Electrochemical activity of α-MoO3 nano-belts as lithium-ion battery cathode†
Uttam Kumar
Sen
and
Sagar
Mitra
*
Electrochemical Energy Laboratory, Department of Energy Science and Engineering, IIT Bombay, Mumbai-400076, India. E-mail: sagar.mitra@iitb.ac.in; Fax: +91 22 2576 4890; Tel: +91 22 2576 7849
First published on 18th September 2012
Abstract
Few metal oxides have seen renewed interest because of their novel reactivity towards Li, leading to a large storage capacity. However, apart from their large capacity gain, it suffers from cycling instability, large polarization loss and poor rate performance. Herein, we report on the structural, morphological and electrochemical properties of α-MoO3 nano belts prepared by a simple hydrothermal method and used as a cathode for lithium-ion battery application. During the electrode preparation, we observed that the MoO3 nano-belt composite sample cast on stainless steel (SS) substrate leads to a better electrochemical performance towards Li compared to aluminium (Al) or nickel (Ni) substrates. The reason behind the poor performance was considered here, due to surface passivation on Al substrates. This report comprises experimental results depicting (i) a sustained reversible capacity of 140 mA h g−1 for over 50 cycles at a rate of 200 mA g−1, (ii) outstanding rate capabilities with reversible capacities as high as 320 mA h g−1 at a rate of 50 mA g−1and (iii) electrochemical stability of α-MoO3 nano belts towards a stainless steel substrate. Being able to make such highly oriented α-MoO3 nano belt-based electrodes, through the hydrothermal process and providing the electrochemical results, together show another efficient way to use MoO3 electrodes as a cathode in lithium-ion batteries.
Introduction
Rechargeable Li-ion batteries can offer high energy density, flexibility, light weight and long cycle life; they are fast gaining popularity as the technology of choice for portable computing and telecommunication equipment for today's requirements.1 Moreover, with the increasing awareness of side issues linked to air pollution combined with the foreseen oil shortage, we are in a new period where the use of renewable energy sources and electric transportation becomes a must.One of the major challenges of next generation Li-ion technologies for high power applications such as hybrid vehicles and clean energy storage are energy density, power density, safety and cost.2 As the demand for performance exceeds the capabilities of the existing Li-ion technology, new electrode materials with superior electrochemical properties, performance and low cost must be developed.
Orthorhombic molybdenum oxide (α-MoO3), which is the most stable form of molybdenum oxides, shows a significant interest because of its unique electrical and electrochemical properties, especially as it undergoes the Li-ion intercalation–deintercalation process in its two dimensional layered structures. MoO3 also exists in two other metastable forms known as β-MoO3 (monoclinic) and h-MoO3 (hexagonal) and these can be easily converted to α-MoO3 upon heating. Due to the ability of Li+ intercalation in the inner layers, α-MoO3 can act as a host material for Li+ and can be used as a cathode material for Li-ion batteries.3 Despite the high lithium mobility even at room temperature, the main disadvantage in the electrochemical application of pure MoO3 is poor electronic conductivity (σ = 1 × 10−3 S cm−1).4,5 Therefore the electrochemical performance of pure α-MoO3 is not well studied. As per the literature, the electrochemical performance of any material can be improved by increasing the utilization rate of active materials i.e. to have a better electrode–current collector interface or by using nano materials.6,7
So far, worldwide attention has been paid to the nanostructured materials as electrodes for Li-ion batteries, due to their attractive properties like small particle sizes, large active surface area and high surface energy.8 As we know, the properties of metal oxides depend closely on the microstructure, including crystal size, orientation, morphology and crystallographic density. For example, enhanced electrochemical activity was observed for LiCoO2 and V2O5 fibers and tubes compared to bulk samples.9–11
Herein, we report on the simple hydrothermal based synthesis of anisotropic α-MoO3 nano-belts. Structural and morphological studies have been conducted as well as reported using different characterization techniques. The electrochemical behaviour was studied using cyclic voltammetry (CV), in situ electrochemical impedance spectroscopy (EIS) and galvanostatic charge–discharge methods. As per our knowledge, 1.7 Li-ions per formula of α-MoO3 can be taken during the first discharge cycle, which is close to the gravimetric capacity of 317 mA h g−1, but a serious issue of capacity fading has been observed in all the previous reports.3,12,13 Here, we observed that the capacity became stabilized within a few initial cycles, giving rise to a capacity of 150 mA h g−1 (47% of the initial capacity) at the 25th cycle, and at the end of 100 cycles the capacity was found to be 127 mA h g−1 (40% of the initial capacity). The results show that α-MoO3 nano-belts exhibit high capacity and excellent cyclic stability compared to previous reports. As per our knowledge, this report will be the first of its kind showing cyclic stability and the role of the current collector on the performance of α-MoO3 nano-belts as a cathode. The present results are encouraging and show an opportunity for α-MoO3 nano-belts to be used as a moderate potential cathode in lithium-ion battery applications.
Experimental section
Synthesis of α-MoO3 nano belts
Anisotropic α-MoO3 nano-belts were prepared by a simple hydrothermal method, as reported by Li et al.14 In a typical experimental procedure, 2.0 g sodium molybdate (Na2MoO4·2H2O, Merck) was dissolved in 10 ml deionized water. Then 5 ml 4 M perchloric acid (HClO4), (Merck) was added dropwise to the molybdate solution under constant stirring. At the first step a colourless solution was obtained, which turned turbid after stirring for an hour. This solution was then transferred into a 35 ml Teflon-lined stainless steel autoclave, which was kept inside a muffle furnace at 180 °C for 24 h. A thick white coloured precipitate was obtained, which was washed several times with distilled water and acetone and then dried over a hot air oven at 60 °C for 12 h. The dried sample was calcined at 300 °C and 500 °C in a pure nitrogen atmosphere for further physical characterizations.Characterization
All solid samples were systematically examined by X-ray diffraction (XRD) at room temperature (25 °C) using a Philips X’pert diffractometer with Cu-Kα radiation (λ = 1.5418 Å) at 40 kV and 40 mA. Different metal–oxygen vibrational modes of α-MoO3 were characterized using a Raman spectrometer (Jobin Yvon HR800) equipped with a 514.5 nm laser at 10 mW power. A field emission gun scanning electron microscope (FEG-SEM, JEOL-7600F) with a resolution of about 1 nm was used to study the surface morphology of the samples. Further investigations were done by the use of a high resolution field emission transmission electron microscope (HR-TEM, JEOL-2100F).Cell fabrication and electrochemical measurements
All the electrochemical performances of the materials were carried out in two electrode Swagelok type cells in a Li/electrolyte/MoO3 cell configuration. The complete cell comprises α-MoO3 as the cathode, borosilicate glass fiber sheet (Whatman GF/D) soaked with a 1 M LiPF6 solution [in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC/1
DMC/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass ratio (LP-30, Merck, Germany)] as a separator and pure Lithium (Alfa Aesar) as a counter as well as a reference electrode. The cells were assembled in an argon-filled glove box (Lab Star, Mbraun, Germany) with a water and oxygen concentration level of ∼1 ppm. The electrode materials were hand ground with 12% carbon black (Super C-65, Timcal, Switzerland) and 8% PVDF (Sigma Aldrich) for half an hour. A slurry of the composite was prepared from the mixed powder by adding a few drops of NMP (Qualigens, India). This slurry was then tape cast on Al foil, stainless steel (SS 304) plates and Ni mesh. Then the electrodes were dried at 120 °C under vacuum for 12 h and pressed, unless otherwise mentioned.
1 mass ratio (LP-30, Merck, Germany)] as a separator and pure Lithium (Alfa Aesar) as a counter as well as a reference electrode. The cells were assembled in an argon-filled glove box (Lab Star, Mbraun, Germany) with a water and oxygen concentration level of ∼1 ppm. The electrode materials were hand ground with 12% carbon black (Super C-65, Timcal, Switzerland) and 8% PVDF (Sigma Aldrich) for half an hour. A slurry of the composite was prepared from the mixed powder by adding a few drops of NMP (Qualigens, India). This slurry was then tape cast on Al foil, stainless steel (SS 304) plates and Ni mesh. Then the electrodes were dried at 120 °C under vacuum for 12 h and pressed, unless otherwise mentioned.
Electrochemical Impedance Spectroscopy (EIS) experiments were carried out at open circuit voltage (OCV) in frequency ranges 1 MHz–0.01 Hz in Bio-logic VMP-3. The in situ EIS experiment was performed in a Bio-logic VMP-3 instrument and the impedance experiment was performed at nine different cathodic polarizations, starting at OCV, 2.8 V, 2.76 V, 2.65 V, 2.45 V, 2.3 V, 2.18 V, 2.0 V, and 1.51 V vs. Li/Li+. At each point, the EIS was taken within the frequency range 1 MHz–0.01 Hz and with voltage perturbation ΔV = 5 mV. A selection of the points and the other details are given later in the results and discussion section, while the points and the discharge curve, along with the potential points where EIS was performed, are shown in Fig. S1, ESI.† The cycling voltammetry (CV) profile was obtained by measuring the I–V response at a scan rate of 0.2 mV s−1, and the cut-off voltage was 1.5 V–3.5 V. Bio-logic VMP-3 was employed for CV measurement. The electrochemical charge–discharge experiments were performed on an Arbin Instrument, USA (BT2000 model) with various current rates. All the electrochemical measurements were done at a constant temperature of 20 °C.
Results and discussions
B Structural characterization
In the literature, α-MoO3 was prepared from peroxomolybdic acid, and various methods15–17 have been used to obtained peroxomolybdic acid. In the first step, the molybdenum salt was converted to molybdic acid (H2MoO4), which gets oxidized by the use of a strong oxidizing agent like H2O2 or HNO3. In the present study we have used HClO4 or HNO3 to form peroxomolybdic acid from sodium salt of molybdate, and used the hydrothermal synthesis process to get pure anisotropic α-MoO3 powder. In this particular work, we have observed, at 180 °C, that the shape of the nano-belts was more uniform and the yield was maximum. It was also observed that when phosphoric acid was used instead of HClO4 or HNO3, a colourless solution was obtained at the end of the hydrothermal reaction. The mechanism of MoO3 formation could be as follows,The XRD pattern of α-MoO3 powder is shown in Fig. 1a, which is indexed as the orthorhombic phase of MoO3 and a space group of Pbnm (JCPDS card No. 35-0609). As explained earlier, the α phase of MoO3 is thermodynamically most stable among the three possible phases. The other two meta-stable phases of MoO3 can be readily converted to the α phase by heating the sample at 450 °C.18,19 The XRD pattern of the as-prepared sample was compared with annealed samples heated at 300 °C and 500 °C, to convert the hexagonal and monoclinic forms, respectively, to the orthorhombic form. Fig. 1a shows that the peak positions and their relative intensities were unchanged during heating at higher temperature, which proves that the as-prepared sample is phase pure α-MoO3. For this reason all the electrochemical performance was done with the as-prepared samples. The diffraction pattern (Fig. 1a) shows that, except for (0k0), all other peaks have very low intensity as compared to the standard data. The observed diffraction patterns with the highest intensities are (020), (040), and (060), which indicates the preferential orientation of the α-MoO3 nano-belts in the [001] direction.12,20
 | ||
| Fig. 1 (a) XRD and (b) Raman spectra of α-MoO3. | ||
The Raman spectroscopy result shown in Fig. 1b also supports the XRD results. The band positions of the as-prepared sample and the annealed samples are the same. The observed band positions are assigned as 290 cm−1(B2g, B3g), 337 cm−1(B1g, Ag), 667 cm−1(B2g, B3g), 819 cm−1(Ag, B1g) and 995 cm−1(Ag, B1g), respectively, which is in good agreement with the result obtained from pure α-MoO3.21,22
From the high end scanning electron micrographs, it has been observed that the α-MoO3 has a belt like morphology (Fig. 2). The FEG-SEM images shown in Fig. 2a and b illustrate that the α-MoO3 belts were formed uniformly, having a width in the range 100–500 nm and a length of several micrometers. The belt thickness was observed to be varying from 20 nm to 100 nm, but careful observation shows that each belt consists of several thin layers of MoO3 (shown in Fig. 2c). Essentially, thin layers of MoO3 are the building blocks that combined to form the nano-belt morphology. The number of MoO3 layers assembled determines the thickness of the individual belt. The number of layers varies from belt to belt, and as a result their thickness also varies. The FEG-TEM image shown in Fig. 2d also shows that α-MoO3 has the morphology of nano-belts, which is in good agreement with SEM analysis. Lattice fringes were observed at a higher resolution in TEM studies, shown in Fig. 2e. The lattice spacing was found to be 3.6 nm and 4.0 nm, which correspond to the d-spacing of the (100) and (001) planes of α-MoO3, respectively.3,13
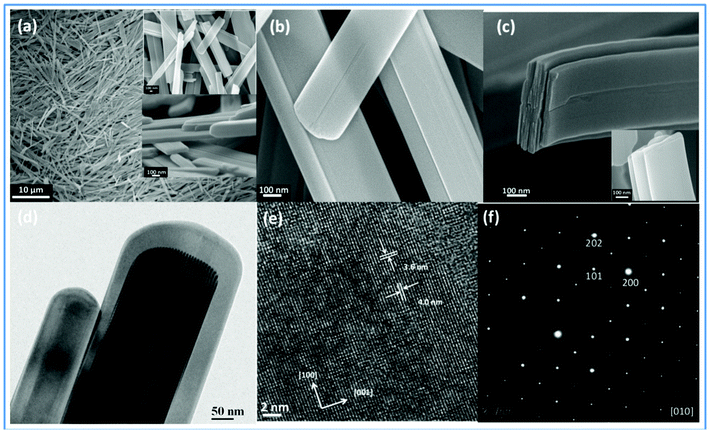 | ||
| Fig. 2 FEG-SEM images are shown in (a), (b) and (c). FEG-TEM images are shown in (d) and (e). (f) SAED pattern of α-MoO3. | ||
The selected area electron diffraction (SAED) pattern recorded perpendicular to the anisotropic growth axis of an individual nano-belt is attributed to the [010] zone axis (shown in Fig. 2f). Combining with the TEM and SAED patterns indicates that the highly crystalline nano-belts of α-MoO3 have grown along the [001] orientation. Because of the gravitational force, the majority of the nano-belts grounded selectively on the (010) base planes of the TEM grid under free sedimentation during TEM sample preparation, and show excellent crystal growth direction along the [001] direction.20
Electrochemical performance
Since the pure α-MoO3 nano-belts are poor electronic conductors, all the samples were initially mixed with conductive carbon (12 wt%) and binder (8 wt%) to perform the electrochemical studies. Before going into the details of electrochemical studies, we have observed enormous capacity fading with the sample cast on aluminium substrate compared to other substrates. Stable capacity retention was observed when the sample was cast on a stainless steel current collector.Selection of current collector
Lithium-ion battery electrodes are composite materials, in which the active mass is bound to a metal current collector with a polymeric binder such as polyvinylidene di-fluoride (PVDF). In addition, the composite electrode contains a conductive additive, usually carbon, and the composite is pasted on the metallic current collector. Current collectors play an important role in the performance of electrode materials for Li-ion batteries, and aluminium (Al) as a current collector is the preferred choice for a cathode material. Apart from Al, chromium (2.5–4.5 V), nickel (≤ 4.5 V) and stainless steel (SS-304) (≤ 5.0 V) have also been used as current collectors for cathode materials.23 For the potential range 1.5 V–3.5 V vs. Li/Li+ the possible current collectors are Al, Ni and SS. In this work, we have observed that α-MoO3 is not compatible with the Al substrate and, as a result, leads to poor electrochemical performance. Cyclic voltammograms (CVs) of α-MoO3 on the Al substrate are shown in Fig. 3a. It was found that during the cathodic process, prominent peaks were observed at 2.7 V and 2.15 V vs. Li/Li+, but in the reverse process (anodic) a weak peak at 2.5 V vs. Li/Li+ was observed. The reduction peak at ∼2.7 V vs. Li/Li+ disappeared after the 1st cycle for all the substrates used in the present study. Previously, the reduction peak was assigned as irreversible formation of the LixMoO3 phase.24 It was well studied by researchers using ex situ XRD and TEM techniques that the irreversible intercalation process occurred at 2.7 V vs. Li/Li+, forming the compound of LixMoO3 (0 < x < 0.25).24,25 XRD and TEM studies also reveal the coexistence of α-MoO3 and Li0.25MoO3 during the 1st irreversible process.24,25 Similar observations were found during the CV of α-MoO3 using Ni as the current collector (shown in Fig. 3b). Two prominent cathodic peaks at 2.44 V and 2.03 V and one anodic peak at 2.57 V vs. Li/Li+ were found. From the 2nd cycle onwards, only one cathodic peak at 2.3 V was observed, whereas the anodic peak position gradually shifted from 2.57 V (in 1st cycle) to 2.52 V (2nd cycle) to 2.46 V (5th cycle). The observed cathode peak positions were at lower potentials compared to the Al substrate. Moreover, we observed one reversible intercalation–deintercalation of Li+ in the potential range 2.15 V–2.25 V vs. Li/Li+, not only in the 1st cycle but also in consecutive cycles with decreasing intensity for all the substrates used currently. The above results agree well with the previous literature by Tsumura et al.24 | ||
| Fig. 3 CVs of α-MoO3 on (a) Al, (b) Ni and (c) SS current collector. (d) EIS of α-MoO3 on different substrates at OCV (in this case OCVs are 3.3 V for Al, 3.1 V for Ni and 3.0 V for SS). | ||
The best electrochemical performance of the α-MoO3 nano-belts was observed when the SS substrate was used as a current collector. For this reason all the electrochemical performance and cyclic stability of α-MoO3 was done on a SS current collector. Fig. 3c shows the cyclic voltammograms (CVs) of the cathode materials within the potential range 1.5 V–3.5 V vs. Li/Li+. In the first cycle, two distinct peaks were observed at 2.55 V and 2.15 V vs. Li/Li+ in the cathodic process (discharge process). However, in the following anodic process (charge process) there was only one distinct peak at 2.56 V, similar to the Al substrate. In consecutive cycles the cathodic peak at 2.55 V disappeared, whereas the 2.15 V peak shifted to 2.23 V. On the other hand, there is not much change in the anodic peak positions, which is consistent with earlier results.3,12,26 According to the literature12,23 the prominent set of peaks (2.23/2.56 V or 2.15/2.56 V) in the first cycle is due to the intercalation of Li-ions into the interlayer spacing of the [MoO6] octahedron layers, whereas the 2.55 V peak in the cathodic process is due to intercalation of the Li-ion into the irreversible sites of the [MoO6] octahedron interlayers. As a result, once the Li-ion was inserted into the spacing of the [MoO6] octahedron interlayers (causing the development of 2.55 V peak), it was trapped inside the cavity, which led to the unavailability of these sites for further Li-ion insertion and as a result no further peak at 2.55 V was observed. But on a closer look we can observe that from the 2nd cycle a new cathodic hump at 2.8 V was observed, which signifies that some unrecoverable sites were still available for Li+ insertion, but on a higher potential side. This extra intensity of the hump/peak gradually diminished and finally disappeared from the 5th cycle. Similar CVs were also observed by Chen et al.12 It was also noticed during the charge–discharge performance (Fig. 3c) that a substantial amount of capacity fading was there in the initial 5 cycles.
Electrochemical impedance spectroscopy (EIS) is one of the most commonly used techniques to elicit the electrochemical processes occurring at the electrode–electrolyte interphase, and has been widely applied to the layered transition metal oxides.27,28 Here, the EIS technique was used to find out the electronic conductivity, electronic structure, phase transitions and their effects on the electrochemical performances of the α-MoO3 electrode on different substrates with approximately equal electrode material loading and similar film thickness. Fig. 3d shows the increase in diameter of the high frequency semicircle when the same composition of MoO3 was cast over the SS, Ni and Al current collectors, respectively. After careful investigation of the EIS spectra, we observed that the semicircle in the high frequency region is a summation of two semicircles (one very small depressed semicircle observed in the very high frequency region and a large semicircle in the high to middle frequency region). These two semicircles are attributed to SEI formation and charge-transfer resistance from the high to the low frequency region, respectively.
It was observed that the charge transfer resistance was a minimum in case of SS and a maximum for Al. Surface passivation on the Al surface could be the reason behind the higher charge transfer resistance. A thin passivating layer of Al2O3 always forms on the Al foil, but during electrode fabrication it might be possible that a thicker layer of oxide is formed. There can be a probability of a conversion reaction among MoO3 and Al during electrode drying at 120 °C, resulting in Mo and Al2O3. The presence of a small amount of Mo nanoparticles can catalyze the electrolyte decomposition, which causes corrosion on the Al surface.23 Zhou et al.29 also report that poor performance on the Al current collector over SS is expected due to corrosion, but the actual reason is not yet understood.
A detailed study of impedance spectroscopy was undertaken for SS supported α-MoO3. As shown in Fig. 4, in situ EIS was carried out for SS supported α-MoO3 during the 1st discharge cycle at a current rate of 50 mA g−1. As discussed earlier, two types of Li-ion intercalation processes are there, the first one is for irreversible Li-ion intercalation, occurring at 2.75 V, which accounts for a Li-ion insertion of 0.25 moles of Li-ions per formula unit of MoO3, while the other one is at 2.3 V vs. Li/Li+. For a better understanding of the intercalation mechanism, the entire discharge profile was divided into three zones, and for each zone three sets of impedance measurements were carried out at three different potentials (shown in Fig. 4a). Zone-I was restricted to the first stable plateau i.e. first intercalation layer (Fig. 4b) while Zone-II (shown in Fig. 4c) represents the second discharge plateau and the sloppy part was represented by Zone-III (Fig. 4d).
 | ||
| Fig. 4 (a) 1st discharge cycles of α-MoO3 on SS substrate at a current rate of 50 mA g−1, (b) EIS at Zone-I (c) EIS at Zone-II (d) EIS at Zone-III at three different cathodic potentials. | ||
 | ||
| Fig. 5 (a) Charge–discharge curves as a function of rate for the first two cycles, (b) charge–discharge curve on SS substrate at 200 mA g−1, (c) charge–discharge cycles of α-MoO3 on different substrates at a current rate of 200 mA g−1, (d) power cycle performance at different current rates. | ||
As per the previous literature, the Nyquist plot of electrochemical intercalation–deintercalation for LiCoO2-based electrodes is commonly observed in three distinct parts: a semicircle in the high frequency range, which is generally attributed to the migration of Li+ across the surface film; another semicircle in the middle frequency range, which is ascribed to the charge transfer process from the electrolyte to the layered structure; and in the low frequency region the observed step inclined line is attributed to the solid-state diffusion process of the Li-ion into the LiCoO2 lattice.30 However, the explanations and observations for the layered cathodes based on EIS experiments are debatable in many ways. According to Nobili et al.,27 the EIS spectra for the layered materials are interpreted in terms of the following physical processes: (1) at very high frequency, the presence of the semicircle is due to the presence of the electrode surface passivating layer, (2) at an intermediate frequency range, the dispersion is due to the charge transfer process, (3) in the low frequency region, the semicircle is associated with the electronic properties of the material and finally, (4) at very low frequency, the inclined line is due to ionic diffusion.31 Very few reports could see four different frequency dispersions and describe the reaction mechanism clearly. As per Fig. 4b, the Nyquist plot of the MoO3 cathode at the open circuit potential (1st EIS at 3.0 V), shows a depressed small semicircle in the high frequency range, as well as a large semicircle in the high to middle frequency range and a slightly inclined line in the low frequency range. With increasing electrode polarization potentials, the depressed small semicircle in the high frequency region does not change significantly. However low frequency dispersion started appearing from 2.8 V onwards, which is a starting point of the co-existence of MoO3-LixMoO3. The middle to low frequency semicircle could be attributed to a change in electronic properties of the electrode materials and this was observed from point 2 (see Fig. 4b). It was also observed that with increasing cathodic electrode polarization, the extremely low frequency inclined line showing resistive behavior (more inclined towards the real axis) is due to an increase of grain boundaries, which reduces Li-ion diffusion. The more resistive nature of ion diffusion is observed from point 2 (see Fig. 4b) and again started inclining towards an imaginary impedance axis from point 8 (see Fig. 4d). After the complete conversion of MoO3, the Li-ion can easily diffuse through the materials, due to nanometric nature of the composite formation. One more interesting point to note is that after point 7 (see Fig. 4d), the diameter of the high to middle frequency semicircle corresponding to charge transfer resistance decreases with more cathodic polarization, which was ascribed to the formation of a more conductive lithiated MoO3 phase. It seems that in the low frequency region the line is not a sloppy one. It has been found that the impedance response in the very low frequency region is relatively more sensitive towards experimental conditions and reproducible 2–5% of the time, even when using the same battery cell.32 So, the last few points can be considered as non-reproducible or an experimental limitation. Now if we neglect the last 3 to 4 points, which are at a very low frequency range, then the impedance spectra (Fig. 4b–d) can easily be explained. During the reduction, at point 1, Li+ starts accumulating at the surface of the active material, due to which a sharp increase in the impedance line (point 2) was observed in the low frequency regions. At 2.7 V, Li+ starts intercalating on the MoO3 layers, which causes two phase (MoO3 and Li0.25MoO3) formation, and as a result solid-state diffusion becomes more sluggish (point 3) as the resistive nature is increased due to an increase in the grain boundaries. But as soon as the first plateau ends and LixMoO3 starts forming (start of 2nd plateau) the small diffusion characteristics are visible and the sloppy nature of the impedance curve becomes more prominent from point 4 to point 6 (Fig. 4c). At the end of the 2nd plateau (Fig. 4d) the lithium diffusion improves due to the formation of lithiated MoO3, and as a result the slope at the low frequency impedance line is improved from point 7 to point 9. After 2.2 V there was no Li intercalation, only accumulation of Li on the surface of the electrode. So, in the very low frequency range (for points 8 and 9) a small sharp increment in the impedance line along the Z′′-axis was observed, which is due to lithium accumulation33 (illustrated in Fig. S2, ESI†).
Rate and cycling performance
The galvanostatic charge discharge properties of α-MoO3 on SS are shown in Fig. 5a and b. Two prominent discharge plateaus are observed in the first cycle, which is in good agreement with the CV experiment. From Fig. 5a, it was shown that the discharge plateau with a higher rate of cycling shifted to a lower potential, and the charge plateau was shifted to a higher potential, which means the polarization increased when the current density increased. A similar observation was also reported by Zhou et al.3 The first discharge capacity recorded was 320 mA h g−1 at a rate of 50 mA g−1 and the capacity was 315 mA h g−1 for 100 mA g−1, corresponding to the ∼1.7 Li+ insertion per mole of MoO3, but the capacity was decreased to 280 mA h g−1 (equivalent to 1.5 Li+ per mole) for the first cycle with a discharge rate of 200 mA g−1. Almost 107 mA h g−1 (∼0.57 Li+/MoO3) capacity loss was observed in the initial 5 cycles, which was due to Li+ insertion in unrecoverable sites of the [MoO6] octahedron, and the observations are consistent with the CV results. In the remaining 95 cycles the capacity fading was smaller (shown in Fig. 5b and c). A total 74% capacity (with respect to the 5th cycle) was retained at the end of 100 cycles, which is the best among all the reported results with any current rate. Initially, charge–discharge performances for α-MoO3 on Al, Ni and SS substrates are shown in Fig. 5c, and the result reflects a similar conclusion as obtained from CV. After the initial few cycles a huge capacity fading was observed for α-MoO3–metal, except for the SS substrate, which means the Li+ reversibility was lost completely due to the complete loss of the MoO3 structure and the formation of a large resistive interface. The reason for this phenomenon is not yet fully understood. A deep investigation is required to reveal the inside chemistry using in situ techniques. When the Al substrate was used as the current collector (Fig. 5c), the reversible capacity reaches almost zero after the initial few cycles. By using the Ni current collector the performance was improved a little in comparison to the Al current collector. The observed capacity decreased gradually in the initial 10 cycles and then stabilized for the remaining cycles with a constant capacity of 52 mA h g−1.To know the electrochemical stability and the quality of capacity retention, a power plot (shown in Fig. 5d) has been made with different current densities starting from 100 mA g−1, 200 mA g−1, 300 mA g−1, 400 mA g−1 and 500 mA g−1, and then reverts back to 100 mA g−1 then 200 mA g−1 for the Li/electrolyte/α-MoO3 half cell.
Fig. 5d shows that at high rate of about 500 mA g−1 (∼2.7C) the material exhibits a discharge capacity of about 111 mA h g−1. The C rate is calculated for the capacity obtained due to 1 Li+ insertion in a formula unit of MoO3 in 1 h. Here, a rate of 1C is equivalent to 186 mA h g−1 capacity gain/loss in 1 h. After reversing the rate to 100 mA g−1, it shows a stable capacity of around 170 mA h g−1 and at the end of the 100th cycle it shows a stable capacity of 128 mA h g−1 with a rate of 200 mA g−1 (which equals to a rate of ∼1.1 C). The above results show the excellent capacity retention and robustness of the electrode material at high as well as low rate performance.
In summary, this kind of work falls within the scope of improving the electrochemical performance of the MoO3 material as a cathode in a lithium-ion battery. In addition to previous findings in the reported literature i.e. (1) electrochemical performance enhancement by using lithiated MoO3 nanostructures,34 (2) by making composites with conductive polymers35 and (3) by imposing oxygen deficiency,5 the present study provides a simple way to improve the electrochemical performance by improving the electrode–current collector interface. A common approach alluded to above is focusing on the realization of better 2D nanostructured materials and a better current collector interface for MoO3 in order to achieve the best electrochemical performance. A real weakness of these materials towards applications is irreversible capacity loss during the initial stage of charge–discharge, poor capacity retention and large polarization. Such limitations were suggested to be due to irreversible structural change during reduction, low electronic conductivity of phase pure α-MoO3 materials and kinetic limitation related either to some intrinsic properties of materials or non-compatibility with current collectors.
Conclusions
The present work emphasizes a few important points. It highlights the advantages of simple preparation of anisotropic α-MoO3-based cathode materials at low temperature using a one step hydrothermal process. Very importantly, the electrochemical studies show that α-MoO3 material is more stable with a stainless steel substrate in terms of capacity retention and rate capabilities, while the reason behind the poor cycling performance of aluminium current collectors was the formation of the resistive interface. Here, we have improved the discharge capacity as much as possible (140 mA h g−1) and the capacity is stable up to 100 cycles at a rate of 200 mA g−1. During electrochemical cycling against Li, we have also observed a two phase reaction, and at the end of the reduction process loss of the MoO3 structure is observed as in previous reports. Furthermore, we have tried to explain the same phenomenon by electrochemical impedance spectroscopy. All the above results show that the α-MoO3 material could be reconsidered as a suitable low potential cathode for lithium-ion battery applications.Acknowledgements
We thank the National Centre for Photovoltaic Research and Education (NCPRE)–Ministry of New and Renewable Energy, Govt. of India and IRCC-IIT Bombay for support. The authors are thankful to the staff members of SAIF, IITB for their assistance with electron diffraction and FEG-SEM analysis.References
- Y. Wang and G. Cao, Adv. Mater., 2008, 20, 2251–2269 CrossRef CAS.
- G. Ceder, MRS Bull., 2010, 35, 693–701 CrossRef CAS.
- L. Zhou, L. Yang, P. Yuan, J. Zou, Y. Wu and C. Yu, J. Phys. Chem. C, 2010, 114, 21868–21872 CAS.
- C. Julien and G. A. Nazri, Solid State Ionics, 1994, 68, 111–116 CrossRef CAS.
- A. M. Hashim, G. H. Wrodnigg, M. H. Askar, M. Winter, J. H. Albering and J. O. Besenhard, Ionics, 2002, 8, 183–191 CrossRef CAS.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J.-M. Tarascon, Nature, 2000, 407, 496–499 CrossRef CAS.
- P. L. Taberna, S. Mitra, P. Poizot, P. Simon and J.-M. Tarascon, Nat. Mater., 2006, 5, 567–573 CrossRef CAS.
- S. Cavaliere, S. Subianto, I. Savych, D. J. Jones and J. Rozière, Energy Environ. Sci., 2011, 4, 4761–4785 CAS.
- L. J. Chen, J. D. Liao, Y. J. Chuang, K. C. Hsu, Y. F. Chiang and Y. S. Fu, J. Appl. Polym. Sci., 2011, 121, 154–160 CrossRef CAS.
- C. R. Sides and C. R. Martine, Adv. Mater., 2005, 17, 125–128 CrossRef CAS.
- D. Yu, C. Chen, S. Xie, Y. Liu, K. Park, X. Zhou, Q. Zhang, J. Li and G. Cao, Energy Environ. Sci., 2011, 4, 858–861 CAS.
- J. S. Chen, Y. L. Cheah, S. Madhavi and X. W. Lou, J. Phys. Chem. C, 2010, 114, 8675–8678 CAS.
- K. Dewangan, N. N. Sinha, P. K. Sharma, A. C. Pandey, N. Munichandraiah and N. S. Gajbhiye, CrystEngComm, 2011, 13, 927–933 RSC.
- X.-L. Li, J.-F. Liu and Y.-D. Li, Appl. Phys. Lett., 2002, 81, 4832–4834 CrossRef CAS.
- G. Li, L. Jiang, S. Pang, H. Peng and Z. Zhang, J. Phys. Chem. B, 2006, 110, 24472–24475 CrossRef CAS.
- M. C. Chakravorti, S. Ganguly and M. Bhattacharjee, Polyhedron, 1993, 12, 55–58 CrossRef CAS.
- A. Phuruangrat, J. S. Chen, X. W. Lou, O. Yayapao, S. Thongtem and T. Thongtem, Appl. Phys. A: Mater. Sci. Process., 2012, 107, 249–254 CrossRef CAS.
- A. Michailovski, F. Krumeich and G. R. Patzke, Chem. Mater., 2004, 16, 1433–1440 CrossRef CAS.
- S. R. Dhage, M. S. Hassan and O. B. Yang, Mater. Chem. Phys., 2009, 114, 511–514 CrossRef CAS.
- X. W. Lou and H. C. Zeng, Chem. Mater., 2002, 14, 4781–4789 CrossRef CAS.
- M. A. Py and K. Maschke, Physica B+C, 1981, 105, 370–374 CrossRef CAS.
- B. C. Windom, W. G. Sawyer and D. W. Hahn, Tribol. Lett., 2011, 42, 301–310 CrossRef CAS.
- S. T. Myung, Y. Hitoshi and Y. K. Sun, J. Mater. Chem., 2011, 21, 9891–9911 RSC.
- T. Tsumura and M. Inagaki, Solid State Ionics, 1997, 104, 183–189 CrossRef CAS.
- Y. Iriyama, T. Abe, M. Inaba and Z. Ogumi, Solid State Ionics, 2000, 135, 95–100 CrossRef CAS.
- W. Li, F. Cheng, Z. Tao and J. Chen, J. Phys. Chem. B, 2006, 110, 119–124 CrossRef CAS.
- F. Nobili, S. Dsoke, F. Croce and R. Marassi, Electrochim. Acta, 2005, 50, 2307–2313 CrossRef CAS.
- F. Nobili, S. Dsoke, M. Minicucci, F. Corce and R. Marassi, J. Phys. Chem. B, 2006, 110, 11310–11313 CrossRef CAS.
- J. Zhou and P. S. Fedkiw, Electrochim. Acta, 2003, 48, 2571–2582 CrossRef CAS.
- M. D. Levi, E. Markevich, C. Wang, M. Koltypin and D. Aurbach, J. Electrochem. Soc., 2004, 151, A848–A856 CrossRef CAS.
- X.-Y. Qiu, Q.-C. Zhuang, Q.-Q. Zhang, R. Cao, P.-Z. Ying, Y.-H. Qiang and S.-G. Sun, Phys. Chem. Chem. Phys., 2012, 14, 2617–2630 RSC.
- J. M. Hawkins and L. O. Barling, Some aspects of battery impedance characteristics, Proceeds of the 17th International Telecommunications Energy Conference, 1995 Search PubMed.
- D. Aurbach, J. Power Sources, 2000, 89, 206–218 CrossRef CAS.
- L. Mai, B. Hu, W. Chen, Y. Qi, C. Lao, R. Yang, Y. Dai and Z. L. Wang, Adv. Mater., 2007, 19, 3712–3716 CrossRef CAS.
- C. V. S. Reddy, Z. R. Deng, Q. Y. Zhu, Y. Dai, J. Zhou, W. Chen and S. I. Mho, Appl. Phys. A: Mater. Sci. Process., 2007, 89, 995–999 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra21373g |
| This journal is © The Royal Society of Chemistry 2012 |

