GNP confinement at the interface of cationic reverse micelles: influence in improving the lipase activity†
Moumita
Ghosh
,
Subhabrata
Maiti
,
Sayanti
Brahmachari
and
Prasanta Kumar
Das
*
Department of Biological Chemistry, Indian Association for the Cultivation of Science Jadavpur, Kolkata, 700032, India. E-mail: bcpkd@iacs.res.in; Fax: +(91)-33-24732805
First published on 26th July 2012
Abstract
The present work reports thiol-assisted confinement of gold nanoparticles (GNPs) at the interface of reverse micelles with the aim to enhance the interfacial area and thereby the efficiency of surface-active Chromobacterium viscosum lipase. The strong gold capping ability of optimally hydrophobic thiols (1-dodecanethiol and 1,6-hexanedithiol) was aptly utilized to pull GNPs (∼3–5 nm) from the water pool to the oil/water interface of cetyltrimethylammonium bromide (CTAB) reverse micelles. These small sized GNPs were fitted at the microscopic interface of CTAB reverse micelles possibly because of the comparable thickness of the interface (∼1–2 nm) to that of the GNP diameter. Lipase solubilized within this augmented interface enjoys a flexible conformation, which resulted in the improvement of its activity (∼2.5 fold) with respect to only CTAB microemulsion. The activity of lipase within CTAB reverse micelles was thoroughly studied in the presence of mono and dithiols with varying chain length, where a greater improvement in activity was observed with dithiols. Bidentate ligand property of dithiols led to firm localization of higher number of GNPs at the interface which enhanced the total space in vicinity of enzyme at the interfacial domain. Fitting fusion of small sized GNPs within CTAB reverse micellar interface was confirmed by microscopic and spectroscopic studies. Smooth localization of lipase at the enhanced interface was also confirmed from the improvement in its secondary structure (α-helical content) in circular dichroism spectroscopic analysis. Interestingly, large sized GNPs (∼8 and 20 nm) were found to be well fitted at the interface of bigger head group-containing surfactants, cetyltriethylammonium bromide (CTEAB) and cetyltripropylammonium bromide (CTPAB). The hydrolytic efficiency of lipase in 1,6-hexanedithiol included GNP (∼20–25 nm)-doped CTPAB reverse micelles improved by ∼3.4 fold compared to that observed in only CTAB.
Introduction
Research activities involving gold nanoparticles (GNPs) are progressively getting attention due to their unique optical,1 electronic2 and molecular recognition properties.3,4 Size-dependent distinct surface properties because of the high surface-to-volume ratio offered by these metal nanoparticles have opened up their widespread applications in biosensing, drug delivery and so forth.5–7 Recently, the influence of GNPs in enzymology is also finding notable importance.8 GNPs have been extensively exploited in diverse applications because of their ease of synthesis and simple method of stabilizing them with different functional agents.9 In this context, alkanethiol and amine have emerged as the most effective stabilizers of GNPs that led to the development of monolayer-protected gold clusters.10 Also, GNPs have been reported to be stabilized by various surfactants, like CTAB, AOT and Triton-X, thereby developing multifunctional soft nanocomposites.11To this end, reverse micelles (also known as water-in-oil (w/o) microemulsions) represent a beautiful self assembly of surfactant molecules enclosing an aqueous core inside. Two distinct domains of w/o microemulsions, namely the water pool and oil–water interface, are capable of entrapping both hydrophilic (trypsin, chymotrypsin) and surface-active enzymes (lipase, horseradish peroxidase (HRP)).12–15 In previous reports, we showed the crucial role of “space” in the vicinity of enzymes in regulating their activity by varying the microstructural parameters, including surfactant head group, tail length, counterion etc.12d–f,13c,15a,c Enzymes located at an augmented domain in reverse micelles always exhibited superior activity due to increased substrate concentration and conformational flexibility of biocatalysts. This very idea was further complemented by solubilizing gold nanomaterials of the appropriate size and dimension within the water pool to enhance the surrounding interfacial area, which consequently resulted in the notable improvement in the activity of interfacially localized lipase.16 Doping of large sized GNPs (20–25 nm, at [Au] = 40–50 μM) within the CTAB reverse micellar water pool (dimension varies from 5–15 nm depending on compositions) could only improve the lipase activity due to the increase in overall interfacial area of (GNP + water)-in-oil microemulsion. However, small sized GNPs (3–5 nm) did not have any influence on lipase activity as they get easily solubilized within the same water pool without affecting the interfacial domain.16a In our continued effort to enhance the space in the vicinity of the surface-active enzyme, it is quite reasonable that small sized GNPs (3–5 nm) could be fit in at the interface (Scheme 1) considering the average thickness of the interfacial domain (varies within a few nm (1–2 nm), depending on the head group size of the surfactant).12f Also, an increase in interfacial area may be achieved by confinement of small GNPs at a low concentration, which would not affect the stability of microemulsions. Moreover, loss of the structural integrity of lipase in close association with large GNPs at higher concentrations could be avoided in the case of smaller sized GNPs integrated at the micellar interface (Scheme 1).16a,17
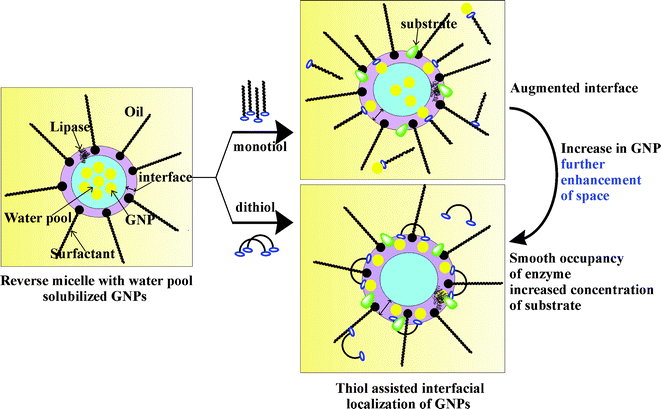 | ||
| Scheme 1 Schematic representations of the confinement of GNPs at the interface of reverse micelles in the presence of monothiol and dithiol. | ||
In the present work, strong gold capping ability of thiols10a–c,18 was exploited for localizing GNPs at the reverse micellar interface having comparable dimension to that of the nanoparticles. The presence of hydrophobic thiols facilitated the transfer of water pool solubilized GNPs to the interface, which resulted in the augmentation of microscopic interfacial area. Lipase within these interfacially confined GNP (3–5 nm)-doped CTAB w/o microemulsions showed a ∼2.5-fold enhancement in its activity compared to that in only CTAB reverse micelles. Interestingly, this improvement in lipase activity was achieved at a low Au concentration (∼10 μM). In the case of bigger head group sized surfactants (CTEAB and CTPAB, Fig. 1), confinement of larger sized GNPs (20–25 nm) at the interface also offered a similar improvement in lipase activity. An overall 3.4-fold increase in lipase activity was achieved in interfacially confined GNP-based systems with respect to CTAB alone. Spectroscopic and microscopic investigations were done to establish the interfacial localization of GNPs, along with their influence on enzyme conformation and activity.
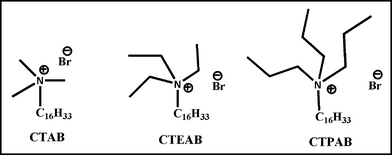 | ||
| Fig. 1 The structures of the surfactants. | ||
Results and discussion
Evolution of a better template with the objective of improving an enzyme's efficiency is in constant demand. For example, in the case of reverse micelles, refurbishment at the component level has been extensively explored to develop a superior host for enzymes by augmenting the space in the vicinity of the biocatalyst. The latest addition in this attempt was doping of large sized (∼25 nm) gold nanomaterials (sphere or rod-shaped with high surface area) inside the water pool to augment the interfacial area of w/o microemulsions.16 Interfacial area could possibly be increased more effectively if GNPs are localized at the interface instead of solubilizing within the water pool. To this end, the present investigation is aimed at increasing the space in the vicinity of the surface-active enzyme by the confinement of GNPs of the appropriate size at the microscopic interface of reverse micelles.Initially, small sized GNPs were synthesized from HAuCl4 solution using sodium borohydride as the reducing agent and citrate as the capping agent.16a The surface plasmon resonance (SPR) peak of the synthesized GNPs in aqueous solution was observed at 508 nm (Fig. 2a). The corresponding TEM image shows ∼3–5 nm dimension of GNP508 (Fig. 2b). GNP508 was doped in a w/o microemulsion of CTAB (50 mM)/isooctane/n-hexanol/water (pH = 6.0, 20 mM phosphate) at z ([n-hexanol]/[surfactant]) = 4.8, W0([water]/[surfactant]) = 48 following the reported experimental protocol.16a A minimum volume of n-hexanol was used to reduce the inhibitory effect of n-hexanol on lipase.14c,15a,e,19 Lipase activity within these nanohybrid systems was followed by sequential addition of enzyme and the substrate (p-nitrophenyl-n-octanoate). The overall concentrations of lipase, substrate and GNP508 were maintained at 1.02 μg mL−1, 3.0 mM and 10 μM, respectively. The hydrolytic efficiency of lipase was determined by measuring the absorbance of the liberated p-nitrophenol at the isosbestic points (Table S1, Electronic Supplementary Information (ESI†)). The lipase activity (second order rate constant, k2) merely improved from 433 ± 7 to 482 ± 6 cm3 g−1 s−1 (Table 1) in GNP508-doped CTAB reverse micelles over that in the only CTAB-based system. Water pool solubilized GNP508 could not influence lipase activity as the small sized nanoparticles were incapable of enhancing the interfacial area, which was earlier achieved by doping large sized GNPs.16a Considering the interfacial thickness of reverse micelles that generally varies between 1–2 nm, we intend to increase the interfacial area of CTAB w/o microemulsion by localizing the same small sized GNP508 at the interface.12f
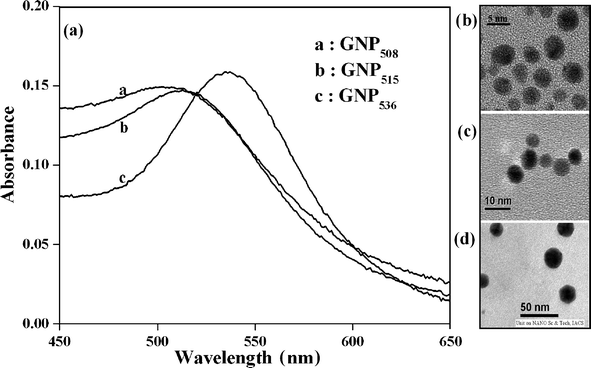 | ||
| Fig. 2 (a) Surface plasmon resonance peaks of GNP508, GNP515 and GNP536. TEM images of the aqueous solution of (b) GNP508, (c) GNP515 and (d) GNP536. | ||
| Only CTAB (in the absence of GNP and thiol) | CTAB + GNP (in the absence of thiol) | CTAB + GNP + thiol | |||||
|---|---|---|---|---|---|---|---|
| C2-thiol (mercapto acetic acid) | C6-thiol (hexane thiol) | C10-thiol (decane thiol) | C12-thiol (dodecane thiol) | C16-thiol (hexadecane thiol) | |||
| a = [surfactant] = 50 mM, [lipase] = 1.02 × 10−6 g mL−1, [substrate] = 3 mM. | |||||||
| GNP508 | 433 ± 7 | 482 ± 6 | 562 ± 9 | 738 ± 7 | 780 ± 8 | 805 ± 9 | 562 ± 7 |
| GNP515 | 433 ± 7 | 500 ± 7 | 519 ± 8 | 562 ± 6 | 607 ± 7 | 712 ± 6 | 541 ± 8 |
| GNP536 | 433 ± 7 | 516 ± 8 | 526 ± 8 | 526 ± 7 | 520 ± 6 | 534 ± 7 | 525 ± 7 |
In this context, the strong gold capping ability of thiols was exploited in pulling the GNPs from the inner aqueous core to the interface.10a–d,18 It was presumed that successful interfacial localization of GNPs would augment the space at the micellar interface (Scheme 1). Different hydrophobic thiols with varying chain length (mercapto acetic acid (C2-thiol), 1-hexanethiol (C6-thiol), 1-decanethiol (C10-thiol), 1-dodecanethiol (C12-thiol) and 1-hexadecanethiol (C16-thiol)) were used for interfacial localization of GNPs. To start with, C2-thiol (100 μM) was added to the GNP508-doped CTAB reverse micelles (z = 4.8, W0 = 48) and vortexed for 1–2 min. Lipase activity marginally improved to 562 ± 9 cm3 g−1 s−1 in the same GNP508-doped CTAB system in the presence of C2-thiol (Table 1). The activity of lipase within thiol-included GNP508-doped reverse micelles also followed second order kinetics, as observed in previously reported w/o microemulsions.14c,15e,f,20 Addition of C6-thiol (100 μM) to the same GNP508-doped CTAB-based system improved the enzyme activity notably by 1.7-fold (k2 = 738 ± 7 cm3 g−1 s−1) over that of native CTAB reverse micelles (Table 1). The lipase activity steadily increased to 780 ± 8 cm3 g−1 s−1 and 805 ± 9 cm3 g−1 s−1 (Table 1) with an increase in the hydrophobicity of the thiols (100 μM) to C10-thiol and C12-thiol, respectively. However, no improvement in lipase activity was observed when these hydrophobic thiols were doped in CTAB reverse micelles in the absence of GNP508. The presence of either of the external agents (small sized GNP or thiol) alone within CTAB reverse micelles could not influence the lipase activity, while the combined presence of both resulted in a marked improvement of the enzyme's efficiency. Hence, enhancement of lipase activity in GNP508-doped CTAB reverse micelles in the presence of hydrophobic thiols might be due to the increase in space in the vicinity of the enzyme by the confinement of GNP508 at the interface. Under identical experimental conditions, the extent of improvement in lipase activity dropped (k2 = 562 ± 7 cm3g−1s−1) with a further increase in the chain length of the alkanethiol to C16. Thiols with increasing hydrophobicity possibly earned the required amphiphilic character to suitably localize themselves at the oil–water interface, thereby leading to the confinement of GNPs at the interfacial domain. Hydrophilic C2-thiol mostly gets solubilized within the water pool, while C12-thiol preferred to be located at the interface, resulting in the highest influence on lipase activity among all of the thiols. In the case of C16-thiol, an increase in hydrophobicity possibly favours its solubilization in the non-polar organic domain instead of being located at the oil–water interface. Accordingly, C16-thiol lost the ability to confine GNP508 at the interface and showed a trivial influence on lipase activity.
Enzyme catalysis was further investigated across the varying concentrations of thiols to determine the optimum amount of thiol needed for exhibiting maximal influence on interfacial GNP localization and subsequently on lipase activity. C12-thiol was chosen as the representative thiol owing to its best influence in improving lipase activity in GNP508-doped CTAB reverse micelles. The concentration of C12-thiol was varied from 10 to 600 μM, keeping the Au concentration fixed at 10 μM (maximum GNP concentration can be reached within CTAB reverse micelles in our experimental conditions). Lipase activity steadily improved from 433 ± 7 to 805 ± 9 cm3 g−1 s−1 as the concentration of C12-thiol gradually increased to 100 μM (Fig. 3). However, beyond 100 μM thiol concentration, k2 slightly decreased to 738 ± 8 cm3 g−1 s−1 up to 600 μM of C12-thiol. Thus, 100μM thiol was found to be the optimum concentration for localizing GNP at the interface and exhibiting maximal influence to improve the lipase activity.
![Variation of the second order rate constant (k2) for the lipase-catalyzed hydrolysis of p-nitrophenyl-n-octanoate in GNP508-doped CTAB reverse micelle at [Au] = 10 μM and with varying C12-thiol and C6-dithiol concentrations at z = 4.8, W0 = 48 and 25 °C. [Surfactant] = 50 mM, [lipase] = 1.02 × 10−6 g mL−1, [substrate] = 3 mM. Experimental errors are within ±1–2%.](/image/article/2012/RA/c2ra21237d/c2ra21237d-f3.gif) | ||
| Fig. 3 Variation of the second order rate constant (k2) for the lipase-catalyzed hydrolysis of p-nitrophenyl-n-octanoate in GNP508-doped CTAB reverse micelle at [Au] = 10 μM and with varying C12-thiol and C6-dithiol concentrations at z = 4.8, W0 = 48 and 25 °C. [Surfactant] = 50 mM, [lipase] = 1.02 × 10−6 g mL−1, [substrate] = 3 mM. Experimental errors are within ±1–2%. | ||
So far, we observed that C12-thiol having a single –SH moiety yielded the best influence in improving lipase activity in GNP-doped CTAB reverse micelles. At this point, we were intrigued to know whether an increase in the –SH moiety would boost the enzyme activity further by firm placement of GNPs at the interface. Alkanedithiols bearing bidentate ligand property are expected to act as better capping agents of GNP than that of the monothiol.21 Also, considering the importance of hydrophobicity of the alkyl chain in localizing the GNPs at the interfacial domain, we used 1,6-hexanedithiol (C6-dithiol) as the GNP capping agent. Under the same experimental conditions as described above, C6-dithiol was doped (instead of monothiol) into the same GNP508-included CTAB reverse micelles (z = 4.8, W0 = 48). Lipase activity within these w/o microemulsions was also studied over a dithiol concentration range of 10–600 μM (Fig. 3). The hydrolytic efficiency of lipase was remarkably enhanced from 433 ± 7 to 1083 ± 8 cm3 g−1 s−1 in the presence of 100 μM of C6-dithiol within GNP508-doped CTAB reverse micelles (Fig. 4). Here, also the enzyme activity increased with increasing concentration of C6-dithiol up to 100 μM, followed by a decrease to 790 ± 7 cm3 g−1 s−1 (Fig. 3) with a further increase in dithiol concentration (600 μM). Most importantly, in the presence of C6-dithiol lipase activity within these GNP508-doped CTAB reverse micelles improved 2.5-fold (compared to only CTAB reverse micelles), which was 1.8-fold in the case of C12-monothiol. The alkyl chain length of dithiol was also varied to find out the essential hydrophobicity required for its interfacial solubilization and their effect on the lipase activity. At z = 4.8, W0 = 48, 1,2-ethanedithiol (C2-dithiol) and 1,4-butanedithiol (C4-dithiol) were separately doped in GNP508-included CTAB w/o microemulsions. The k2 increased to 650 ± 8 cm3 g−1 s−1 (1.5-fold) and 866 ± 8 cm3 g−1 s−1 (2.0-fold) in the presence of C2-dithiol and C4-dithiol, respectively (Fig. 4). Here also, lipase activity improved with enhanced hydrophobic character of the dithiol, being highest for C6-dithiol. However, in the case of 1,8-octanedithiol (C8-dithiol) with a further increase in hydrophobicity lipase activity trivially decreased to 986 ± 8 cm3 g−1 s−1 compared to that observed in C6-dithiol (1083 ± 8 cm3 g−1 s−1), probably due to similar reasons as those described in the case of C16-thiol. Importantly, in the case of dithiols, the extent of improvement in enzyme activity is notably higher compared to the analogous monothiols. Even in the presence of C4-dithiol, lipase activity (866 ± 8 cm3 g−1 s−1) exceeded the highest enzyme efficiency (805 ± 9 cm3 g−1 s−1) observed using the optimal monothiol (C12-thiol). Thus, the stronger capping ability of dithiols possibly ensured localization of a higher number of GNPs at the interface that significantly enhanced the total space in the vicinity of lipase. Smooth localization of enzyme and increased concentration of substrate at this augmented interface remarkably increased the lipase activity within these GNP–thiol-included CTAB reverse micelles.
![Variation of the second order rate constant (k2) for the lipase-catalyzed hydrolysis of p-nitrophenyl-n-octanoate in GNP508, GNP515 and GNP536-doped CTAB reverse micelles having [Au] = 10 μM and dithiols (100 μM) of varying chain length at z = 4.8, W0 = 48 and 25 °C. [Surfactant] = 50 mM, [lipase] = 1.02 × 10−6 g mL−1, [substrate] = 3 mM. Experimental errors are within ±1–2%.](/image/article/2012/RA/c2ra21237d/c2ra21237d-f4.gif) | ||
| Fig. 4 Variation of the second order rate constant (k2) for the lipase-catalyzed hydrolysis of p-nitrophenyl-n-octanoate in GNP508, GNP515 and GNP536-doped CTAB reverse micelles having [Au] = 10 μM and dithiols (100 μM) of varying chain length at z = 4.8, W0 = 48 and 25 °C. [Surfactant] = 50 mM, [lipase] = 1.02 × 10−6 g mL−1, [substrate] = 3 mM. Experimental errors are within ±1–2%. | ||
However, as reported earlier in several instances, GNP alone was found to influence enzyme activity.8e,f,g Thus, in our present study, to ascertain whether the observed increase in lipase activity was due to the proximity of the enzyme with the GNP surface, we monitored the activity of lipase in the presence of (10 μM) GNPs in aqueous medium. Interestingly, k2 remained almost the same in the presence of GNP508 compared to that without GNP (Table S2, ESI†). Also, the thiol/dithiol (10–600 μM) did not have any influence on the lipase activity in water, both in presence and absence of GNP508. This result clearly delineates that small sized GNP508 alone fails to persuade any activity improvement in aqueous medium. Even in reverse micelles, water pool solubilized GNP508 did not exhibit any notable influence on lipase activity in the absence of thiols. Alternatively, in reverse micelles, 1.8-fold and 2.5-fold higher activities were achieved at a much lower thiol concentration (100 μM). Hence, the enhanced lipase activity in GNP508-doped CTAB reverse micelles in the presence of thiol can be attributed to the smooth conformation attained by the enzyme at the increased interfacial domain.
Thiol-assisted enhancement in lipase activity in GNP508-doped CTAB systems was also observed even in the presence of a higher concentration of n-hexanol (z = 6.4), which is a competitive inhibitor of lipase. At W0 = 44, k2 increased by 1.7-fold from 373 ± 7 to 636 ± 9 cm3 g−1 s−1 in the case of C12-thiol, while C6-dithiol improved the activity by 2.1-fold (768 ± 8 cm3 g−1 s−1), following a similar trend to that observed at lower n-hexanol content (z = 4.8). The activity of lipase did not alter across the varying W0 ranges of 40–52 and 36–48 at z = 4.8 and 6.4, respectively, in thiol-included GNP-doped CTAB reverse micelles. In a previous report, it was observed that in the absence of thiols inclusion of large sized GNPs (∼20–25 nm) inside the water pool resulted in augmentation of the surrounding interfacial area, which was further enhanced with increasing W0, leading to an improvement of lipase activity.16a However, in the presence of thiol, unaltered lipase activity with increasing W0 established the fact that GNP508 is localized at the micellar interface, where additional water could not increase the interfacial area, similar to that of only CTAB reverse micelles (in the absence of both thiol and GNP).
To confirm the thiol-assisted localization of GNP508 at the microscopic interface of w/o microemulsions, we tested the efficacy of thiols to pull GNPs from the aqueous medium in a macroscopic biphasic mixture of water/isooctane. The aqueous layer comprised of GNP508 and C2-thiol, C12-thiol and C6-dithiol was added separately to isooctane and the entire mixture was vortexed for ∼2 min (Fig. 5a). In the absence of thiol, the red colour of GNP expectedly remained in the aqueous phase. GNPs were not found to move from the aqueous phase to organic phase or at the interface in the presence of C2-thiol. However, with the addition of C12-thiol, GNPs (red colour) were simultaneously distributed within both the aqueous phase and isooctane (Fig. 5a). Importantly, in the presence of C6-dithiol, GNPs tend to reside at the water/isooctane interface with a distinct colour change from red to violet.21a Thus, C2-thiol cannot facilitate the localization of GNP at the oil–water interface. However, C12-thiol is efficient enough in pulling GNPs from water to the non-aqueous phase, while C6-dithiol has the best efficacy in placing GNPs at the isooctane–water interface. Interestingly, this finding is in complete concurrence with the observed trend in thiol-assisted improvement of lipase activity within GNP508-doped CTAB reverse micelles. This hydrophobic thiol-assisted interfacial localization of GNP was further investigated by taking transmission electron microscopic (TEM) images of GNP508-doped CTAB reverse micelles. In the absence of thiol, the TEM images of GNP508 solubilized within the water pool showed no specific orientation (Fig. 5b) and it was almost similar to that of GNPs in water (Fig. 2). However, in the presence of C12-thiol a circular spatial arrangement of GNP508 was observed, which vividly indicates their localization at the periphery of the spherical aqueous core of reverse micelles (Fig. 5c and S1, ESI†). Also, the diameter of this circle formed by ordered positioning of GNP508 matches quite well with the diameter of the reverse micellar water pool (∼10–15 nm, Fig. 5c).15g,h These images further confirm the thiol-assisted confinement of GNP508 at the oil–water interface of reverse micelles. Interestingly, TEM images of the C6-dithiolated reverse micelles showed a circular orientation of a much higher number of GNP508 around the aqueous core than that of the monothiolated systems (Fig. 5d). Better enzyme activity in dithiolated reverse micellar systems could be attributed to the confinement of a higher number of GNP508 at the microscopic interface. This subsequently led to greater augmentation of space in the vicinity of the enzyme, resulting in higher lipase activity than that of the monothiolated systems (Scheme 1).
![(a) Optical images of GNP508 in a water/isooctane biphasic mixture (without thiol, in the presence of C2-thiol, C12-thiol and C6-dithiol), [GNP] = 0.25 mM; [thiols] = 2.5 mM. TEM images of GNP508 in CTAB reverse micelles (b) in the absence of thiol; (c) in the presence of C12-thiol and d) C6-dithiol.](/image/article/2012/RA/c2ra21237d/c2ra21237d-f5.gif) | ||
| Fig. 5 (a) Optical images of GNP508 in a water/isooctane biphasic mixture (without thiol, in the presence of C2-thiol, C12-thiol and C6-dithiol), [GNP] = 0.25 mM; [thiols] = 2.5 mM. TEM images of GNP508 in CTAB reverse micelles (b) in the absence of thiol; (c) in the presence of C12-thiol and d) C6-dithiol. | ||
The interfacial localization of GNPs in the presence of thiols was also monitored by fluorescence spectroscopy using the sodium salt of fluorescein (Na-fl). The hydrophilic fluorescent probe Na-fl is known to localize itself close to the interfacial domain of reverse micelles.22 This intrinsic property of Na-fl was utilized for confirming the position of GNPs within the thiol-incorporated newly developed self-assembled soft nanocomposites. The fluorescence quenching ability of GNP is quite well-established, particularly when the probes are closely associated with the nanoparticle.23 Here also, the emission intensity of Na–fl in aqueous solution steadily dropped with increasing concentration of GNP508 (Fig. S2a, ESI†). Hence, we studied the fluorescence emission of Na-fl in GNP508-doped CTAB reverse micelles in the presence and absence of C6-dithiol. In the presence of only C6-dithiol (without GNP), the emission intensity of Na-fl was found to be comparable to that observed in only CTAB w/o microemulsions. The fluorescence intensity of the probe quenched when GNP508 was doped in CTAB reverse micelles (without thiol), probably due to the certain extent of the proximity of the water pool-entrapped GNPs with the fluorophore (Fig. 6, Scheme 1). However, the emission intensity of Na-fl drastically dropped upon addition of C6-dithiol into the same GNP508-doped reverse micelles. This might be due to thiol-directed shifting of GNPs from the aqueous core to the interface, which in turn brought the nanoparticle in close proximity to the fluorophore residing near the interface. The entire fluorescence behaviour of Na-fl can be explained only if thiol-capped GNPs localize themselves at the interfacial domain of reverse micelles.
![Fluorescence spectra of the sodium salt of fluorescein (Na-fl) within CTAB (50 mM) reverse micelles in the presence of C6-dithiol (100 μM) with GNP508 ([Au] = 10 μM) at z = 6.4, W0 = 44.](/image/article/2012/RA/c2ra21237d/c2ra21237d-f6.gif) | ||
| Fig. 6 Fluorescence spectra of the sodium salt of fluorescein (Na-fl) within CTAB (50 mM) reverse micelles in the presence of C6-dithiol (100 μM) with GNP508 ([Au] = 10 μM) at z = 6.4, W0 = 44. | ||
The microscopic and spectroscopic evidences are in ideal concurrence in support of thiol-assisted confinement of GNPs at the reverse micellar interface, which enhanced the space in the vicinity of the enzyme. We were curious to find out the influence of this augmented space on the conformation of lipase within the thiol-included GNP-doped microemulsions. Circular dichroism (CD) spectroscopic analysis of the enzyme at the far UV region give an idea about the secondary structure of the protein and the mean residual ellipticity (MRE) value at 222 nm demonstrates its α-helical content.16,24 The smaller the MRE value is, the higher the α-helical content of the protein is. The MRE value of lipase was found to be in a comparable range both in CTAB and GNP508 (10 μM)-doped CTAB reverse micelles (Fig. 7). Thus, water pool solubilized GNP508 does not have any notable impact on the secondary structure of the enzyme. Accordingly, similar enzyme activity was also observed in the presence and absence of GNP508. However, in C12-thiol-included GNP508-doped CTAB reverse micelles, the MRE value at 222 nm decreased by a small extent, indicating an increase in the α-helical content of lipase. Notably, upon the addition of C6-dithiol in place of C12-thiol to the same GNP508-doped microemulsion, a distinct decrease in MRE was observed (Fig. 7), delineating a further increase in the α-helical content of lipase in dithiolated systems. Interestingly, the observed enhancement in lipase activity (Table 1 and Fig. 4) in thiol-included GNP-doped w/o microemulsions followed the same trend in accordance with the gradual improvement in the secondary structure of lipase in presence of mono- and dithiol. Hence, the marked improvement in the enzyme conformation at the augmented interface is also responsible for the enhanced lipase activity in thiol-included GNP-doped w/o microemulsions.
![CD spectra of CV-lipase in GNP508 ([Au] = 10 μM)-doped CTAB (50 mM) reverse micelles in the presence of C12-thiol and C6-dithiol (100 μM).](/image/article/2012/RA/c2ra21237d/c2ra21237d-f7.gif) | ||
| Fig. 7 CD spectra of CV-lipase in GNP508 ([Au] = 10 μM)-doped CTAB (50 mM) reverse micelles in the presence of C12-thiol and C6-dithiol (100 μM). | ||
In our quest for augmenting the interfacial area by entrapping GNPs of suitable dimensions, an attempt was made to accommodate large sized GNPs at the micellar interface. Earlier, solubilization of large sized GNPs within the water pool was proven to be an efficient method in enhancing the interfacial area. In this regard, GNPs having dimensions of 6–8 nm and 20–25 nm (Fig. 2c,d) with SPR peaks at 515 and 536 nm, respectively, (Fig. 2a) were synthesized.16a Lipase activity was measured within GNP515- and GNP536- (10 μM) doped CTAB reverse micelles in the absence and presence of thiols. A maximum 1.6-fold improvement (k2 = 712 ± 6 cm3 g−1 s−1, Table 1) in enzyme activity was observed in GNP515-doped CTAB reverse micelles in the presence of C12-thiol. In the case of all other monothiols having shorter or longer alkyl chains, the k2 did not improve significantly. In contrast, the presence of any monothiol was found to be incompetent to notably increase lipase activity within GNP536-doped CTAB reverse micelles. The k2 remained almost unaltered (516 ± 8 to 534 ± 7 cm3 g−1 s−1 in the presence of thiols with varying hydrophobicity (Table 1). In case of dithiols, the activation effect was expectedly a little higher than that observed with monothiols (Fig. 4). In C6-dithiol-included GNP515- and GNP536-doped CTAB w/o microemulsions, the best lipase activity was found to be 2.1-fold (k2 = 906 ± 8 cm3 g−1 s−1) and 1.7-fold (k2 = 736 ± 8 cm3 g−1 s−1) higher than only CTAB reverse micelles, respectively, (Fig. 4).
The preceding results clearly illustrate that large sized GNPs could not be well fitted within the CTAB reverse micellar interface, which was successfully achieved for small sized GNP. GNP515 having a 6–8 nm diameter might have been confined in close proximity to the interfacial domain in the presence of hydrophobic thiols, which in turn enhanced the interfacial area to a certain extent and consequently the lipase activity. In contrast, GNP536 with a much larger diameter (20–25nm) could not accommodate itself at the CTAB reverse micellar interface, having a thickness of ∼1–2 nm, leading to a negligible influence on the enhancement of lipase activity. These results further support the fact that with the assistance of hydrophobic thiols, GNP508 (∼3–5 nm diameter) was best fitted at the CTAB reverse micellar interface (∼1–2 nm), simply because of well-matched fusion in their dimension (Scheme 1). This fitting confinement resulted in maximum (2.5-fold) improvement in lipase activity owing to the augmentation of space in the vicinity of the enzyme at the interface. In the present study, the notable improvement in lipase activity was achieved with thiol-assisted interfacial localization of small sized GNPs (GNP508) at a very low concentration (∼10 μM), which was unable to produce any influence on lipase activity in the absence of thiol capping agents (Fig. 4).
At this point, it would be really intriguing to investigate whether large sized GNPs (GNP515 and GNP536) could be appropriately localized within a bigger interfacial domain compared to that of CTAB reverse micelles. To this end, w/o microemulsions were prepared with surfactants having a larger head group size, cetyltriethylammonium bromide (CTEAB) and cetyltripropylammonium bromide (CTPAB, Fig. 1). At z = 4.8, W0 = 48, lipase entrapped within CTEAB and CTPAB reverse micelles exhibited higher activity (582 ± 8 and 790 ± 8 cm3 g−1 s−1, respectively) compared to that of CTAB w/o microemulsions (Fig. 8) due to the enhanced interfacial area created by large head groups.12f,24c However, in the presence of varying sized GNPs (GNP508, GNP515 and GNP536) at a concentration of 10 μM within the water pool of these reverse micelles, a maximum 1.2-fold improvement in k2 was observed (Table S3, ESI†). Once again, it affirms that GNPs located inside the water pool have negligible influence in altering the interfacial area and thereby the lipase activity. Hence, to place the GNPs at the interface, C6-dithiol was used because of its proven efficacy among all thiols used in the present investigation. In CTAB microemulsion among all three GNPs, GNP508 was best fitted at the interface having the highest k2 = 1083 ± 8 cm3 g−1 s−1. Interestingly, in the case of CTEAB, GNP515 was found to be most appropriately integrated at the interface, where lipase activity improved to 1048 ± 10 cm3 g−1 s−1 (Fig. 8). Although thiol-assisted interfacial localization of the other two GNPs enhanced the k2, those activities are lower compared to that in the presence of the thiol-included GNP515-doped CTEAB system. It is evident that appropriate interfacial confinement of a GNP of a particular dimension depends on the thickness of the reverse micellar interface. However, in the combined system of CTEAB and thiol-capped GNP515, lipase activity could not be improved beyond the value that was observed in analogous systems of CTAB and GNP508. Therefore, along with GNP508 and GNP515, an attempt was made to incorporate considerably large sized (20–25 nm) GNP536 at the interface of CTPAB reverse micelles with higher interfacial thickness compared to CTAB and CTEAB. Lipase activity steadily improved with increasing size of GNP in the presence of C6-dithiol (Fig. 8). Most fascinatingly, lipase showed the highest activity in the GNP536-integrated CTPAB system (k2 = 1462 ± 7 cm3 g−1 s−1). Thus, in comparison to the CTAB w/o microemulsion, a remarkable improvement (by ∼3.4 fold) in hydrolytic efficiency of lipase was achieved within GNP536-doped CTPAB reverse micelles (Fig. 8). These results further establish the concept that efficient confinement of GNP at the micellar interface is dependent on the matching dimensions of both GNP and interfacial area. Although the interfacial thickness of CTEAB and CTPAB reverse micelles cannot not be compared to that of larger sized GNP diameter, the improvement in lipase activity was obtained presumably due to very close placement of GNP515 in CTEAB and GNP536 in the CTPAB micellar interface. Confinement of large sized GNP515 and GNP536 in reverse micelles of CTEAB and CTPAB in the presence of C6-dithiol were also studied using fluorescence spectroscopy, as investigated in the case of CTAB. In both cases, maximum quenching of interfacially located Na-fl was observed in the presence of C6-dithiol compared to that in the absence of thiol, as well as both thiol and GNP (Fig. S2b,c, ESI†). This finding once again confirmed the thiol-assisted confinement of GNPs at the interface of reverse micelles that remarkably improved the efficiency of surface-active lipase due to the enhancement of space in the vicinity of the enzyme.
![Variation of the second order rate constant (k2) for the lipase-catalyzed hydrolysis of p-nitrophenyl-n-octanoate in GNP-doped CTAB, CTEAB and CTPAB reverse micelles having [Au] = 10 μM and [C6-dithiol] = 100 μM at z = 4.8, W0 = 48 and 25 °C. [Surfactant] = 50 mM, [lipase] = 1.02 × 10−6 g mL−1, [substrate] = 3 mM. Experimental errors are within ±1–2%.](/image/article/2012/RA/c2ra21237d/c2ra21237d-f8.gif) | ||
| Fig. 8 Variation of the second order rate constant (k2) for the lipase-catalyzed hydrolysis of p-nitrophenyl-n-octanoate in GNP-doped CTAB, CTEAB and CTPAB reverse micelles having [Au] = 10 μM and [C6-dithiol] = 100 μM at z = 4.8, W0 = 48 and 25 °C. [Surfactant] = 50 mM, [lipase] = 1.02 × 10−6 g mL−1, [substrate] = 3 mM. Experimental errors are within ±1–2%. | ||
Conclusion
In summary, thiol-assisted confinement of appropriately sized GNPs at the reverse micellar interface of comparable thickness led to an enhancement in the overall interfacial area of w/o microemulsions. Optimally, hydrophobic thiols served the purpose of pulling the water pool-solubilized GNPs at the reverse micellar interface. In this regard, dithiols with better capping ability were found to be more proficient in localizing a higher number of GNPs at the micellar interface compared to monothiols. Also, large sized GNPs were suitably fitted within the reverse micellar interface constituted by larger head group sized surfactants in the presence of hydrophobic thiols. These newly developed soft nanocomposite systems with augmented interface served as a superior host for surface active lipase owing to its smooth localization, as well as increased concentration of substrate. Development of GNP-included self-assembled nanohybrid systems exhibiting higher enzyme activity can play an important role in nanobiotechnological advancements.Experimental section
Materials
Chromobacterium viscosum lipase (E.C.3.1.1.3 Type XII) (size = 4.5 nm × 5 nm × 4.5 nm),14g HAuCl4 (30 wt%) solution, all monothiols and dithiols were purchased from Sigma, USA and were used as received. Analytical grade CTAB was purchased from Spectrochem (India) and it was crystallized three times from methanol/diethyl ether. Recrystallized CTAB was without minima in its surface tension plot. HPLC-grade isooctane, n-hexanol, NaBH4 and all other reagents were purchased from Spectrochem and SRL (India) and were of the highest analytical grade. Trisodium citrate was procured from Merck (India). The UV-visible absorption spectra were recorded on a Perkin Elmer Lambda 25 spectrophotometer. Fluorescence spectra were recorded in a Varian Cary Eclipse luminescence spectrometer. Circular dichroism spectroscopy was carried out in a Jasco J-815 spectrometer. CTEAB, CTPAB and p-nitrophenyl-n-octanoate were synthesized following previously reported protocols.12f,24cPreparation of different GNP solutions
I) Synthesis of GNP508. An aqueous solution (5 mL) of 0.3 mM HAuCl4 was prepared containing 0.3 mM trisodium citrate. To this solution, 150 μL of ice-cold, freshly prepared NaBH4 solution (0.1 M) was added under stirring conditions to maintain the final concentration of NaBH4 at 3 mM. The concentration ratio of [Au]:[citrate]:[NaBH4] was 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The solution immediately turned to pink after the addition of NaBH4, indicating formation GNP. The SPR peak of that solution was at 508 nm (GNP508).
10. The solution immediately turned to pink after the addition of NaBH4, indicating formation GNP. The SPR peak of that solution was at 508 nm (GNP508).
II) Synthesis of GNP515. A similar procedure was followed during the preparation of GNP515 to that mentioned above, except the volume of 0.1 M NaBH4 solution. 70 μL of ice-cold, freshly prepared NaBH4 solution (0.1 M) was used as the reducing agent. Here, the concentration ratio of [Au]:[citrate]:[NaBH4] was 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5. The solution turned to wine-red. The SPR peak of that solution was observed at 515 nm (GNP515).
4.5. The solution turned to wine-red. The SPR peak of that solution was observed at 515 nm (GNP515).
III) Synthesis of GNP536. The same method was followed as that described above, where 30 μL of ice-cold, freshly prepared 0.1 M NaBH4 solution was used as the reducing agent. The final concentration of NaBH4 was 0.6 mM and the concentration ratio of [Au]:[citrate]:[NaBH4] was 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The solution turned to wine-red and the SPR peak of that solution was found at 536 nm (GNP536).
2. The solution turned to wine-red and the SPR peak of that solution was found at 536 nm (GNP536).
Lipase activity in reverse micelles. The second-order rate constant (k2) in lipase-catalyzed hydrolysis of p-nitrophenyl-n-octanoate in cationic w/o microemulsions was determined spectrophotometrically at the isosbestic points, as described previously.12c,13f,14c,16 In a typical experiment, 4.5 μL of the aqueous enzyme stock solution (0.34 mg mL−1) and 10 μL of the substrate stock solution (0.45 M) in isooctane were added to the w/o microemulsion (1.5 mL) previously prepared with the desired surfactant concentration and pH (pH refers to the pH of the aqueous buffer solutions used for preparing the w/o microemulsions; pH within the water pool of w/o microemulsions did not vary significantly <1 unit), in a cuvette to attain the particular W0 and reactant concentrations.15h,25 Gentle shaking produced clarification of the microemulsion within 1 min. The initial linear rate of increase in absorbance of liberated p-nitrophenol was then recorded at the isosbestic points (λiso) of the corresponding system. λiso and the molar extinction coefficients (ε) at λiso of the p-nitrophenol/p-nitrophenolate couple in GNP-doped w/o microemulsions of different surfactants CTAB, CTEAB and CTPAB are given in Table S1, ESI†. The overall concentrations of lipase and p-nitrophenyl-n-octanoate were 1.02 × 10−6 g mL−1 and 3 ×10−3 M, respectively. Although the lipase was essentially confined to the dispersed water droplets (at the oil/water interface), concentrations of reactants were referred to the overall concentration within the cuvette to avoid the complexity of the volume fraction of water droplets in the w/o microemulsions and the partitioning coefficient of the substrate. Hydrolytic efficiency of lipase was estimated by measuring the second-order rate constant (k2) instead of first-order Michaelis–Menten catalytic constant (kcat), since the initial rate of lipase-catalyzed hydrolysis of p-nitrophenyl-n-octanoate was observed to be first order with respect to the substrate concentration.14c,15f,20 The working equation for lipase catalyzed hydrolysis is:
| d[P]/dt = k2[S][E] |
Activity of lipase in water + 4% EtOH. The activity of lipase was also checked in aqueous medium. The k2 of the lipase catalysed hydrolysis of p-nitrophenyl n-hexanoate in water + 4% EtOH medium was obtained spectrophotometrically at the corresponding isosbestic points. For complete solubilization of the substrate, the concentration of the enzyme and the substrate were kept 50-fold lower than that for reverse micelles. The final concentration of substrate and the enzyme were kept at 0.06 mM and 0.02 × 10−6 g cm−3, respectively, and the thiol concentration was varied (10–600 μM) with respect to a fixed GNP concentration (10 μM). The isosbestic point and molar extinction coefficient at λiso (340 nm) in water + 4% EtOH without GNP and with GNP were 6500 and 6250 M−1 cm−1, respectively.16a
TEM studies. 4 μL of GNP-doped reverse micellar solutions in the absence and presence of thiol were placed on 300-mesh Cu coated TEM grid and dried under vacuum for 4h before taking TEM images. Similarly, GNPs in water were also placed on a Cu-coated TEM grid. TEM measurements were performed on a JEOL JEM 2010 microscope.
Fluorescence spectroscopy. The luminescence spectra of Na-fl was taken by exciting at wavelength of 480 nm all of the thiol-doped GNP-included CTAB, CTEAB and CTPAB reverse micelles at z = 6.4, W0 = 44. To the previously prepared reverse micelles, 2 μL of Na-fl stock solution was added to keep the final concentration of the fluorophore at 1 μg mL−1. The excitation and emission slits were kept at 5 nm. In aqueous medium fluorescence spectra were observed over a range of GNP concentration 5–40 μM keeping the fluorophore concentration the same.
Circular dichroism spectra. The CD spectra of lipase entrapped within CTAB and GNP-doped CTAB reverse micelles in the presence and absence of thiol were recorded in Jasco J-815 using a 2 mm path length cell at wavelength 220–280 nm with a scan speed of 50 nm min−1 (CD spectra could not be measured below 220 nm due to off-scale signal). All of the spectra were corrected by subtracting a blank spectrum (without enzyme) and accumulated 6 times. Results were expressed in terms of mean residue ellipticity (deg cm2 dmol−1). The final concentration of the lipase was kept at 25 μg mL−1 in reverse micelles.
Acknowledgements
P. K. D. is thankful to the Council of Scientific and Industrial Research (CSIR), India for financial assistance (01(2471)/11/EMR-II). M. G., S. M. and S. B. acknowledge the CSIR, India, for their Research Fellowships.References
- P. K. Jain, K. S. Lee, I. H. El-Sayed and M. A. El-Sayed, J. Phys. Chem. B, 2006, 110, 7238 CrossRef CAS.
- G. Schmid and U. Simon, Chem. Commun., 2005, 697 RSC.
- (a) U. Drechsler, B. Erdogan and V. M. Rotello, Chem.–Eur. J., 2004, 10, 5570 CrossRef CAS; (b) Z. Li, S. W. Chung, J. M. Nam, D. S. Ginger and C. A. Mirkin, Angew. Chem., 2003, 115, 2408 CrossRef ; Angew. Chem. Int. Ed., 2003, 42, 2306.
- M. Sakamoto, T. Tachikawa, M. Fujitsuka and T. Majima, Adv. Funct. Mater., 2007, 17, 857 CrossRef CAS.
- C. Avelino, G. Hermenegildo, M. N. Pedro, P. Ana, C. J. Jose and T. Susana, Chem.–Eur. J., 2007, 13, 6359 CrossRef CAS.
- (a) B. Van de Broek, N. Devoogdt, A. D'Hollander, H. L. Gijs, K. Jans, L. Lagae, S. Muyldermans, G. Maes and G. Borghs, ACS Nano, 2011, 6, 4319 Search PubMed; (b) S. Maiti, S. Dutta and P. K. Das, Chem.–Eur. J., 2011, 17, 7538 Search PubMed.
- B. Kang, M. A. Mackey and M. A. El-Sayed, J. Am. Chem. Soc., 2010, 132, 1517 CrossRef CAS.
- (a) A. Verma, J. M. Simard, J. W. E. Worrall and V. M. Rotello, J. Am. Chem. Soc., 2004, 126, 13987 CrossRef CAS; (b) C. C. You, R. R. Arvizo and V. M. Rotello, Chem. Commun., 2006, 2905 RSC; (c) R. Bonomi, A. Cazzolaro, A. Sansone, P. Scrimin and L. J. Prins, Angew. Chem., 2011, 123, 2355 Search PubMed ; Angew. Chem. Int. Ed., 2011, 50, 2307; (d) T. Serizawa, Y. Hiraiab and M. Aizawab, Mol. BioSyst., 2010, 6, 1565 RSC; (e) J. L. Brennan, N. S. Hatzakis, T. R. Tshikhudo, N. Dirvianskyte, V. Razumas, S. Patkar, J. Vind, A. Svendsen, R. J. M. Nolte, A. E. Rowan and M. Brust, Bioconjugate Chem., 2006, 17, 1373 CrossRef CAS; (f) J. L. Brennan, A. G. Kanaras, P. Nativo, T. R.Tshikhudo, C. Rees, L. C. Fernandez, N. Dirvianskyte, V. Razumas, M. Skjøt, A. Svendsen, C. I. Jørgensen, R. Schweins, M. Zackrisson, T. Nylander, M. Brust and J. Barauskas, Langmuir, 2010, 26, 13590 CrossRef CAS; (g) J. Deka, A. Paul and A. Chattopadhyay, RSC Adv., 2012, 2, 4736 RSC.
- (a) J. Turkevich, P. C. Stevenson and J. Hillier, Discuss. Faraday Soc., 1951, 11, 55 RSC; (b) I. Ojea-Jime'nez, F. M. Romero, N. G. Bastu's and V. Puntes, J. Phys. Chem. C, 2010, 114, 1800 CrossRef; (c) L. Polavarapu and Q. H. Xu, Nanotechnology, 2009, 2, 185606 Search PubMed; (d) R. N. Mitra and P. K. Das, J. Phys. Chem. C, 2008, 112, 8159 CrossRef CAS.
- (a) T. Sainsbury, T. Ikuno, D. Okawa, D. Pacile', J. M. J. Fre'chet and A. Zettl, J. Phys. Chem. C, 2007, 111, 12992 CrossRef CAS; (b) P. Pandey, S. P. Singh, S. K. Arya, V. Gupta, M. Datta, S. Singh and B. D. Malhotra, Langmuir, 2007, 23, 3333 CrossRef CAS.
- (a) W. Cheng, S. Dong and E. Wang, Langmuir, 2003, 19, 9434 CrossRef CAS; (b) A. M. Alkilany and C. J. Murphy, Langmuir, 2009, 25, 13874 CrossRef CAS; (c) V. Arcoleo and V. Turco Liver, Chem. Phys. Lett., 1996, 258, 223 CrossRef CAS.
- (a) A. S. Bommarius, T. A. Hatton and D. I. C. Wang, J. Am. Chem. Soc., 1995, 117, 4515 Search PubMed; (b) E. Abuin, E. Lissi and C. Calderon, J. Colloid Interface Sci., 2007, 308, 573 Search PubMed; (c) M. A. Biasutti, E. B. Abuin, J. J. Silber, N. M. Correa and E. A. Lissi, Adv. Colloid Interface Sci., 2008, 136, 1 CrossRef CAS; (d) S. Debnath, A. Dasgupta, R. N. Mitra and P. K. Das, Langmuir, 2006, 22, 8732 CrossRef CAS; (e) R. N. Mitra, A. Dasgupta, D. Das, S. Roy, S. Debnath and P. K. Das, Langmuir, 2005, 21, 12115 CrossRef CAS; (f) D. Das, S. Roy, R. N. Mitra, A. Dasgupta and P. K. Das, Chem.–Eur. J., 2005, 11, 4881 CrossRef CAS; (g) S. Mahiuddin, A. Renoncourt, P. Bauduin, D. Touraud and W. Kunz, Langmuir, 2005, 21, 5259 Search PubMed; (h) Z. Guo, N. Hauser, A. Moreno, T. Ishikawa and P. Walde, Soft Matter, 2011, 7, 180 RSC; (i) D. Mitra, I. Chakraborty, S. C. Bhattacharya, S. P. Moulik, S. Roy, D. Das and P. K. Das, J. Phys. Chem. B, 2006, 110, 11314 Search PubMed.
- (a) V. Brissos, E. P. Melo, J. M. G. Martinho and J. M. S. Cabral, Biochim. Biophys. Acta, 2008, 1784, 1326 Search PubMed; (b) R. Biswas, A. R. Das, T. Pradhan, D. Touraud, W. Kunz and S. Mahiuddin, J. Phys. Chem. B, 2006, 112, 6620 Search PubMed; (c) S. Debnath, D. Das, S. Dutta and P. K. Das, Langmuir, 2010, 26, 4080 CrossRef CAS; (d) K. Martinek, A. V. Levashov, Y. L. Khmelnitsky, N. L. Klyachko and I. V. Berezin, Science, 1982, 218, 889 CrossRef CAS; (e) C. Oldfield, R. B. Freedman and B. H. Robinson, Faraday Discuss., 2005, 129, 247 RSC; (f) A. Dasgupta, D. Das and P. K. Das, Biochimie, 2005, 87, 1111 Search PubMed; (g) A. Harrar, O. Zech, A. Klaus, P. Bauduin and W. Kunz, J. Colloid Interface Sci., 2011, 362, 423 Search PubMed; (h) S. Fornera, T. Bauer, A. D. Schluter and P. Walde, J. Mater. Chem., 2012, 22, 502 RSC.
- (a) C. M. L. Carvalho, M. R. Aires-Barros and J. M. S. Cabral, Langmuir, 2000, 16, 3082 Search PubMed; (b) A. Shome, S. Roy and P. K. Das, Langmuir, 2007, 23, 4130 CrossRef CAS; (c) A. Dasgupta, D. Das, R. N. Mitra and P. K. Das, J. Colloid Interface Sci., 2005, 289, 566 CrossRef CAS; (d) T. Maruyama, T. Hosogi and M. Goto, Chem. Commun., 2007, 43, 4450 Search PubMed; (e) B. Minofar, R. Vacha, A. Wahab, S. Mahiuddin, W. Kunz and P. Jungwirth, J. Phys. Chem. B, 2006, 110, 15939 CrossRef CAS; (f) Z. Guo, H. Regger, R. Kissner, T. Ishikawa, M. Willeke and P. Walde, Langmuir, 2009, 25, 11390 CrossRef CAS; (g) D. Lang, B. Hofmann, L. Haalck, H. J. Hecht, F. Spener, R. D. Schmid and D. Schomburg, J. Mol. Biol., 1996, 259, 704 CrossRef CAS.
- (a) D. Das and P. K. Das, Langmuir, 2003, 19, 9114 CrossRef CAS; (b) M. C. Pinna, P. Bauduin, D. Touraud, M. Monduzzi, B. W. Ninham and W. Kunz, J. Phys. Chem. B, 2005, 109, 16511 CrossRef CAS; (c) S. Roy, A. Dasgupta and P. K. Das, Langmuir, 2006, 22, 4567 Search PubMed; (d) C. Schirmer, Y. Liu, D. Touraud, A. Meziani, S. Pulvin and W. Kunz, J. Phys. Chem. B, 2002, 106, 7414 Search PubMed; (e) D. Das and P. K. Das, Langmuir, 2009, 25, 4421 CrossRef CAS; (f) P. K. Das and A. Chaudhuri, Langmuir, 2000, 16, 76 Search PubMed; (g) S. S. Atik and J. K. Thomas, J. Am. Chem. Soc., 1981, 103, 4367 CrossRef CAS; (h) E. M. Corbeil and N. E. Levinger, Langmuir, 2003, 19, 7264 Search PubMed.
- (a) S. Maiti, D. Das, A. Shome and P. K. Das, Chem.–Eur. J., 2010, 16, 1941 CrossRef CAS; (b) S. Maiti, M. Ghosh and P. K. Das, Chem. Commun., 2011, 47, 9864 RSC.
- L. Fei and S. Perrett, Int. J. Mol. Sci., 2009, 10, 647 Search PubMed.
- (a) T. K. Mandal, M. S. Fleming and D. R. Walt, Nano Lett., 2002, 2, 3 CrossRef; (b) A. Badia, L. Cuccia, L. Demers, F. Morin and R. B. Lennox, J. Am. Chem. Soc., 1997, 119, 2682 CrossRef CAS.
- (a) T. R. J. Jenta, T. Batts, G. D. Rees and B. H. Robinson, Biotechnol. Bioeng., 1997, 54, 416 CrossRef CAS; (b) G. W. Zhou, G. Z. Li, J. Xu and Q. Sheng, Colloid Surf., A, 2001, 41, 194 Search PubMed.
- P. D. I. Fletcher, B. H. Robinson, R. B. Freedman and C. Oldfield, J. Chem. Soc., Faraday Trans. 1, 1985, 81, 2667 RSC.
- (a) Z. Tang, B. Xu, B. Wu, M. W. Germann and G. Wang, J. Am. Chem. Soc., 2010, 132, 3367 CrossRef CAS; (b) M. Brust, D. Bethell, D. J. Schffrin and C. J. Kiely, Adv. Mater., 1995, 7, 795 CAS; (c) C. Guarise, L. Pasquato and P. Scrimin, Langmuir, 2005, 21, 5537 CrossRef CAS.
- (a) G. B. Dutta, J. Chem. Phys., 2008, 129, 014501 Search PubMed; (b) G. B. Dutt, J. Phys. Chem. B, 2008, 112, 7220 CrossRef CAS.
- (a) N. Nerambourg, R. Praho, M. H. V. Werts and M. B. Desce, Nano Lett., 2006, 6, 530 CrossRef CAS; (b) H. I. Peng, C. M. Strohsahl, K. E. Leach, T. D. Krauss and B. L. Miller, ACS Nano, 2009, 3, 2265 CrossRef CAS; (c) J. R. Lakowicz, Anal. Biochem., 2001, 298, 1 CrossRef CAS.
- (a) S. Debnath, D. Das and P. K. Das, Biochem. Biophys. Res. Commun., 2007, 356, 163 CrossRef CAS; (b) P. Walde, D. Han and P. L. Luisi, Biochemistry, 1993, 32, 4029 CrossRef CAS; (c) D. Das, D. Das and P. K. Das, Biochimie, 2008, 90, 820 Search PubMed.
- (a) P. K. Das, A. Chaudhuri, S. Saha and A. Samanta, Langmuir, 1999, 15, 4765 Search PubMed; (b) P. K. Das and A. Chaudhuri, Langmuir, 1999, 15, 8771 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Isosbestic points and corresponding molar extinction coefficients; lipase activity in CTAB, CTEAB and CTPAB reverse micelles in presence of GNPs of varying sizes; fluorescence spectra of sodium salt of fluorescein (Na-fl) in water, CTEAB and CTPAB reverse micelles. See DOI: 10.1039/c2ra21237d |
| This journal is © The Royal Society of Chemistry 2012 |
