One-step preparation of giant lipid vesicles with high encapsulation efficiency using an electrospray technique†
Tatsuo
Maruyama
*,
Yu
Fukui
,
Eiko
Tsuchiya
and
Akihiro
Fujii
Department of Chemical Science and Engineering, Kobe University, 1-1 Rokkodai, Nada-ku, Kobe 657-850, Japan. E-mail: tmarutcm@crystal.kobe-u.ac.jp.; Fax: +81-(0)78-803-6070
First published on 5th October 2012
Abstract
The one-step preparation of giant lipid vesicles (few micrometers in diameter) was achieved using a coaxial electrospray technique, with no volatile organic solvents. We also succeeded in encapsulating a variety of water-soluble and insoluble substrates (calcein, dextran, microbeads, and cells) in the giant lipid vesicles.
Giant lipid vesicles (giant liposomes) are spherical, closed molecular bilayers that entrap an aqueous compartment with a diameter of several micrometers. Giant lipid vesicles have attracted attention in a variety of research fields as artificial biological systems, microreactors, drug carriers, immobilization carriers, and as potential candidates for the origin of life,1,2 since they are biocompatible and biodegradable. They can also co-encapsulate hydrophobic molecules within the phospholipid bilayer, and hydrophilic substrates in the vesicle interior. Because of their large size, giant vesicles can be studied as a model for cells, and can be observed using optical microscopy, allowing the direct visualization of the distribution and transport of molecules of interest.3 Despite their large size, only a few reports exist describing micrometer-sized substrate encapsulation in giant lipid vesicles, probably owing to the limitations of conventional giant-vesicle preparation methods.4
To date, several methods for the preparation of giant lipid vesicles have been proposed; these include gentle hydration, electroformation, W/O emulsion methods, microchannel methods, and a pulsed-flow method.1–5 However, there are disadvantages to preparing giant lipid vesicles using these methods. For example, some of them use toxic organic solvents (chloroform and methanol) to dissolve phospholipids and another surfactant to help the formation of emulsions. These chemicals need to be removed by the subsequent procedures. Some require a specifically designed apparatus or have low productivity (like a small test-tube scale or a countable number of vesicles) and low production yields. Others require many steps involving phase transitions (evaporation or freezing), which prevent continuous and automated production. In some cases, the encapsulation efficiency is low (25–27%),6 or it is hard to encapsulate a physicochemically unstable substrate and micrometer-sized substrates.
The electrospray technique, which is mainly used as an ionization technique in mass spectroscopy, shows great promise for microcapsule preparation, because of the simplicity of the apparatus, its high productivity, its efficacy for continuous production, and the fact that it is easy to set up.7 The electrospray technique can also produce monodisperse microcapsules with high encapsulation efficiency. Previously we reported the preparation of polyelectrolyte (e.g., alginate) microcapsules and the encapsulation of various substrates in the microcapsules using the electrospray technique.7e In the present study, we extended the methodology to prepare giant lipid vesicles, and succeeded in the continuous preparation of giant lipid vesicles without using any toxic organic solvents.
Scheme 1 illustrates the experimental setup, and shows a schematic for the giant lipid vesicle formation. To avoid the use of toxic organic solvents, lecithin was dissolved in polyethylene glycol (PEG; M.W. 200). A lecithin solution (1 wt%) containing rhodamine-labeled phospholipids (0.004 wt%), and an aqueous solution containing 10 wt% poly(allylamine hydrochloride) (PAH) were electrosprayed from the outer and inner needles, respectively, using a coaxial electrospray apparatus. The ejected droplet (a mixture of the lecithin solution and the PAH solution) fell into a receiving buffer (5 mM citrate buffer, pH 7). In the receiving buffer, the following three phenomena took place simultaneously: (i) PEG diffused to the bulk aqueous and inner PAH-rich phases, (ii) phosphate ions invaded the PAH-rich phase to form a microgel-like complex between the PAH and multivalent ions,8 and (iii) phospholipids formed bilayers on the PAH-rich phase. The inset photo in Scheme 1 shows the successful formation of a Taylor cone on the tip of the coaxial needle. The PEG solution (outer phase) and the calcein-containing PAH solution (inner phase) stably and simultaneously formed cones.7c,d,9
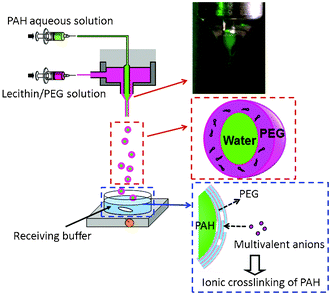 | ||
| Scheme 1 Schematic diagram of the preparation of giant lipid vesicles using an electrospray technique. Inset shows a Taylor cone formed on the tip of a coaxial needle. | ||
Fig. 1a and b show CLSM and differential interference images of giant lipid vesicles prepared with a 10 wt% PAH solution, using the electrospray technique. Spherical, hollow microspheres (giant lipid vesicles) were observed. The mean diameter of the vesicles was 3.2 μm (C.V. 15%). The size distribution was relatively narrow, probably because the droplets were formed by electrospraying. The prepared vesicles were stable for more than 2 days. The giant-vesicle formation proposed here was based on the formation of a microgel-like complex between PAH and multivalent anions. We then used a phosphate buffer (5 mM, pH 7.4) as a receiving buffer, instead of a citrate buffer. Giant vesicles were also observed with this buffer, but the size distribution became broader (mean diameter of 2.2 μm, and C.V. of 27%). When aqueous buffers composed of monovalent anions (acetate, Tris-HCl, and HEPES) were used as a receiving buffer, no giant vesicles were produced. These results support our hypothesis given above.
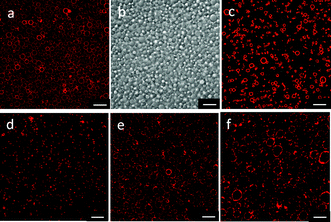 | ||
| Fig. 1 CLSM images (a, c–f), and differential interference image (b) of giant lipid vesicles prepared using the electrospray technique. (a, b) Vesicles prepared with a 10 wt% PAH solution and a citrate buffer. (c) Vesicles prepared with 10 wt% PAH solution and a phosphate buffer. (d, e, f) Vesicles prepared with a citrate buffer and a PAH solution (1, 20 and 30 wt%, respectively). The scale bars represent 10 μm. | ||
PAH also played an important role in the electrospraying and in the formation of a microgel-like complex. The PAH concentration was varied from 1 wt% to 30 wt%. All of the PAH concentrations used produced giant vesicles, with the size depending on the PAH concentration (Table 1). The diameter of the giant vesicles decreased with decreasing the PAH concentration. Low concentrations of PAH would form only a loose complex with the citrate ions, resulting in fractured microspheres, which would produce giant vesicles with a relatively smaller diameter. The control of the droplet size is usually achieved by the operating conditions of the electrospray.10 We are currently studying the control of the vesicle size by varying other spraying conditions (nozzle size, feed rate etc.).
A possible mechanism for the formation of giant lipid vesicles in the present study is proposed as follows: the electrospray produced microdroplets (composed of the PAH aqueous solution and the lecithin/PEG solution) with a relatively narrow size distribution. Once the microdroplets fell into a citrate buffer, PEG diffused to the bulk phase, and lecithin could not remain dissolved, and this led to the formation of lipid bilayers (see ESI†). Citrate ions entered the microdroplet to form a microgel-like complex with the PAH. Coinciding with the movement of citrate ions, PAH moved towards the bulk phase. However, the diffusion of a polymer in aqueous solution is typically 10 times slower than that of small ions.11 The viscosity of the 10 wt% PAH solution was quite high (23 mPa s at 25 °C), which would have helped to retain the PAH-rich microdroplet in the citrate buffer for a while. As a result, a microgel-like complex formed, and its surface was covered by lecithin bilayers to form a giant vesicle.
One of the important issues in the preparation of vesicles is the encapsulation of substrates. We attempted to encapsulate a variety of substrates in giant vesicles using the electrospray technique. The substrates were dissolved or dispersed in PAH solutions, and this was followed by electrospraying. Fig. 2a and b show the encapsulation of calcein and FITC-dextran (40 kDa) in the giant vesicles. Green fluorescence was observed in the lecithin layer, suggesting an interaction between the FITC-dextran and the lecithin layer. The encapsulation efficiencies of calcein and FITC-dextran were 82% and 99%, which were notably high compared with conventional methods.6 These high encapsulation efficiencies, and the higher efficiency for FITC-dextran, agreed with a previous study on polyelectrolyte microcapsules prepared using electrospraying;7e these results showed that the aqueous solution sprayed from the inner part of the coaxial nozzle was retained in the vesicles. The successful encapsulation of small molecules (calcein) also indicates the formation of a lipid barrier without defect. These results support our proposed mechanism for the formation of the giant vesicles. Interestingly, fluorescent polymer microparticles (0.5 μm diameter) and Escherichia coli cells (dyed with SYTO9 after vesicle preparation) were also successfully encapsulated in the giant vesicles (Fig. 2c and d). Half of the giant vesicles contained the microparticles, and non-entrapped microparticles were not found in the microscope observations. E. coli cells were encapsulated in 2% of the vesicles. The encapsulated polymer microparticles and cells did not leak out of the vesicles at least during 24 h. The present method was able to encapsulate water-insoluble and susceptible micrometer-sized substrates in giant lipid vesicles in a single step, even though there was a microgel-like complex in the giant lipid vesicles. Jesorka et al. reported the inclusion of hydrogel inside a giant vesicle to produce a vesicle with a temperature-responsiveness.12 They also discussed the high polymer concentration inside a vesicle as a model of cytoplasm. The giant vesicles filled with microgel prepared in the present study would also be applied to stimuli-responsive vesicles and cytoplasm-like microenvironments.
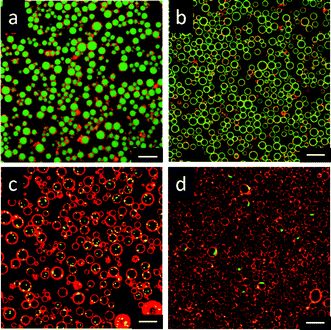 | ||
| Fig. 2 CLSM images of giant lipid vesicles encapsulating (a) calcein, (b) FITC-dextran, (c) 0.5 μm fluorescent microparticles, and (d) E. coli K12 cells (dyed with SYTO9). The PAH concentration was 10 wt%, and a citrate buffer was used as a receiving phase. The scale bars represent 10 μm. | ||
In conclusion, we proposed a novel method to prepare giant lipid vesicles using a coaxial electrospray. The present method has several advantages; no use of volatile organic solvents to dissolve phospholipids, a single step to produce giant phospholipid vesicles, a relatively narrow size distribution, the ability to be applied in continuous production, and the ability to encapsulate a wide variety of substrates (from small molecules to bacterial cells). Water-soluble substrates can be encapsulated with high efficiency, and without critical damage to the substrates, because no toxic organic solvents are used, and no phase transitions (e.g., drying) are required. Owing to the simplicity of the electrospray setup and the attractive properties of this technique, the present method is expected to be practical for the industrial production of giant lipid vesicles. The setup and the mechanism for electrospray are the same as or similar to those of electrospinning. The energetic studies on electrospinning in the past decade have provided a number of new findings and novel applications of nano- and micromaterials using a wide variety of matrices.13 The findings in the present study will also be useful for the encapsulation of substrates in electrospinning.
The authors thank Prof. H. Matsuyama for his technical help with CLSM observations. This work was supported financially by Special Coordination Funds for Promoting Science and Technology, Creation of Innovation Centers for Advanced Interdisciplinary Research Areas (Innovative Bioproduction Kobe), MEXT, Japan. This work was partially supported by the Kao Foundation for Arts and Sciences.
References
- (a) D. E. Discher and A. Eisenberg, Science, 2002, 297, 967 CrossRef CAS; (b) M. Antonietti and S. Förster, Adv. Mater., 2003, 15, 1323 CrossRef CAS; (c) V. P. Torchilin, Nat. Rev. Drug Discovery, 2005, 4, 145 CrossRef CAS.
- (a) M. I. Angelova and D. S. Dimitrov, Faraday Discuss. Chem. Soc., 1986, 81, 303 RSC; (b) D. S. Dimitrov and M. I. Angelova, Bioelectrochem. Bioenerg., 1988, 19, 323 CrossRef CAS; (c) T. Tanaka, Y. Tamba, S. M. Masum, Y. Yamashita and Y. Yamazaki, Biochim. Biophys. Acta, Biomembr., 2002, 1564, 173 CrossRef CAS; (d) A. Moscho, O. Orwar, D. T. Chiu, B. P. Modi and R. N. Zare, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 11443 CrossRef CAS; (e) A. Karlsson, R. Karlsson, M. Karlsson, A. S. Cans, A. Stromberg, F. Ryttsen and O. Orwar, Nature, 2001, 409, 150 CrossRef CAS; (f) A. S. Cans, N. Wittenberg, R. Karlsson, L. Sombers, M. Karlsson, O. Orwar and A. Ewing, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 400 CrossRef CAS; (g) S. Sugiura, T. Kuroiwa, T. Kagota, M. Nakajima, S. Sato, S. Mukataka, P. Walde and S. Ichikawa, Langmuir, 2008, 24, 4581 CrossRef CAS; (h) T. Maruyama, H. Yamamura, M. Hiraki, Y. Kemori, H. Takata and M. Goto, Colloids Surf., B, 2008, 66, 119 CrossRef CAS.
- (a) C. Decher, H. Ringsdorf, J. Venzmer, D. Bitter-Suermann and C. Weisgerber, Biochim. Biophys. Acta, Biomembr., 1990, 1023, 357–364 CrossRef; (b) F. M. Menger and N. Balachander, J. Am. Chem. Soc., 1992, 114, 5862 CrossRef CAS; (c) E. Farge and P. F. Devaux, Biophys. J., 1992, 61, 347 CrossRef CAS; (d) V. Noireaux and A. Libchaber, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 17669 CrossRef CAS.
- (a) K. Funakoshi, H. Suzuki and S. Takeuchi, J. Am. Chem. Soc., 2007, 129, 12608 CrossRef CAS; (b) Y. C. Tan, K. Hettiarachchi, M. Siu, Y. R. Pan and A. P. Lee, J. Am. Chem. Soc., 2006, 128, 5656 CrossRef CAS.
- (a) S. Kim and G. M. Martin, Biochim. Biophys. Acta, Biomembr., 1981, 646, l CrossRef; (b) N. Oku and R. C. MacDonald, Biochim. Biophys. Acta, Biomembr., 1983, 734, 54 CrossRef CAS; (c) H. H. Hub, U. Zimmermann and H. Ringsdorf, FEBS Lett., 1982, 140, 254 CrossRef; (d) P. Muller, T. F. Chien and B. Rudy, Biophys. J., 1983, 44, 375 CrossRef; (e) K. Higashi, S. Suzuki, H. Fujii and Y. Kirino, J. Biochem., 1987, 101, 433 CAS.
- A. Wagner, K. Vorauer-Uhl and H. Katinger, Eur. J. Pharm. Sci., 2002, 54, 213 CAS.
- (a) B. Bugarski, Q. Li, M. F. A. Goosen, D. Poncelet, R. J. Neufeld and G. Vunjak, AIChE J., 1994, 40, 1026 CrossRef; (b) V. A. Nedovic, B. Obradovic, I. Leskosek-Cukalovic, O. Trifunovic, R. Pesic and B. Bugarski, Process Biochem., 2001, 37, 17 CrossRef CAS; (c) I. G. Loscertales, A. Barrero, I. Guerrero, R. Cortijo, M. Marquez and A. M. Ganan-Calvo, Science, 2002, 295, 1695 CrossRef CAS; (d) G. Larsen, R. Velarde-Ortiz, K. Minchow, A. Barrero and I. G. Loscertales, J. Am. Chem. Soc., 2003, 125, 1154 CrossRef CAS; (e) Y. Fukui, T. Maruyama, Y. Iwamatsu, A. Fujii, T. Tanaka, Y. Ohmukai and H. Matsuyama, Colloids Surf. A, 2010, 370, 28 CrossRef CAS.
- R. K. Rana, V. S. Murphy, J. Yu and M. S. Wong, Adv. Mater., 2005, 17, 1145 CrossRef CAS.
- A. G.Marin, I. G. Loscertales, M. Marquez and A. Barrero, Phys. Rev. Lett., 2007, 98, 14502 CrossRef.
- A. J. Rulison and R. C. Flagan, J. Colloid Interface Sci., 1994, 167, 135 CrossRef CAS.
- (a) C. R. Wilke and P. Chang, AIChE J., 1955, 1, 264 CrossRef CAS; (b) A. Masuda, K. Ushida, H. Koshino, K. Yamashita and T. Kluge, J. Am. Chem. Soc., 2001, 123, 11468 CrossRef CAS.
- L. Osinkina, M. Markstrom, O. Orwar and A. Jesorka, Langmuir, 2010, 26, 1 CrossRef CAS.
- (a) W.-E. Teo, R. Inai and S. Ramakrishna, Sci. Technol. Adv. Mater., 2011, 12, 013002 CrossRef; (b) C. J. Luo, S. D. Stoyanov, E. Stride, E. Pelan and M. Edirisinghe, Chem. Soc. Rev., 2012, 41, 4708 RSC.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental section and SAXS measurement. See DOI: 10.1039/c2ra21423g |
| This journal is © The Royal Society of Chemistry 2012 |
