Green synthesis of highly UV-orange emitting ZnSe/ZnS:Mn/ZnS core/shell/shell nanocrystals by a three-step single flask method†
Bich
Thi Luong
,
Eunsu
Hyeong
,
Seokhwan
Ji
and
Nakjoong
Kim
*
Organic Photonic and Electronic Materials Lab., Department of Chemistry and Research Institute for Natural Sciences, Hanyang University, 17 Haengdang-Dong, Seongdong-Gu, Seoul 133-791, South Korea. E-mail: marialuongbich@gmail.com; Fax: +82-2-2295-0572; Tel: +82-2-2220-0935
First published on 31st October 2012
Abstract
New water-soluble and highly luminescent ZnSe/ZnS:Mn/ZnS core/shell/shell nanocrystals with color-tunable emission range between 580 and 600 nm were synthesized via a three-step in single flask method.
Colloidal semiconductor nanocrystals (NCs) have attracted great attention in recent years for their unique electrical and optical properties. Interestingly, direct band gap semiconductor NCs are inorganic lumophores that have high quantum efficiencies, color-tunable and narrow emission spectra, and excellent chemical stability.1 So far, these properties have created many applications for NCs in light-emitting diodes (LEDs), lasers, biological and chemical sensing, and solar cells.2 However, the band gap of most emissive semiconductors is either too high or too low to easily make visible light emitting NCs, the exception in systems generally being cadmium chalcogenide.3 Unfortunately, cadmium is toxic, so these systems have limited application due to their effect on human health. However, recent reports have demonstrated visible phosphorescence from NCs with ZnS coated shell which have wider band gaps (3.67 eV for bulk ZnS), such as CdTe/ZnS core/shell NCs system. These can both protect against the degradation of the surrounding environment by cadmium and improve the quantum yield, which is especially interesting when observed in nontoxic doped NCs using cadmium. A two-step method for the synthesis of water-soluble ZnSe/ZnS quantum dots, which are potential candidates for blue emitting LEDs and biological labels, was also presented with a high degree of crystallinity and quantum efficiencies.4 Doping with atomic impurities is an efficient way to generate luminescent NCs, due to their strong dopant emission. To date, colloidal NCs have successfully been doped with transition metals (such as Fe, Ni, Mn, Cu) and lanthanides (such as Eu, Er, Tm, Tb) to alter their electronic, optical or magnetic properties.5 Light emission is independent of the band gap of the host materials and largely immune to the thermal and chemical variations of the surrounding medium.6 In order for these doped NCs to be luminescent at the colours of the dopant, the excitation energy of the dopant needs to be smaller than the band gap of the host material to enable energy transfer of an exciton in the NC core to the impurity dopant. In this vein, procedures for doping of high band gap materials, such as zinc selenide or zinc sulfide with manganese (ZnSe:Mn, ZnS:Mn NCs), were developed, which result in visible phosphorescence from the Mn2+ 4T1 → 6A1 transition centered at ∼580 nm.5,14 Furthermore, when the NC host material is a wide band gap semiconductor, such as ZnSe and ZnS, thin films of NCs do not absorb in the visible wavelength region, making them compatible with the construction of transparent devices. The ZnSe/ZnS:Mn/ZnS core/shell/shell NCs are well-known to offer the photo- and chemical stability and high photoluminescence (PL) quantum yield (QY) needed for alternating current thin-film electroluminescent device (ACTFEL) applications.8 The high PL QY of these ZnSe/ZnS:Mn/ZnS NCs is due to their thick ZnS outer shell, which localizes the excited state on the Mn-dopant atom by preventing energy transfer to surface states or the surrounding medium. The thick shell also improves the stability of the NC, preventing migration of the Mn dopant.8,14 The various Mn2+ doping concentrations can change the PL QYs and the emission colours under UV light (365 nm). These NCs are soluble in water due to their secondary shell of zinc sulfide for use in nontoxic chemical sensing.7 However, either these core/multi-shell NCs were synthesized in organic solvent at high temperature,7,8 which is not environmentally friendly, or ZnSe:Mn/ZnS core/shell NCs were prepared in water under microwave irradiation with mild conditions.9,10 These synthetic conditions are still complex. The conventional aqueous synthesis approach is reproducible and cheap, and the reaction conditions are mild. Furthermore, this approach is suitable for large-scale and economic synthesis of highly luminescent NCs, which is also very important for future applications.
In this work, a green, three-step single-flask method for synthesis of low toxicity ZnSe/ZnS:Mn/ZnS core/shell/shell NCs in the aqueous phase is presented using mercaptopropionic acid (MPA) as a stabilizer at low temperature (80–100 °C). Under mild reaction conditions, the core/multi-shell NCs have high quantum yield (42.9% with 45% doping Mn concentration relative to the Zn of the first shell), small particle size , good crystallization, color-tunable property, and good water-solubility. This is a facile route to get ZnSe/ZnS:Mn/ZnS core/(doped) shell/shell NCs which emit in the range of 580–600 nm. This synthetic method represents a more versatile, cost-effective, and safe approach for fabricating high-quality luminescent NCs, which is presented in detail in the electronic supplementary information (ESI†).
ZnSe/ZnS:Mn/ZnS core/shell/shell NCs were synthesized by a three-step single-flask method, as shown in Fig. 1, without any washing steps between each growth step. MPA is usually chosen to serve as a capping ligand in the preparation of ZnSe or other II–IV semiconductor NCs by the aqueous route, due to its preferable binding capacity.11 One molecule of MPA can coordinate to one metal atom site to form the most favourable hexagonal configuration, thus enhancing the colloidal stability of the formed NCs which is favourable for PL performance. The addition of Zn2+ and Mn2+ in the second step is undertaken slowly in order to grow the doped Mn in the ZnS first shell onto the ZnSe core. Moreover, Zn2+ and Mn2+ ions are inclined to be soft Lewis acids, and the RSH group in MPA is a soft Lewis base. The RSH groups prefer to bind to the Zn2+ and Mn2+ ions compared with RCOOH of MPA, because soft acids tend to associate with soft bases.9 The hydrophilic carboxylic groups at the outer surface render excellent water solubility for NCs after the thiol group has bonded to the zinc and manganese ions on their surfaces.4 In a similar way, the second shell of ZnS was deposited on the outer layer of the first doped shell of ZnS:Mn to form the core/(doped) shell/shell nanostructure. The RSH groups prefer to bind to the Zn2+ ions compared with RCOOH of MPA and form water-soluble NCs with the hydrophilic carboxylic groups at the outer surface of the ZnSe/ZnS:Mn/ZnS core/shell/shell NCs.
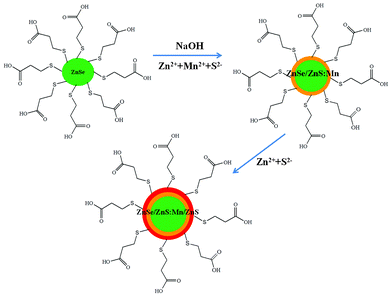 | ||
| Fig. 1 Schematic illustration for the synthesis of the ZnSe/ZnS:Mn/ZnS core/(doped) shell/shell NCs. | ||
Fig. 2(a) shows the UV-Vis absorption and PL spectra of the ZnSe cores, ZnSe/ZnS:Mn core/(doped) shell and ZnSe/ZnS:Mn/ZnS core/(doped) shell/shell with 45.0% Mn doping concentration relative to Zn of the first shell NCs. As can be seen, the absorption red-shifts with every added layer, which is indicative of shell formation.4 The relative diameter size of the core, core/shell (cs), and core/shell/shell (css) NCs were also considered using dynamic light scattering (DLS) measurements, which show the increase in the relative diameter size in the core, cs and css NCs in Fig. 2(b), and were obtained from well-dispersed aqueous NCs colloidal solutions. Fig. 2(c) shows that the ZnSe core NCs produce light blue-emission at 400–420 nm, while the weaker trap emission shows at 500 nm. Furthermore, Mn2+ centered emission at ∼580–600 nm is increased dramatically from surface passivation as seen in the ZnSe/ZnS:Mn to ZnSe/ZnS:Mn/ZnS spectra. The importance of doping Mn in ZnS shell is that the emission of Mn2+ ions is strong enough to overcome the trap emission at 500 nm in the PL spectra, which results in the high UV-orange emission seen in Fig. 2(c). Real images of ZnSe core, ZnSe/ZnS:Mn(45%) cs and ZnSe/ZnS:Mn(45%)/ZnS css NCs are also shown in Fig. 2d. The PL quantum yield increased from 35.6% for ZnSe/ZnS:Mn(45%) cs NCs to 42.9% for ZnSe/ZnS:Mn(45%)/ZnS css NCs. Fig. 3(a,b) show that the PL intensities increase according to the increasing Mn doping concentrations, due to formation of a larger number of Mn luminescent centers and corresponding enhancement of Mn2+ emission. Furthermore, Fig. 3(d) shows that the emission wavelengths are slightly red-shifted, which makes css NCs slightly color-tunable with increasing Mn dopant concentration. The absorptions of css NCs at different Mn doping concentrations, shown in Fig. 3(c), are not changeable. This means that the average size of css NCs may not be affected by Mn dopant concentrations at a range of 11.7–45.0% relative to Zn of the first doped shell. These core/(doped) shell/shell phosphor NC developed materials rely on several observations. First, small semiconductor clusters are more resistant to impurity doping than larger NCs. Further, it is known that the binding, and thus residence time, of guest impurities on a growing NC surface has a large effect on the ability to envelop that impurity within a host NC framework. As it has been predicted from theoretical modeling that the manganese has a greater interaction with a zinc sulfide surface versus zinc selenide, doping a ZnS shell should result in better incorporation of Mn2+ into the NC system.12,13 Impurity emitters are also known to be more efficient and less prone to self-quenching if they are diluted within the outer edges of a NC (or located in the shell of a core/shell material).14 Our method combines all of these effects in an approach to impregnate impurity phosphors within the outer shell of a strongly binding material. In the course of this work, we discovered that shell-doped NCs are also very sensitive to the aqueous passivating layer on their surface, through the observation of phosphorescence quenching upon dilution of ZnSe/ZnS:Mn NCs and their subsequent brightening in MPA water solution. More interestingly, if the amount of MPA is too great, the Zn2+ and Mn2+ ions do not subsequently grow onto the outer ZnSe core NCs and ZnSe/ZnS:Mn core/(doped) shell NCs. Therefore, the MPA was added at the beginning or during the first step to form ZnSe cores, as it must be large enough for these ions to interact with each other and overcoat the ZnSe cores.
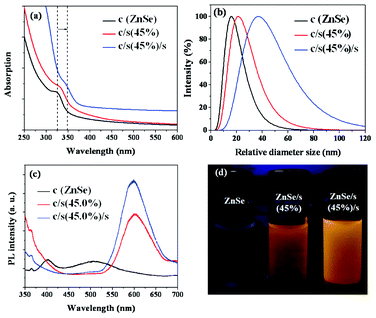 | ||
| Fig. 2 (a) UV-Vis absorbance, (b) DLS spectra, (c) PL spectra and (d) real images under 365 nm wavelength of the ZnSe core, ZnSe/ZnS:Mn(45%) cs, and ZnSe/ZnS:Mn(45%)/ZnS css NCs. | ||
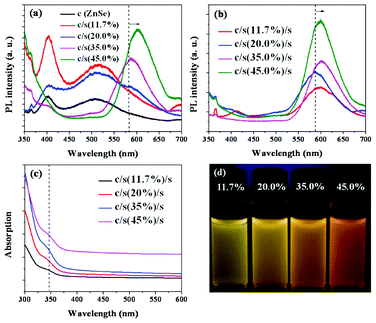 | ||
| Fig. 3 PL spectra of (a) the ZnSe core and ZnSe/ZnS:Mn cs NC; (b) PL, (c) UV spectra and (d) real images under 365 nm wavelength of ZnSe/ZnS:Mn/ZnS css NCs at different Mn doping concentrations. | ||
Typical high resolution TEM (HRTEM) images for the MPA-stabilized ZnSe, ZnSe/ZnS:Mn core/doped shell and ZnSe/ZnS:Mn/ZnS core/doped shell/shell NCs are shown in Fig. 4. The figures show that all NCs possessed a good crystalline structure, and their diameters were about 4.6(± 0.2) and 5.5(± 0.3) and 7.2(± 0.2) nm, for ZnSe, ZnSe/ZnS:Mn and ZnSe/ZnS:Mn/ZnS NCs, respectively. Fig. 4 confirmed the size of the core/(doped) shell/shell NCs. There was not any precise HRTEM image which could distinguish the core and shell layers in the core/shell/shell NCs, due to the similar crystalline structures of multilayers.10 However, since the core and the shell have similar electron densities and lattice parameters, the image contrast also may not be used to distinguish the shell and the core.9 In addition, the three kinds of particles have the same selected area electron diffraction (SAED) pattern, presented in Fig. 4(d), which matched the pattern of zinc-blende ZnSe NCs reported in previous communications.6,15Fig. 5(a) shows the powder XRD patterns for the ZnSe core-a, ZnSe/ZnS:Mn(45%) cs-b, and ZnSe/ZnS:Mn(45%)/ZnS css-c NCs, which reveal that the ZnSe core, ZnSe/ZnS:Mn cs, and ZnSe/Zns:Mn/ZnS css possess zinc-blende crystal structures, due to the display of XRD patterns at the (111), (220) and (311) planes. This supports the hypothesis that Mn2+ can be more easily incorporated in cubic ZnS NCs than in wurtzite and rock-salt crystals.12 In addition, we can conlude that doping Mn2+ into the host ZnS of the shell NCs does not bring about a phase transformation of the crystal structure. All of these crystal structure features are consistent with the growth of ZnS (MnS) shell on the surface of ZnSe core and the ZnSe/ZnS:Mn core/(doped) shell particles. The broad peaks are attributed to the size distribution of the nanoparticles. We found that the diffraction peaks shifted to larger angles as the shell thickness increased from zero to 1.7 nm.4,9
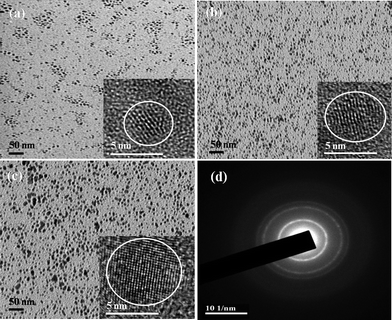 | ||
| Fig. 4 TEM and HRTEM images of the ZnSe core (a), ZnSe/ZnS:Mn(45%) cs (b), ZnSe/ZnS:Mn(45)/ZnS css (c), (d) SAED pattern of NCs. | ||
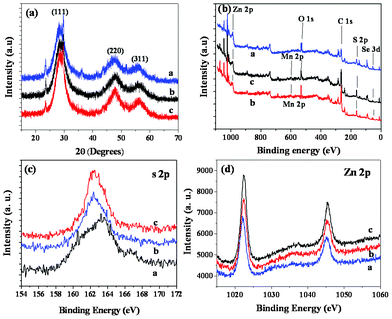 | ||
| Fig. 5 XRD patterns (a) and XPS spectra (b, c, d) of the ZnSe core-a, ZnSe/ZnS:Mn core/doped shell-b, and ZnSe/ZnS:Mn/ZnS core/(doped) shell/shell-c NCs. | ||
The X-ray photoelectron spectra (XPS) results confirmed the proposed ZnSe/ZnS:Mn/ZnS core/shell/shell structure. Fig. 5(b,c,d) show that the coordination of Zn-S in the ZnSe/ZnS:Mn core/shell and ZnSe/ZnS:Mn/ZnS core/shell/shell NCs is different from that of Zn-SR (i.e., thiols) in ZnSe core NCs. Fig. 5(c) shows that the binding energy assigned to s 2p shifted from 163.5 eV for the ZnSe NCs to 162.5 ev for the ZnSe/ZnS:Mn and ZnSe/ZnS:Mn/ZnS NCs, which verifies the proposed core/shell/shell structure and is consistent with previous reports.16 Furthermore, XPS provides further evidence for growth of the ZnS:Mn(S) on the ZnSe cores, and ZnS shell on the ZnSe/ZnS:Mn core/doped shell. The XPS information on Zn intensity shows that this has obviously increase from ZnSe core, to ZnSe/ZnS:Mn core/(doped) shell and ZnSe/ZnS:Mn/ZnS core/(doped) shell/shell NCs due to the layers of the shell increasing. It is shown in Fig. 5(d) that the Zn concentration is clearly increased in the core NCs, core/shell NCs and core/shell/shell NCs.
Conclusions
In summary, we report a green and facile three-step process in a single flask for synthesis of water-soluble ZnSe/ZnS:Mn/ZnS NCs in aqueous phase. The obtained NCs produce emissions ranging from 580 to 600 nm and possess a high degree of crystallinity, high quantum efficiencies, and narrow size distribution, making them potential candidates for orange LEDs, ACTFEL devices and biological labels.Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation (NRF) of Korea funded by the Ministry of Education, Science and Technology (2011-0004106) and the Korea government (MEST) through the Active Polymer Center for Pattern Integration (No. R11-2007-050-00000-0)References
- A. P. Alivisatos, Perspectives on the physical chemistry of semiconductor nanocrystals, J. Phys. Chem., 1996, 100, 13226–13239 CrossRef CAS.
- S. Coe, W. K. Woo, M. G. Bawendi and V. Bulovíc, Nature, 2002, 420, 800–803 CrossRef CAS.
- C. B. Murray, D. J. Norris and M. G. Bawendi, J. Am. Chem. Soc., 1993, 115, 8706–8715 CrossRef CAS.
- B. Dong, L. Cao, G. Su and W. Liu, Chem. Commun., 2010, 46, 7331–7333 RSC.
- D. J. Norris, N. Yao, F. T. Charnock and T. A. Kennedy, Nano Lett., 2001, 1, 3–7 CrossRef CAS.
- N. Pradhan, D. Goorskey, J. Thessing and X. Peng, J. Am. Chem. Soc., 2005, 127, 17586–17587 CrossRef CAS.
- R. Thakar, Y. C. Chen and P. T. Snee, Nano Lett., 2007, 7, 3429–3432 CrossRef CAS.
- V. Wood, J. E. Halpert, M. J. Panzer, M. G. Bawendi and V. Bulovíc, Nano Lett., 2009, 9, 2367–2371 CrossRef CAS.
- D. Zhu, X. X. Jiang, C. Zhao, X. L. Sun, J. R. Zhang and J. J. Zhu, Chem. Commun., 2010, 46, 5226–5228 RSC.
- Z. Fang, P. Wu, X. Zhong and Y. J. Yang, Nanotechnology, 2010, 21(305604), 9pp Search PubMed.
- A. Shavel, N. Gaponik and A. Eychmüller, J. Phys. Chem. B, 2004, 108, 5905–5908 CrossRef CAS.
- S. C. Erwin, L. Zu, M. I. Haftel, A. L. Efros, T. A. Kennedy and D. J. Norris, Nature, 2005, 436, 91–94 CrossRef CAS.
- L. Zu, D. J. Norris, T. A. Kennedy, S. C. Erwin and A. L. Efros, Nano Lett., 2006, 6, 334–340 CrossRef CAS.
- Y. Yang, O. Chen, A. Angerhofer and Y. C. Cao, J. Am. Chem. Soc., 2006, 128, 12428–12429 CrossRef CAS.
- N. Pradhan and X. G. Peng, J. Am. Chem. Soc., 2007, 129, 3339–3347 CrossRef CAS.
- Y. He, H. T. Lu, L. M. Sai, Y. Y. Su, M. Hu, C. H. Fan, W. Huang and L. H. Wang, Adv. Mater., 2008, 20, 3416–3421 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra21309e |
| This journal is © The Royal Society of Chemistry 2012 |
