et al.29,30 fabricated silver nanocomposite films or hydrogels impregnated with curcumin, which are potentially useful in treating infection because of their superior antibacterial properties. Gopinath et al.31 prepared curcumin incorporated collagen films to support dermal wound healing.
However, curcumin is insoluble in water or aqueous solutions, which limits its applications that would utilise its bioactivity. Therefore, many attempts have been made to overcome this barrier by increasing its solubility in water and improving its bioavailability. The explored approaches involve: the use of adjuvants like piperine that interfere with glucuronidation; the use of liposomal curcumin; curcumin nanoparticles; the use of curcumin phospholipid complex; the use of structural analogues of curcumin.32 However, these methods seem to be expensive or complicated or have a low performance. Therefore, to enlarge the applicable potential of curcumin in biomedicine, a simple and effective method to incorporate curcumin into polymers is still desired.
In view of the pharmacological applications and safety of curcumin and the excellent mechanical properties of cellulose films, an attempt in the present work was made to incorporate a natural antibacterial food material into cellulose and make composite films and study their mechanical properties, thermal stability and antibacterial activity. The composite films have great potential in food packaging and medical applications.
Experimental
Materials
Cotton linter pulp with an average degree of polymerization of 620 was supplied by Hubei Chemical Company Limited (Xiangfan, China). Curcumin with a minimum assay of 98% supplied by Sigma Aldrich (Bengaluru, India) was used. The ionic liquid, AmimCl, was synthesized in our laboratory by the method described in our previous work,14 and the water content in the resultant ionic liquid was less than 0.3% as measured by the Carl–Fisher method. Cotton linters and curcumin were dried at 80 °C for 6 h prior to use.Preparation of cellulose/curcumin composite films
To prepare cellulose/curcumin composite films, a known weight of curcumin and 0.8 g of cotton linter pulp were dispersed into 19.2 g AmimCl in a flask connected to a vacuum pump. The mixtures were heated at 100 °C and stirred for 1.5 h until the cellulose samples were completely dissolved, yielding transparent solutions with about 4% cellulose concentration. The solutions were cast onto a glass plate to give a thickness of about 0.50 mm, then immediately coagulated in deionized water, giving transparent regenerated cellulose/curcumin composite films. To remove residual ionic liquid in samples, the films were further washed with distilled water at least three times until no Cl− ions were detectable by the AgNO3 test. After drying in a vacuum oven at 80 °C for 12 h, the cellulose/curcumin composite films with different curcumin content (0, 1, 2, 3, 4, 5 wt%) were obtained and kept in a dessicator prior to characterization.Characterization
Polarized optical microscopy was used to evaluate the solubility of curcumin in ionic liquid and was conducted on a Leica DMLP polarizing microscope (Leica company, German). The 40× magnification was chosen for recording the micrographs. A droplet of solution sandwiched between a clean glass slide and a cove slip was used for observation.Scanning electron microscopy (SEM) was used to observe the fracture surface of cellulose/curcumin composite films and was carried out on a JSM-6700F JEOL scanning electron microscope at an accelerating voltage of 10 KV. The brittle fractured samples for SEM observation were chosen randomly from films. The cross-sections of the specimens were coated with platinum before observation.
Tensile testing was performed on an Instron 3365 with a 5 kN load cell at a crosshead speed of 2 mm min−1. The specimens were cut into rectangular-shaped strips with 10 mm widths and 50 mm gauge lengths. The average values and standard deviations were calculated for five samples.
Thermogravimetric analysis of the composites was performed using a Pyris 1 thermal analyzer (Perkin Elmer Instruments, USA). The samples (3 ± 0.5 mg) cut from films were heated in a Pt crucible from 30 to 600 °C in a N2 atmosphere at a heating rate of 20 °C min−1.
Antibacterial activity
The antibacterial activity of the composite films under study was investigated by a disc method using the standard procedure.29 Nutrient agar medium was prepared by mixing peptone (5.0 g), beef extract (3.0 g), and sodium chloride (NaCl) (5.0 g) in 1000 ml distilled water and the pH was adjusted to 7.0. Finally, agar (15.0 g) was added to the solution. The agar medium was sterilized in a conical flask at a pressure of 15 lbs for 30 min. This medium was transferred into sterilized Petri dishes in a laminar air flow chamber (Microfilt Laminar Flow Ultra Clean Air Unit, Mumbai, India). After solidification of the media, the E. coli (50 μl) culture was spread on the solid surface of the media. Over this inoculated Petri dish, small pieces of cellulose and composite films were placed and incubated for 2 days at 37 °C in the incubation chamber. After this period, the observed inhibition zones were photographed.Results and discussion
Dissolution of curcumin in AmimCl and preparation of cellulose/curcumin composite films
To prepare the cellulose/curcumin composites, dissolution of curcumin in cellulose solvents is the prerequisite. Although quite soluble in organic solvents such as DMSO, ethanol, methanol or acetone, curcumin is poorly soluble in aqueous solvents.33 So far, no work has been reported on the solubility of curcumin in ionic liquids. Therefore, in the present study, we first evaluated the solubility of curcumin in the ionic liquid AmimCl, a powerful non-derivatizing solvent for cellulose. The dissolution procedure of curcumin in AmimC1 was monitored by polarized microscopy and the micrographs of the solutions at different dissolution times are presented in Fig. 1. From these micrographs, it can be seen that the initial solid crystalline structure of curcumin gradually disappeared as the dissolution process proceeded. The minimum time taken for the dissolution of curcumin in this solvent was found to be 30 min. It is evident that curcumin can be well dissolved in AmimCl within a short period. | ||
| Fig. 1 The polarized micrographs of curcumin/AmimCl (1%) solutions. | ||
The dissolution of cellulose in AmimC1 was well established by us.14 Both cellulose and curcumin were also found to be uniformly soluble in AmimCl, which was further evidenced by the polarized optical micrographs of the solution. It was found that the addition of curcumin into the cellulose solution in AmimCl did not result in the precipitation of cellulose. Fig. 2 presents the photographs of cellulose, curcumin and cellulose/curcumin solutions prepared with AmimCl solvent. It can be seen that all the solutions were uniform and clear, and the solutions containing curcumin have a distinct yellow color.
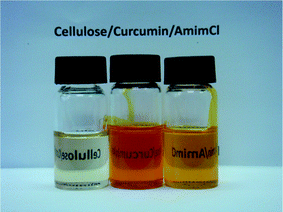 | ||
| Fig. 2 The photographs of cellulose (left, 4%), curcumin (middle, 1%) and cellulose/curcumin solutions (right, 3% curcumin for cellulose) prepared with AmimCl solvent. | ||
As AmimCl is completely miscible with water in any ratio, the cellulose/curcumin composite gels could be prepared using a water coagulation bath. After thoroughly drying the gels, the cellulose/curcumin composite films were obtained. The photographs of cellulose/curcumin (3 wt%) composite gel and its dried film are shown in Fig. 3. It can be seen that the composite film containing curcumin was transparent lemon yellow in color compared with the pure cellulose film.
 | ||
| Fig. 3 Photos of cellulose/curcumin composite gel (a), cellulose (b) and cellulose/curcumin composite (c) films with 3 wt% curcumin. | ||
SEM observation for fractured surface of cellulose/curcumin composite films
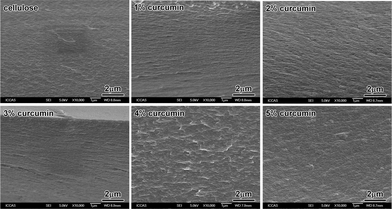
To evaluate the dispersion of the curcumin component in cellulose, SEM observations of the fracture surface of cellulose/curcumin composite films was carried out and the results are shown in Fig. 4. It can be seen that, when the curcumin content was below 4 wt%, the fracture surfaces of composites were compact and relatively smooth, which is similar to that of pure regenerated cellulose film. When the curcumin content was above 4 wt%, the fracture surface of cellulose/curcumin composite films became somewhat rough, but no obvious aggregation or dispersed particle could be seen. The SEM observations indicate the uniform distribution of curcumin in the matrix.Mechanical properties of cellulose/curcumin composite films
The tensile stress–strain curves of the cellulose and cellulose/curcumin composite films are shown in Fig. 5. The obtained average Young's modulus E (GPa), tensile strength (MPa), and % elongation at break of the composite films with different curcumin content are presented in Table 1.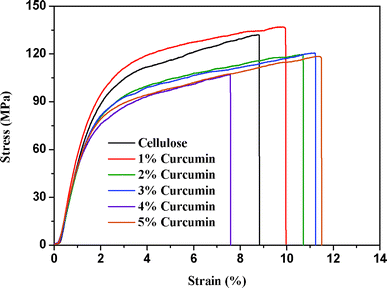 | ||
| Fig. 5 Stain–stress curves of cellulose and cellulose/curcumin composite films. | ||
| Code | Cellulose (wt%) | Curcumin (wt%) | Tensile modulus/GPa | Tensile strength/MPa | Elongation (%) |
|---|---|---|---|---|---|
| RC-0 | 100 | 0 | 6.7 ± 0.5 | 130 ± 7 | 9.1 ± 1.3 |
| RC-1 | 99 | 1 | 7.2 ± 0.3 | 131 ± 5 | 8.1 ± 1.8 |
| RC-2 | 98 | 2 | 6.3 ± 0.1 | 115 ± 4 | 8.4 ± 1.4 |
| RC-3 | 97 | 3 | 6.3 ± 0.1 | 114 ± 4 | 8.6 ± 1.7 |
| RC-4 | 96 | 4 | 6.2 ± 0.1 | 110 ± 2 | 8.7 ± 0.9 |
| RC-5 | 95 | 5 | 6.0 ± 0.6 | 112 ± 4 | 10.1 ± 2.5 |
From Fig. 5, it can be seen that no yield point existed in the tensile process for the composite films, indicating the brittle nature of the composite films. Interestingly, from Fig. 5 and Table 1, it is evident that the tensile properties of cellulose/curcumin composite films decreased only marginally compared to pure cellulose film. Even for a curcumin content of 5 wt%, the composite films possessed a tensile strength and modulus greater than 110 MPa and 6 GPa, respectively, and the elongation at break reached 8% in the absence of a plasticizer. In fact, the incorporation of curcumin as a small molecular compound is expected to decrease the tensile properties of composite films. The good mechanical properties of cellulose/curcumin composite films can be explained by the uniform dispersion of curcumin in cellulose and the good interfacial interaction between them. It should be noted that the tensile properties of these cellulose/curcumin composite films are much higher than that of the commonly used polyolefin-based packaging materials,34 whose tensile strengths are usually in a range of 20–40 MPa. Transparency, uniformity, high strength etc. are often the main criteria required for packaging films. All these results indicate the potential application of cellulose/curcumin films for packaging applications.
Thermal properties
In order to verify the thermal stability of the composite films under study, their thermal properties were evaluated by thermogravimetric analysis (TGA). The primary thermograms of cotton linter, regenerated cellulose, curcumin and their composite films are presented in Fig. 6, and some of their thermal parameters are listed in Table 2.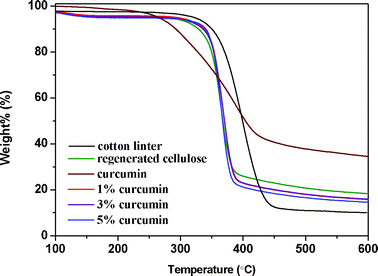 | ||
| Fig. 6 TGA curves of cotton linters, regenerated cellulose and cellulose/curcumin composites. | ||
| Code | Cellulose (wt%) | Curcumin (wt%) | T onset/°C | T max/°C | Residue (%) |
|---|---|---|---|---|---|
| Cellulose | 100 | 0 | 361 | 400 | 10.1 |
| Curcumin | 0 | 100 | 282 | 385 | 34.6 |
| RC | 100 | 0 | 341 | 365 | 18.4 |
| RC-1 | 99 | 1 | 346 | 369 | 15.8 |
| RC-2 | 98 | 2 | 340 | 368 | 19.0 |
| RC-3 | 97 | 3 | 346 | 370 | 15.9 |
| RC-4 | 96 | 4 | 346 | 367 | 13.2 |
| RC-5 | 95 | 5 | 347 | 366 | 14.5 |
From the thermograms, it can be seen that the pure cellulose and regenerated cellulose (RC) samples start to decompose at 360 °C and 340 °C, respectively, while the curcumin starts to decompose at 282 °C. The temperature at the maximum degradation rate for curcumin is about 385 °C, which is lower than original cellulose (400 °C) but higher than regenerated cellulose (365 °C). The char residue of curcumin is much higher than that of both original and regenerated cellulose, which should be due to the phenyl rings in curcumin. Pure curcumin has a lower onset temperature of degradation and a higher temperature of maximum decomposition compared with cellulose. However, its addition did not have an obvious influence on the thermal stability of cellulose/curcumin films. All cellulose/curcumin composite samples were thermally stable up to 340 °C, which is the same for pure regenerated cellulose. The good thermal stability of cellulose/curcumin composites may be attributed to the good dispersion of curcumin in the matrix and the quite strong interaction between these two components. These observations indicate that the composite films under study can also be used at elevated temperatures.
Antibacterial properties
Curcumin is known to kill several pathogenic gram-positive bacteria such as Staphylococcus aureus, Staphylococcus epidermidis and Enterococcus, and some Gram-negative bacteria such as Escherichia coli and Dysentery Bacilli.35 This is because FtsZ is essential for bacterial cell division and viability, which is a prokaryotic homologue of eukaryotic cytoskeletal protein tubulin.36 Curcumin can disturb the FtsZ functions, leading to the inhibition of bacterial proliferation,37 which in turn can result in anti-bacterial activity.The photographs of the films showing antibacterial activity are presented in Fig. 7. From this figure, it is clearly evident that no inhibition zone was apparent for neat cellulose film, whereas these zones were observed for all the composite films. The measured lengths of inhibition zones of the composite films of varying curcumin content are present in Table 3. From this table, it is clearly evident that the zone diameter increased with increasing curcumin content. These observations clearly indicate the antibacterial activity of the cellulose/curcumin films. Varaprasad et al.30 also observed that the presence of curcumin enhanced the antibacterial activity of sodium carboxylmethyl cellulose silver nanocomposite films impregnated with curcumin. In our study, we found that the cellulose/curcumin composite films showed antibacterial activity even after 50 days of their preparation. Hence, these cellulose based films can also be considered for some medical and food packaging applications.
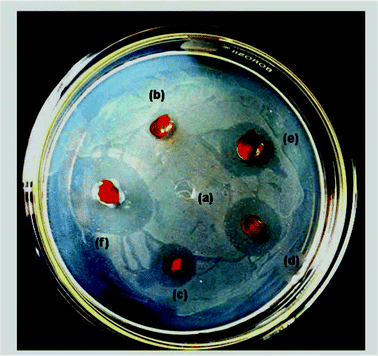 | ||
| Fig. 7 Antibacterial activity of composite films with (a) 0 wt%, (b) 1 wt%, (c) 2 wt%, (d) 3 wt%, (e) 4wt % and (f) 5wt % curcumin against E. coli. | ||
| Sample (curcumin) | a (blank) | b (1%) | c (2%) | d (3%) | e (4%) | f (5%) |
|---|---|---|---|---|---|---|
| Antibacterial inhibition zone on E. coli/cm | — | 0.7 | 1.2 | 1.6 | 1.7 | 2.3 |
Conclusions
Cellulose/Curcumin biocomposite films were prepared using an ionic liquid AmimCl as a solvent. The ionic liquid showed good solubility for cellulose and curcumin. The composite films were transparent with a bright yellow color. SEM results showed that curcumin had good compatibility with cellulose, and it dispersed uniformly in the cellulose matrix without any phase separation. Further, the films had excellent mechanical properties, with tensile strength, modulus and elongation at break higher than 110 MPa, 6 GPa and 8%, respectively. The addition of curcumin did not decrease the mechanical properties of composite films appreciably because of its good dispersion and the strong interfacial adhesion. For the same reason, the composite films were found to be thermally stable up to 340 °C which is the same as for regenerated cellulose. Further, these films exhibited good antibacterial activity against E. Coli bacteria even after 50 days of their preparation. These completely biodegradable composite films possessing transparency, good mechanical properties and antibacterial activity can be considered as potential candidates for food packaging and medical applications.Acknowledgements
J.Z. acknowledges financial support from of the National Natural Science Foundation of China (21174151, and 50873111), Chinese Academy of Sciences (KJCX2-YW-H30-03) and the National Basic Research Program of China (973 Program) (No. 2010CB934705). AVR wishes to thank the Council of Scientific and Industrial Research (CSIR) of India for the award of an Emeritus Scientist Scheme [21(0842)/11/EMR-II dt:10-05-2011].References
- M. J. John and S. Thomas, Carbohydr. Polym., 2008, 71, 343–364 CrossRef CAS.
- K. S. Mikkonen, J. S. Stevanic, C. Joly, P. Dole, K. Pirkkalainen, R. Serimaa, L. Salmen and M. Tenkanen, Cellulose, 2011, 18, 713–726 CrossRef CAS.
- J. Li, Y. Lu, D. J. Yang, Q. F. Sun, Y. X. Liu and H. J. Zhao, Biomacromolecules, 2011, 12, 1860–1867 CrossRef CAS.
- Y. Cao, H. Q. Li, Y. Zhang, J. Zhang and J. S. He, J. Appl. Polym. Sci., 2010, 116, 547–554 CrossRef CAS.
- N. Reddy and Y. Q. Yang, Green Chem., 2005, 7, 190–195 RSC.
- Y. S. Lu, L. H. Weng and X. D. Cao, Macromol. Biosci., 2005, 5, 1101–1107 CrossRef CAS.
- H. Miyamoto, C. Yamane, M. Seguchi and K. Okajima, Food Sci. Technol. Res., 2009, 15, 403–412 CrossRef CAS.
- J. Kim, Z. J. Cai, H. S. Lee, G. S. Choi, D. H. Lee and C. Jo, J. Polym. Res., 2011, 18, 739–744 CrossRef CAS.
- T. J. Park, Y. J. Jung, S. W. Choi, H. Park, H. Kim, E. Kim, S. H. Lee and J. H. Kim, Macromol. Res., 2011, 19, 213–215 CrossRef CAS.
- T. Liebertin Cellulose Solvents: For Analysis, Shaping and Chemical Modification, ed. T. F. Liebert, T. J. Heinze and K. J. Edgar, Amer Chemical Soc, Washington, 2009, vol. 1033, pp. 3-54 Search PubMed.
- J. G. Huddleston, A. E. Visser, W. M. Reichert, H. D. Willauer, G. A. Broker and R. D. Rogers, Green Chem., 2001, 3, 156–164 RSC.
- S. Aparicio, M. Atilhan and F. Karadas, Ind. Eng. Chem. Res., 2010, 49, 9580–9595 CrossRef CAS.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS.
- H. Zhang, J. Wu, J. Zhang and J. S. He, Macromolecules, 2005, 38, 8272–8277 CrossRef CAS.
- Y. Cao, J. Wu, J. Zhang, H. Q. Li, Y. Zhang and J. S. He, Chem. Eng. J., 2009, 147, 13–21 CrossRef CAS.
- R. J. Moon, A. Martini, J. Nairn, J. Simonsen and J. Youngblood, Chem. Soc. Rev., 2011, 40, 3941–3994 RSC.
- N. Hameed and Q. P. Guo, Cellulose, 2010, 17, 803–813 CrossRef CAS.
- M. G. Freire, A. R. R. Teles, R. A. S. Ferreira, L. D. Carlos, J. A. Lopes-da-Silva and J. A. P. Coutinho, Green Chem., 2011, 13, 3173–3180 RSC.
- C. Tsioptsias, A. Stefopoulos, I. Kokkinomalis, L. Papadopoulou and C. Panayiotou, Green Chem., 2008, 10, 965–971 RSC.
- H. Zhang, Z. G. Wang, Z. N. Zhang, J. Wu, J. Zhang and J. S. He, Adv. Mater., 2007, 19, 698–704 CrossRef CAS.
- B. B. Aggarwal, A. Kumar, M. S. Aggarwal and S. ShishodiaIn: Preuss H, ed. Phyto-pharmaceuticals in cancer chemoprevention, Boca RattonL CRC Press, 2005, pp.349 Search PubMed.
- K. S. Parvathu, P. S. Negi and P. Srinivas, Antioxidant, Food Chem., 2009, 115, 265–271 Search PubMed.
- R. A. Sharma, A. J. Gescher and W. P. Steward, Eur. J. Cancer, 2005, 41, 1955–1968 CrossRef CAS.
- R. De, P. Kundu, S. Swarnakar, T. Ramamurthy, A. Chowdhury, G. B. Nair and A. K. Mukhopadhyay, Antimicrob. Agents Chemother., 2009, 53, 1592–1597 CrossRef CAS.
- A. Goel, A. B. Kunnumakkara and B. B. Aggarwal, Biochem. Pharmacol., 2008, 75, 787–809 CrossRef CAS.
- O. Suwantong, P. Opanasopit, U. Ruktanonchal and P. Supaphol, Polymer, 2007, 48, 7546–7557 CrossRef CAS.
- O. Suwantong, U. Ruktanonchai and P. Supaphol, J. Biomed. Mater. Res. A, 2010, 94A, 1216–1225 CAS.
- Y. Chen, J. Lin, Y. N. Fei, H. B. Wang and W. D. Gao, Fibers Polym., 2010, 11, 1128–1131 CrossRef CAS.
- K. Varaprasad, K. Vimala, S. Ravindra, N. N. Reddy, G. V. S. Reddy and K. M. Raju, J. Mater. Sci.: Mater. Med., 2011, 22, 1863–1872 CrossRef CAS.
- K. Varaprasad, Y. M. Mohan, K. Vimala and K. M. Raju, J. Appl. Polym. Sci., 2011, 121, 784–796 CrossRef CAS.
- D. Gopinath, M. R. Ahmed, K. Gomathi, K. Chitra, P. K. Sehgal and R. Jayakumar, Biomaterials, 2004, 25, 1911–1917 CrossRef CAS.
- P. Anand, A. B. Kunnumakkara, R. A. Newman and B. B. Aggarwal, Mol. Pharmaceutics, 2007, 4, 807–818 CrossRef CAS.
- H. H. Tonnesen and J. Karisen, Z. Lebensm.-Unters. Forsch., 1985, 180, 402–404 CrossRef CAS.
- H. Ding, Handbook of Plastic Industry, Chemical Industry Press, Beijing, 1995 Search PubMed.
- S. Mishra, U. Narain, R. Mishra and K. Misra, Bioorg. Med. Chem., 2005, 13, 1477–1486 CrossRef CAS.
- D. RAI, J. Kumar Singh, N. Roy and D. Panda, Biochem. J., 2008, 410, 147–155 CrossRef CAS.
- S. Kaur, N. H. Modi, D. Panda and N. Roy, Eur. J. Med. Chem., 2010, 45, 4209–4214 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |