Copper acetate monohydrate: a cheap but efficient oxidant for synthesizing multi-substituted indolizines from pyridinium ylides and electron deficient alkenes†
Huayou
Hu
*ab,
Junjun
Feng
a,
Yulan
Zhu
a,
Ning
Gu
*b and
Yuhe
Kan
*a
aDepartment: Jiangsu Key Laboratory for Chemistry of Low-Dimensional Materials, School of Chemistry and Chemical Engineering, Huaiyin Normal University, Huaian, 223300, P. R. China. E-mail: njuhhy@hotmail.com; kyh@hytc.edu.cn; Fax: (+)86(517)83525100-1; Tel: (+)86(517)83525100-4
bState Key Laboratory of Bioelectronics, School of Biological Science and Medical Engineering, Southeast University, Nanjing, 210096, P. R. China. E-mail: guning@seu.edu.cn
First published on 14th August 2012
Abstract
We report a highly practical one-pot method for synthesizing multi-substituted indolizines from α-halide carbonyl compounds, pyridines and electron deficient alkenes in the presence of copper acetate monohydrate and sodium acetate in DMF. A variety of function groups are tolerable in standard reaction conditions, including aldehyde. 36 examples were presented. The yield of indolizine was from moderate to high. Furthermore, multi-substituted indolizines can be prepared at gram scale by this method.
Introduction
The indolizine skeleton is abundant in many natural products,1 such as Erythrinan alkaloids,2a swainsonine,2b slaframine,2c gephyrotoxine,2d cryptowoline,2e and Myrmicarin.2f These natural products as well as their synthetic derivatives have been found to exhibit a variety of biological activities, including antibacterial,3a phosphatase and aromatase inhibiting,3b–c antioxidant,3d antidepressant3e and antitumor activities.3f They are also known as calcium entry blockers3g and 5-hydroxytryptamine receptor antagonists.3h Furthermore, indolizine derivatives were observed to have long wavelength absorption and fluorescence with high quantum efficiency in the visible light region.4a–c Therefore, the successful synthesis of these types of compounds has facilitated the development of novel classes of dyes and biological markers.4It is well recognized that 1,3-dipolar cycloaddition5 was one of the most efficient methods to construct five member heterocycles.6 This synthetic strategy has been applied to synthesizing indolizines since the work of Boekelheide and co-workers in 1961.7 However, this kind of reaction has drawbacks relating to the reaction scope due to the involvement of electron deficient alkynes. Compared with electron deficient alkynes, electron deficient alkenes are more readily available. Therefore, replacing electron deficient alkynes with electron deficient alkenes can dramatically extend the reaction scope and decrease the cost of synthesis. However, when electron deficient alkenes are applied to synthesize indolizines through 1,3-dipolar cycloaddition, the corresponding indolizines can be obtained only by adding an excess amount of oxidant, including freshly prepared MnO28 and tetrakispyridinecobalt(II) dichromate (TPCD).9 These two kinds of reagents are not commercially available and need to be prepared in the laboratory. Furthermore, indolizines were synthesized from pyridine in two steps instead of through a more efficient one-pot reaction. Therefore, a cheap and commercially available oxidant which could be applied in a one-pot reaction for synthesizing indolizines from electron deficient alkenes is in great demand.
Copper acetate monohydrate [Cu(OAc)2·H2O] is the kind of reagent that meets the above requirement. It's priced at $350.0 per 5 Kg by Alfa Aesar in the US as chemical reagent10 and at about $4000 per ton in China as an industrial product.11 Copper acetate monohydrate has also been known as an oxidant for many reactions for a long time, including dehydrogenated aromatization reactions.12 As a follow-up to our reported method,13 we herein report that copper acetate monohydrate is an efficient oxidant for synthesizing multi-substituted indolizines from electron deficient alkenes.
Results and discussion
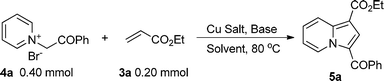
In the preliminary study, in order to optimize the reaction conditions, we chose 1-(2-oxo-2-phenylethyl)pyridinium bromide 4a (2.0 eq.) and ethyl acrylate 3a (1.0 eq.) as our model reaction system. When potassium carbonate was used as the base and N,N-dimethylformamide (DMF) as the solvent, the known product 5a was isolated in good yield (Table 1, entry 1).14a The result showed that copper acetate monohydrate was the most efficient reagent among five kinds of copper salts (Table 1, entries 1–5). Encouraged by this result, we then optimized the reaction conditions by changing the solvent and base.| Entry | Cu Salt (equiv.) | Base (equiv.) | Solvent | Yield(%)a |
|---|---|---|---|---|
| a Isolated yield. b DMSO = dimethyl sulfoxide. c DMA = N,N-dimethylacetamide. d NMP = 1-methylpyrrolidin-2-one. e Salt 4a was synthesized in situ by mixing pyridine 1a with 2-bromo-1-phenylethanone 2a at 60 °C for 2 h and without further purification. | ||||
| 1 | Cu(OAc)2·H2O, 3.0 | K2CO3, 4.0 | DMF | 82 |
| 2 | Cu(NO3)2·3H2O, 3.0 | K2CO3, 4.0 | DMF | 53 |
| 3 | CuSO4, 3.0 | K2CO3, 4.0 | DMF | 77 |
| 4 | CuBr2, 3.0 | K2CO3, 4.0 | DMF | trace |
| 5 | CuCl2, 3.0 | K2CO3, 4.0 | DMF | trace |
| 6 | Cu(OAc)2·H2O, 3.0 | NaHCO3, 4.0 | DMF | 89 |
| 7 | Cu(OAc)2·H2O, 3.0 | t-BuOK, 4.0 | DMF | 69 |
| 8 | Cu(OAc)2·H2O, 3.0 | NaH, 4.0 | DMF | 88 |
| 9 | Cu(OAc)2·H2O, 3.0 | Li2CO3, 4.0 | DMF | 90 |
| 10 | Cu(OAc)2·H2O, 3.0 | Na2CO3, 4.0 | DMF | 93 |
| 11 | Cu(OAc)2·H2O, 3.0 | NaOAc, 4.0 | DMF | 98 |
| 12 | Cu(OAc)2·H2O, 3.0 | No | DMF | 76 |
| 13 | Cu(OAc)2·H2O, 3.0 | NaOAc, 4.0 | 1,4-dioxane | 56 |
| 14 | Cu(OAc)2·H2O, 3.0 | NaOAc, 4.0 | benzene | trace |
| 15 | Cu(OAc)2·H2O, 3.0 | NaOAc, 4.0 | DMSOb | 60 |
| 16 | Cu(OAc)2·H2O, 3.0 | NaOAc, 4.0 | DMAc | 86 |
| 17 | Cu(OAc)2·H2O, 3.0 | NaOAc, 4.0 | MeCN | 75 |
| 18 | Cu(OAc)2·H2O, 3.0 | NaOAc, 4.0 | NMPd | 92 |
| 19 | Cu(OAc)2·H2O, 3.0 | NaOAc, 4.0 | H2O | 6 |
| 20 | Cu(OAc)2·H2O, 2.0 | NaOAc, 4.0 | DMF | 74 |
| 21e | Cu(OAc)2·H2O, 3.0 | NaOAc, 4.0 | DMF | 96 |
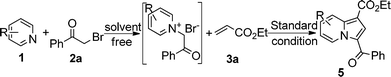
Entries 6–19 in Table 1 demonstrated that DMF was the best solvent and sodium acetate was the best base, while entry 20 showed that when the amount of copper acetate decreased from 3.0 equiv. to 2.0 equiv., the yield of 5a also decreased from 98 to 74%. Notably, when the isolated salt 4a was replaced by in situ formed salt 4a, the yield of 5a did not change markedly (Table 1, entry 21). This result indicated that more efficient one-pot reaction could be applied to this transformation.
With the optimal reaction conditions in hand (Table 1, entry 21), the scope and generality of pyridine derivatives in this transformation were examined (Table 2). A wide range of substituents, either electron-donating or electron-withdrawing groups in the pyridine ring, such as p-CO2Me, o-Me, m-CN, p-Me, p-NMe2, etc., were tolerable in this transformation. Interestingly, 2-methylpyridine 1b gave corresponding indolizine 5b in a high yield of 90%, although 2-phenylindolizine was also found in the reaction mixture. This result should be attributed to the reaction speed of pyridinium ylide with ethyl acrylate, which was much faster than its intermolecular cyclization under standard conditions (Table 2, entry 1). When the pyridine bore a cyano group at the meta position, two isomers (5d and 5d′) were isolated in the ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (Table 2, entry 3). Fused pyridines also gave the corresponding fused indolizine with good yield (Table 2, entries 6–8). The reason that 4-vinylpyridine 1k did not give any designed product could be attributed to polymerization of 1k.
5 (Table 2, entry 3). Fused pyridines also gave the corresponding fused indolizine with good yield (Table 2, entries 6–8). The reason that 4-vinylpyridine 1k did not give any designed product could be attributed to polymerization of 1k.
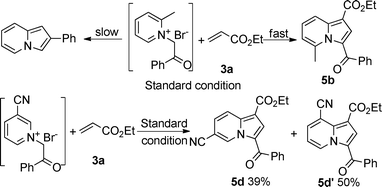 | ||
| Scheme 1 The formation of 5b, 5d and 5d′. | ||
| Entry | Pyridine | Product | Yield (%)a | Ref. |
|---|---|---|---|---|
| a Isolated yield. b DMAP = N,N-dimethylpyridin-4-amine. | ||||
| 1 | 2-methylpyridine 1b |
 5b
5b
|
90 | — |
| 2 | 4-methylpyridine 1c |
 5c
5c
|
54 | — |
| 3 | nicotinonitrile 1d |
 5d;
5d; 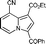 5d′ 5d′ |
39; 50 | — |
| 4 | DMAP 1eb |
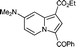 5e
5e
|
50 | — |
| 5 | methyl isonicotinate 1f |
 5f
5f
|
91 | — |
| 6 | quinoline 1g |
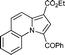 5g
5g
|
63 | 14b |
| 7 | 4-methylquinoline 1h |
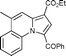 5h
5h
|
61 | — |
| 8 | isoquinoline 1i |
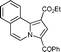 5i
5i
|
95 | 14c |
| 9 | 4-chloropyridine 1j |
 5j
5j
|
26 | — |
| 10 | 4-vinylpyridine 1k | Complex mixture | N. D.d | — |
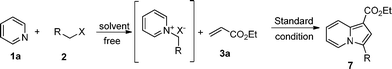
Then, we turned to check the scope of alkenes. The results were listed in Table 3. Many kinds of alkenes bearing a variety of electron-withdrawing groups, including ester, aldehyde, amide, cyano group, ketone and nitro group, were applied in this reaction. Both terminal and non-terminal alkenes reacted with pyridinium ylide smoothly. Some non-terminal alkenes such as 3l and 3p, however, couldn't give corresponding indolizines at high yield even when the reaction time was extended to 72 h. The fused conjugated alkenes 3l also gave indolizine at low yield. Moreover, cyclic alkenes 3m and 3k gave complex mixtures from which the corresponding indolizines could not be isolated. Interestingly, aldehyde could survive under standard reaction conditions and gave corresponding inlodizine at moderate yield (Table 3, entries 17–18). Furthermore, alkenes bearing a nitro group could also be applied in this transformation. When (E)-(2-nitrovinyl) benzene 3t was used, two isomers were isolated. One of the products 6t, which keeps the nitro group, was isolated in a yield of 54%, while the other product 6t′, which loses the nitro group, was isolated in a yield of 28%. When (E)-(2-nitroprop-1-enyl) benzene 3u was used, the only product 6u′ was isolated in a yield of 72%. This result should be attributed to the trend of aromatizing the tetra-hydrogen indolizine intermediate.
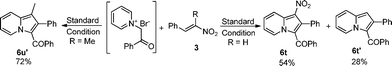 | ||
| Scheme 2 The formation of 6t, 6t′ and 6u′. | ||
| Entry | Alkene | Product | Yield (%)a | Ref. |
|---|---|---|---|---|
| a Isolated yield. b Complex mixture. c 72 h, the yield in parentheses was based on recovered starting material. d Two isomers were isolated. | ||||
| 1 | methyl acrylate 3b |
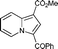 6b
6b
|
95 | 14a |
| 2 | tert-butyl acrylate 3c |
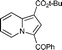 6c
6c
|
80 | — |
| 3 | n-butyl acrylate 3d |
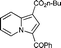 6d
6d
|
85 | 14a |
| 4 | acrylonitrile 3e |
 6e
6e
|
78 | 14a |
| 5 | N,N-dimethylacrylamide 3f |
 6f
6f
|
82 | 14d |
| 6 | (E)-methyl but-2-enoate 3g |
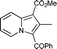 6g
6g
|
90 | — |
| 7 | diethyl fumarate 3h |
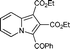 6h
6h
|
92 | 14a |
| 8 | dimethyl maleate 3i |
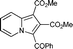 6i
6i
|
80 | 14a |
| 9 | dibutyl maleate 3j |
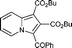 6j
6j
|
80 | — |
| 10 | 1-phenyl-1H-pyrrole-2,5-dione 3k | Complex mixture | C. M.b | — |
| 11 | 2H-chromen-2-one 3l |
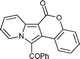 6l
6l
|
43 (59)c | 13c |
| 12 | naphthalene-1,4-dione 3m | Complex mixture | C. M.b | |
| 13 | (E)-1-phenyl-3-p-tolylprop-2-en-1-one 3n |
 6n
6n
|
66 | — |
| 14 | (E)-3-(4-fluorophenyl)-1-phenylprop-2-en-1-one 3o |
 6o
6o
|
50 | — |
| 15 | ethyl cinnamate 3p |
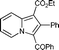 6p
6p
|
25 (32)c | 14e |
| 16 | (1E,4E)-1,5-diphenylpenta-1,4-dien-3-one 3q | 6q′ (see Scheme 3) | 35 | — |
| 17 | (E)-but-2-enal 3r |
 6r
6r
|
54 | — |
| 18 | cinnamaldehyde 3s |
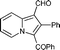 6s
6s
|
39 | — |
| 19 | (E)-(2-nitrovinyl)benzene 3t |
 6t;
6t;  6t′ 6t′ |
45; 28d | —; 14f |
| 20 | (E)-(2-nitroprop-1-enyl)benzene 3u |
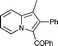 6u′
6u′
|
72 | — |
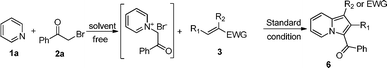
At last, to demonstrate the scope of the reaction, we replaced 2-bromo-1-phenylethanone by several similar reagents. The results were presented in Table 4. All of them gave indolizines at moderate to high yield.
| Entry | R, X | Product | Yield (%)a | Ref. |
|---|---|---|---|---|
| a Isolated yield. | ||||
| 1 | 4-nitrobenzoyl, Br 2b |
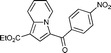 7b
7b
|
80 | — |
| 2 | 4-methoxybenzoyl, Br 2c |
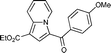 7c
7c
|
88 | 14g |
| 3 | 2-bromo-1-(4-bromophenyl)ethanone 2d |
 7d
7d
|
96 | — |
| 4 | CO2Et, Cl 2e |
 7e
7e
|
75 | 14h |
| 5 | CO2Et, Br 2f | 7e | 38 | 14h |
| 6 | CN, Br 2g |
 7g
7g
|
55 | — |
| 7 | 1-(bromomethyl)-4-nitrobenzene 2h |
 7h
7h
|
58 | 14i |
| 8 | 1,3-dichloropropan-2-one 2i | 7i′ (see Scheme 3) | 33 | — |
Moreover, (E)-(2-nitroprop-1-enyl)benzene 3q gave (1,1′-carbonylbis(2-phenylindolizine-3,1-diyl))bis(phenylmethanone) 6q′ in a yield of 35% (Table 3, entry 16), and 1,3-dichloropropan-2-one 2i also gave diethyl 3,3′-carbonyldiindolizine-1-carboxylate 7i′ in a yield of 33% (Table 4, entry 8).
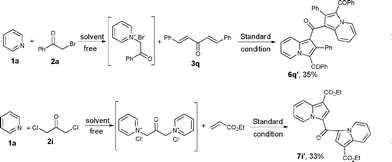 | ||
| Scheme 3 The formation of 6q′ and 7i′. | ||
Furthermore, the reaction scale was easily enlarged to gram scale and the yield did not decrease (Scheme 4).
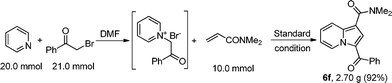 | ||
| Scheme 4 The synthesis of indolizine 6f on the gram scale. | ||
Conclusions
In conclusion, we have demonstrated that copper acetate monohydrate is a cheap and efficient reagent for synthesizing multi-substituted indolizines from simple starting materials, such as pyridines, α-halide ketones and electron-deficient alkenes. The reaction runs under mild conditions and is easy to handle. This reaction can be applied on the gram scale and without reducing the yield. At last, further study of a catalytic version of this transformation is now under thorough investigation.Experimental
General
Unless otherwise noted, all commercial reagents and solvents were obtained from commercial providers and used without further purification. 1H NMR and 13C NMR spectra were recorded on Bruker 400 MHz spectrometers. Chemical shifts were reported relative to internal tetramethylsilane (δ 0.00 ppm) or CDCl3 (δ 7.26 ppm) for 1H NMR and CDCl3 (δ 77.0 ppm) for 13C NMR. Flash column chromatography was performed on 300–400 mesh silica gels. Analytical thin layer chromatography was performed with pre-coated glass baked plates (250 μ) and visualized by fluorescence. HRMS was recorded on a Bruker micrOTOF-Q spectrometer. IR spectra were recorded on a Nicolet Avatar 360 spectrometer.General procedure for the preparation of indolizines 4
Pyridine (1a) 0.40 mmol and 2-bromo-1-phenylethanone (2a) 0.42 mmol were mixed in a test tube and the mixture was heated to 60 °C for 4 h. Then copper acetate monohydrate 0.60 mmol, NaOAc 1.20 mmol, DMF 2.0 mL and ethyl acrylate (3a) 0.20 mmol were added to the mixture. The mixture was then heated at 80 °C for another 4 h (the reaction course was monitored by TLC). Then the mixture was cooled to r.t., poured into water and extracted with CHCl3 (10 mL × 3). The extractions were combined and washed with saturated brine, dried with Na2SO4, and filtered. The solvent was removed by reduce pressure. The residue was purified by flash chromatography on silica gel using petroleum ether/ethyl acetate (the ratio depends on product's polarity) as eluate.Preparation of indolizine 6f at gram scale
Pyridine (1a) 20.0 mmol and 2-bromo-1-phenylethanone (2a) 21.0 mmol and 10.0 mL DMF were mixed in a round bottom flask at r.t.. The mixture was stirred for 2 h (the temperature of mixture rose to 80 °C automatically and then cooled down to r.t.), and white solid was precipitated. Then copper acetate monohydrate 30.0 mmol, NaOAc 60.0 mmol, DMF 70.0 mL and N,N-dimethylacrylamide (3f) 10.0 mmol were added to the mixture. The mixture was then heated at 80 °C for another 4 h (the reaction course was monitored by TLC). Then the mixture was cooled to r.t., poured into 160 mL water and filtered. The mother liquid was extracted with CHCl3 (30 mL × 3) and the filter cake was washed with CHCl3 (10 mL × 3). The organic layers were combined and washed with saturated brine, dried with Na2SO4, and filtered. Then the solvent was removed by reduce pressure. The residue was purified by flash chromatography on silica gel using petroleum ether/ethyl acetate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v
1, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) as eluate to give the corresponding indolizine 6f as a yellow solid.
v) as eluate to give the corresponding indolizine 6f as a yellow solid.
Ethyl 3-benzoyl-7-methylindolizine-1-carboxylate (5b)
Yellow solid; mp 154–155 °C; 1H NMR (CDCl3, 400 MHz): 9.87 (d, J = 7.1 Hz, 1H), 8.20 (s, 1H), 7.82 (dd, J = 6.9, 1.4 Hz, 2H), 7.78 (s, 1H), 7.59 (t, J = 7.2 Hz, 1H), 7.52 (t, J = 7.3 Hz, 2H), 6.94 (dd, J = 7.1, 1.6 Hz, 1H), 4.38 (q, J = 7.1 Hz, 2H), 2.51 (s, 3H), 1.41 (t, J = 7.1 Hz, 3H); 13C NMR (CDCl3, 100 MHz): 185.2, 164.2, 140.5, 140.1, 139.4, 131.3, 129.3, 128.9, 128.7, 128.3, 122.2, 118.3, 117.7, 105.2, 60.0, 21.6, 14.6; IR (NaCl): 2978, 2919, 1697, 1643, 1611, 1575, 1524, 1481, 1464, 1428, 1344; HRMS Calculated for [C19H17NO3+Na]+: 330.1101, Found: 330.1089.Ethyl 3-benzoyl-5-methylindolizine-1-carboxylate (5c)
Yellow oil; 1H NMR (CDCl3, 400 MHz): 8.39 (d, J = 8.8 Hz, 1H), 8.06 (d, J = 7.2 Hz, 2H), 7.76 (s, 1H), 7.65 (t, J = 7.4 Hz, 1H), 7.55 (t, J = 7.5 Hz, 2H), 7.44 (dd, J = 8.6, 7.1 Hz, 1H), 6.94 (d, J = 6.9 Hz, 1H), 4.37 (q, J = 7.1 Hz, 2H), 2.62 (s, 3H), 1.39 (t, J = 7.1 Hz, 3H); 13C NMR (CDCl3, 100 MHz): 182.9, 164.1, 141.9, 139.8 138.6, 132.6, 130.3, 129.6, 128.4, 127.4, 125.4, 117.2, 116.7, 105.5, 60.0, 23.2, 14.6; IR (NaCl): 3059, 2977, 2926, 1698, 1632, 1596, 1577, 1524, 1480, 1422, 1335; HRMS Calculated for [C19H17NO3+Na]+: 330.1101, Found: 330.1105.Ethyl 3-benzoyl-6-cyanoindolizine-1-carboxylate (5d)
Yellow solid; mp 163–164; 1H NMR (CDCl3, 400 MHz): 10.39 (s, 1H), 8.49 (d, J = 9.2 Hz, 1H), 7.94 (s, 1H), 7.85 (d, J = 7.2 Hz, 2H), 7.65 (t, J = 7.4 Hz, 1H), 7.57 (t, J = 7.5 Hz, 2H), 7.50 (dd, J = 9.3, 1.2 Hz, 1H), 4.42 (q, J = 7.1 Hz, 2H), 1.43 (t, J = 7.1 Hz, 3H); 13C NMR (CDCl3, 100 MHz): 185.9, 163.3, 138.9, 138.7, 134.8, 132.3, 129.8, 128.0, 128.6, 126.5, 123.4, 120.5, 116.3, 108.3, 101.3, 60.7, 14.5; IR (NaCl): 3102, 2963, 2918, 2849, 2235, 1704, 1630, 1598, 1576, 1541, 1481, 1431, 1366, 1326; HRMS Calculated for [C19H14N2O3+Na]+: 341.0897, Found: 341.0879.Ethyl 3-benzoyl-8-cyanoindolizine-1-carboxylate (5d′)
Yellow solid; mp 147–148; 1H NMR (CDCl3, 400 MHz): 10.19 (d, J = 6.7 Hz, 1H), 7.93 (d, J = 7.6 Hz, 1H), 7.90 (s, 1H), 7.82 (d, J = 7.2 Hz, 2H), 7.62 (d, J = 7.4 Hz, 1H), 7.55 (t, J = 7.2 Hz, 2H), 7.15 (t, J = 7.2 Hz,1H), 4.47 (q, J = 7.1 Hz, 2H), 1.42 (t, J = 7.1 Hz, 3H); 13C NMR (CDCl3, 100 MHz):185.8, 162.7, 139.0, 136.8, 134.4, 133.0, 132.2, 130.4, 129.1, 128.6, 123.2, 116.3, 113.7, 108.6, 104.7, 60.8, 14.4; IR (NaCl): 3114, 2979, 2925, 2233, 1715, 1627, 1599, 1527, 1476, 1436, 1355; HRMS Calculated for [C19H14N2O3+Na]+: 341.0897, Found: 341.0919.Ethyl 3-benzoyl-7-(dimethylamino)indolizine-1-carboxylate (5e)
Yellow solid; mp 162–164 °C; 1H NMR (CDCl3, 400 MHz): 9.77 (d, J = 7.8 Hz, 1H), 7.80 (d, J = 6.7 Hz, 2H), 7.67 (s, 1H), 7.56–7.46 (m, 3H), 7.37 (d, J = 2.7 Hz, 1H), 6.63 (dd, J = 7.8, 2.8 Hz, 1H), 4.33 (q, J = 7.1 Hz, 2H), 3.14 (s, 6H), 1.38 (t, J = 7.1 Hz, 3H); 13C NMR (CDCl3, 100 MHz): 183.8, 164.6, 149.5, 143.3, 140.6, 130.8, 130.7, 130.3, 128.8, 128.2, 120.8, 104.2, 102.8, 95.6, 59.6, 39.9, 14.6; IR (NaCl): 3058, 2978, 2930, 1690, 1646, 1597, 1532, 1490, 1440, 1340; HRMS Calculated for [C20H20N2O3+H]+: 337.1547, Found: 337.1533.1-Ethyl 7-methyl 3-benzoylindolizine-1,7-dicarboxylate (5f)
Yellow solid; mp 149–151 °C; 1H NMR (CDCl3, 400 MHz): 9.88 (d, J = 7.3 Hz, 1H), 9.00 (s, 1H), 7.87–7.79 (m, 3H), 7.63–7.55 (m, 2H), 7.52 (t, J = 7.4 Hz, 2H), 4.40 (q, J = 7.1 Hz, 2H), 3.98 (s, 3H), 1.42 (t, J = 7.1 Hz, 3H); 13C NMR (CDCl3, 100 MHz): 185.8, 165.1, 163.5, 139.4, 138.2, 131.9, 129.0, 128.9, 128.49, 128.46, 128.0, 123.6, 121.7, 114.1, 109.1, 60.5, 52.7, 14.5; IR (NaCl): 2983, 1724, 1627, 1573, 1527, 1473, 1432, 1347; HRMS Calculated for [C20H17NO5+Na]+: 374.0999, Found: 374.0988.Ethyl 1-benzoyl-5-methylpyrrolo[1,2-a]quinoline-3-carboxylate (5h)
Yellow solid; mp 164–166 °C; 1H NMR (CDCl3, 400 MHz): 8.21 (s, 1H), 8.14–8.09 (m, 3H), 7.95 (dd, J = 8.0, 0.9 Hz, 1H), 7.67 (t, J = 7.4 Hz, 1H), 7.62 (s, 1H), 7.61–7.50 (m, 4H), 4.38 (q, J = 7.1 Hz, 2H), 2.71 (s, 3H), 1.41 (t, J = 7.1 Hz, 3H); 13C NMR (CDCl3, 100 MHz): 184.7, 164.2, 140.5, 138.6, 136.5, 133.0, 132.7, 130.1, 129.9, 128.5, 128.4, 127.9, 125.4, 125.3, 125.2, 120.6, 117.3, 106.7, 60.1, 19.6, 14.6; IR (NaCl): 3060, 2978, 2926, 2855, 1699, 1633, 1597, 1556, 1538, 1499, 1465, 1447, 1416; HRMS Calculated for [C23H19NO3+Na]+: 380.1257, Found: 380.1256.Ethyl 3-benzoyl-7-chloroindolizine-1-carboxylate (5j)
Yellow solid; mp 134–136 °C; 1H NMR (CDCl3, 400 MHz): 9.91 (d, J = 7.5 Hz, 1H), 8.41 (d, J = 2.0 Hz, 1H), 7.83 (d, J = 7.0 Hz, 2H), 7.82 (s, 1H), 7.62 (t, J = 7.3 Hz, 1H), 7.54 (t, J = 7.4 Hz, 2H), 7.07 (dd, J = 7.4, 2.2 Hz, 1H), 4.40 (q, J = 7.1 Hz, 2H), 1.42 (t, J = 7.1 Hz, 3H); 13C NMR (CDCl3, 100 MHz): 185.6, 163.7, 139.9, 139.5, 134.5, 131.7, 129.7, 129.3, 129.0, 128.5, 122.7, 118.6, 116.5, 106.1, 60.3, 14.5; IR (NaCl): 3123, 2980, 1700, 1619, 1523, 1473, 1442, 1343; HRMS Calculated for [C18H14ClNO3+H]+: 328.0735, Found: 328.0737.tert-Butyl 3-benzoylindolizine-1-carboxylate (6c)
Yellow solid; mp 125–126 °C; 1H NMR (CDCl3, 400 MHz): 9.99 (d, J = 7.0 Hz, 1H), 8.36 (d, J = 8.9 Hz, 1H), 7.83 (d, J = 7.1 Hz, 2H), 7.80 (s, 1H), 7.58 (t, J = 7.2 Hz, 1H), 7.52 (t, J = 7.4 Hz, 2H), 7.44 (dd, J = 7.7, 7.0 Hz, 1H), 7.08 (t, J = 6.8 Hz, 1H), 1.63 (s, 9H); 13C NMR (CDCl3, 100 MHz): 185.5, 163.5, 140.0, 139.6, 131.4, 129.24, 129.18, 129.0, 128.4, 127.4, 122.3, 119.6, 115.1, 108.0, 80.6, 28.5; IR (NaCl): 2976, 2931, 1696, 1616, 1521, 1479, 1449, 1367, 1343; HRMS Calculated for [C20H19NO3+Na]+: 344.1257, Found: 344.1264.3-Benzoyl-N,N-dimethylindolizine-1-carboxamide (6f)
Yellow solid; mp 91–93 °C; 1H NMR (CDCl3, 400 MHz): 9.94 (d, J = 7.0 Hz, 1H), 8.06 (d, J = 8.6 Hz, 1H), 7.79 (d, J = 7.2 Hz, 2H), 7.55 (t, J = 7.2 Hz, 1H), 7.48 (t, J = 7.4 Hz, 2H), 7.44 (s, 1H), 7.35 (dd, J = 8.1, 7.6 Hz, 1H), 7.04 (t, J = 6.9 Hz, 1H), 3.14 (s, 6H); 13C NMR (CDCl3, 100 MHz): 185.1, 166.5, 140.2, 139.3, 131.3, 128.9, 128.7, 128.3, 126.44, 126.37, 121.5, 119.4, 115.0, 109.7; IR (NaCl): 3057, 2928, 1713, 1609, 1573, 1526, 1474, 1415, 1346; HRMS Calculated for [C18H16N2O2+Na]+: 315.1104, Found: 315.1106.Dibutyl 3-benzoylindolizine-1,2-dicarboxylate (6j)
Yellow oil. 1H NMR (CDCl3, 400 MHz): 9.63 (d, J = 7.1 Hz, 1H), 8.42 (d, J = 9.0 Hz, 1H), 7.71 (d, J = 7.1 Hz, 2H), 7.55 (t, J = 7.4 Hz, 1H), 7.42–7.48 (m, 3H), 7.10 (dd, J = 6.9, 1.0 Hz, 1H), 4.29 (t, J = 6.6 Hz, 2H), 3.55 (t, J = 6.8 Hz, 2H), 1.70 (qu, J = 7.1 Hz, 2H), 1.49–1.35 (m, 4H), 1.31–1.20 (m, 2H), 0.94 (t, J = 7.4 Hz, 3H), 0.87 (t, J = 7.3 Hz, 3H); 13C NMR (CDCl3, 100 MHz): 186.8, 165.0, 163.1, 139.6, 138.5, 131.9, 131.7, 128.8, 128.5, 128.1, 127.9, 120.7, 120.0, 115.9, 104.3, 65.7, 64.4, 30.8, 30.1, 19.2, 19.1, 13.7; IR (NaCl): 3059, 2959, 2872, 1738, 1702, 1624, 1577, 1506, 1447, 1376, 1344; HRMS Calculated for [C25H27NO5+Na]+: 444.1781, Found: 444.1780.(2-p-Tolylindolizine-1,3-diyl)bis(phenylmethanone) (6n)
Yellow solid; 181–182 °C; 1H NMR (CDCl3, 400 MHz): 9.68 (d, J = 7.1 Hz, 1H), 8.15 (d, J = 8.9 Hz, 1H), 7.45 (d, J = 7.3 Hz, 2H), 7.41–7.32 (m, 3H), 7.20 (t, J = 7.4 Hz, 1H), 7.13 (t, J = 7.4 Hz, 1H), 7.08–7.02 (m, 3H), 6.97 (t, J = 7.7 Hz, 2H), 6.73 (d, J = 7.9 Hz, 2H), 6.49 (t, J = 7.8 Hz, 2H), 2.00 (s, 3H); 13C NMR (CDCl3, 100 MHz): 192.9, 188.5, 139.4, 139.2, 139.0, 136.5, 131.5, 131.3, 130.9, 130.3, 129.5, 129.4, 129.4, 127.8, 127.7, 127.5, 127.4, 127.0, 121.1, 119.1, 115.1, 114.1, 20.9; IR (NaCl): 3060, 2923, 1633, 1612, 1597, 1576, 1499, 1386, 1343; HRMS Calculated for [C29H21NO2+Na]+: 438.1465, Found: 438.1469.(2-(4-Fluorophenyl)indolizine-1,3-diyl)bis(phenylmethanone) (6o)
Yellow solid. 1H NMR (CDCl3, 400 MHz): 9.69 (d, J = 7.1 Hz, 1H), 8.18 (d, J = 8.9 Hz, 1H), 7.47–7.38 (m, 3H), 7.35 (d, J = 7.3 Hz, 2H), 7.24 (t, J = 7.4 Hz, 1H), 7.18 (t, J = 7.4 Hz, 1H), 7.11–7.05 (m, 3H), 7.01 (t, J = 7.6 Hz, 2H), 6.82 (dd, J = 8.4, 5.6 Hz, 2H), 6.41 (t, J = 8.6 Hz, 2H); 13C NMR (CDCl3, 100 MHz): 192.6, 188.2, 162.9, 160.5, 139.2, 139.1, 139.1, 137.9, 133.2, 133.1, 131.6, 131.3, 129.4, 129.3, 127.8, 127.7, 127.6, 127.4, 121.3, 119.2, 115.4, 114.2, 114.2, 114.0; IR (NaCl): 3062, 1606, 1576, 1525, 1496, 1426, 1386; HRMS Calculated for [C28H18FNO2+Na]+: 442.1214, Found: 442.1162.(1,1′-Carbonylbis(2-phenylindolizine-3,1-diyl))bis(phenylmethanone) (6q′)
Yellow solid. 1H NMR (CDCl3, 400 MHz): 9.41 (d, J = 7.0 Hz, 2H), 7.86 (d, J = 8.8 Hz, 2H), 7.30–7.22 (m, 6H), 7.09 (t, J = 7.3 Hz, 2H), 6.96–6.88 (m, 6H), 6.85–6.78 (m, 6H), 6.71 (t, J = 7.4 Hz, 4H); 13C NMR (CDCl3, 100 MHz): 187.9, 187.0, 139.2, 138.5, 138.1, 132. 7, 131.2, 131.0, 129.3, 127.5, 127.3, 126.8, 126.6, 126.3, 121.0, 118.7, 116.9, 114.7; IR (NaCl): 3058, 2923, 2851, 1659, 1603, 1575, 1492, 1465, 1449, 1429, 1387, 1340, 1316; HRMS Calculated for [C43H28N2O3+H]+: 621.2173, Found: 621.2185.3-Benzoyl-2-methylindolizine-1-carbaldehyde (6r)
Yellow solid; mp 171–173 °C; 1H NMR (CDCl3, 400 MHz): 10.17 (s, 1H), 9.55 (d, J = 7.0 Hz, 1H), 8.47 (d, J = 8.8 Hz, 1H), 7.70 (d, J = 7.2 Hz, 2H), 7.60 (t, J = 7.3 Hz, 1H), 7.56–7.46 (m, 3H), 7.08 (t, J = 6.7 Hz, 1H), 2.21 (s, 3H); 13C NMR (CDCl3, 100 MHz): 187.7, 184.2, 140.8, 139.3, 137.8, 132.1, 129.1, 128.7, 128.7, 128.6, 122.8, 118.6, 115.8, 113.6, 12.4; IR (NaCl): 3058, 2724, 2854, 1714, 1655, 1609, 1503, 1441, 1391, 1335; HRMS Calculated for [C17H13NO2+Na]+: 286.0838, Found: 286.0834.3-Benzoyl-2-phenylindolizine-1-carbaldehyde (6s)
Yellow solid; mp 183–185 °C; 1H NMR (CDCl3, 400 MHz): 9.79 (s, 1H), 9.76 (d, J = 7.0 Hz, 1H), 8.69 (d, J = 8.8 Hz, 1H), 7.59 (t, J = 7.8 Hz, 1H), 7.41 (d, J = 7.3 Hz, 2H), 7.23–7.17 (m, 2H), 7.17–7.08 (m, 5H), 7.04 (t, J = 7.7 Hz, 2H); 13C NMR (CDCl3, 100 MHz): 188.0, 186.7, 142.6, 139.0, 138.3, 131.5, 131.5, 131.3, 129.4, 129.2, 128.6, 127.9, 127.7, 127.6, 121.5, 119.9, 116.5, 113.2; IR (NaCl): 3058, 2921, 2793, 1657, 1612, 1575, 1497, 1467, 1435, 1390, 1334; HRMS Calculated for [C22H15NO2+Na]+: 348.0995, Found: 348.0983.(1-Nitro-2-phenylindolizin-3-yl)(phenyl)methanone (6t)
Yellow solid; mp 139–140 °C; 1H NMR (CDCl3, 400 MHz): 9.50 (d, J = 7.0 Hz, 1H), 8.69 (d, J = 9.0 Hz, 1H), 7.68 (dd, J = 8.2, 7.5 Hz, 1H), 7.42 (d, J = 7.4 Hz, 2H), 7.27–7.17 (m, 4H), 7.11–7.05 (m, 5H); 13C NMR (CDCl3, 100 MHz): 188.4, 138.6, 134.6, 133.9, 132.0, 131.1, 130.9, 130.3, 129.3, 128.2, 128.1, 127.7, 127.7, 127.5, 121.5, 119.4, 116.4; IR (NaCl): 3152, 3056, 2963, 2922, 1624, 1598, 1576, 1500, 1469, 1409, 1370, 1327; HRMS Calculated for [C21H14N2O3+Na]+: 365.0897, Found: 365.0905.(1-Methyl-2-phenylindolizin-3-yl)(phenyl)methanone (6u′)
Yellow solid. 1H NMR (CDCl3, 400 MHz): 9.90 (d, J = 7.1 Hz, 1H), 7.55 (d, J = 8.8 Hz, 1H), 7.36 (d, J = 7.4 Hz, 2H), 7.20 (dd, J = 8.0, 7.4 Hz, 1H), 7.11 (t, J = 7.4 Hz, 1H), 7.05–6.95 (m, 7H), 6.91 (t, J = 6.8 Hz, 1H), 2.26 (s, 3H); 13C NMR (CDCl3, 100 MHz): 186.3, 140.5, 137.8, 136.5, 135.0, 131.0, 130.0, 129.2, 128.3, 127.4, 127.2, 126.4, 123.5, 120.3, 116.8, 113.6, 110.7, 9.2; IR (NaCl): 3058, 2920, 2851, 1593, 1571, 1520, 1452, 1432, 1388, 1371; HRMS Calculated for [C22H17NO+Na]+: 334.1202, Found: 334.1208.Ethyl 3-(4-nitrobenzoyl)indolizine-1-carboxylate (7b)
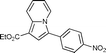
Yellow solid; mp 144–145 °C; 1H NMR (CDCl3, 400 MHz): 10.01 (d, J = 7.0 Hz, 1H), 8.46 (d, J = 8.9 Hz, 1H), 8.40 (d, J = 8.6 Hz, 2H), 7.98 (d, J = 8.6 Hz, 2H), 7.76 (s, 1H), 7.56 (dd, J = 8.1, 7.1 Hz, 1H), 7.20 (t, J = 6.9 Hz, 1H), 4.41 (q, J = 7.1 Hz, 2H), 1.43 (t, J = 7.1 Hz, 3H); 13C NMR (CDCl3, 100 MHz): 182.9, 163.7, 149.4, 145.4, 140.4, 129.7, 129.3, 129.2, 128.6, 123.7, 121.9, 119.7, 116.0, 107.3, 60.3, 14.5; IR (NaCl): 3110, 2924, 2852, 1706, 1619, 1596, 1521, 1482, 1341; HRMS Calculated for [C18H14N2O5+Na]+: 361.0795, Found: 368.0801.Ethyl 3-(4-bromobenzoyl)indolizine-1-carboxylate (7d)
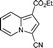
Yellow solid; mp 109–110 °C; 1H NMR (CDCl3, 400 MHz): 9.95 (d, J = 7.0 Hz, 1H), 8.42 (d, J = 8.9 Hz, 1H), 7.79 (s, 1H), 7.71 (d, J = 8.5 Hz, 2H), 7.67 (d, J = 8.5 Hz, 2H), 7.48 (dd, J = 8.0, 7.5 Hz, 1H), 7.12 (t, J = 7.0 Hz, 1H), 4.40 (q, J = 7.1 Hz, 2H), 1.42 (t, J = 7.1 Hz, 3H); 13C NMR (CDCl3, 100 MHz): 184.1, 163.9, 140.0, 138.7, 131.7, 130.5, 129.2, 128.8, 127.9, 126.2, 122.2, 119.6, 115.5, 106.6, 60.2, 14.6; IR (NaCl): 3123, 2979, 1700, 1615, 1585, 1521, 1480, 1430, 1342; HRMS Calculated for [C18H14BrNO3+H]+: 372.0230, Found: 372.0239.Ethyl 3-cyanoindolizine-1-carboxylate (7g)
White solid; mp 102–103 °C; 1H NMR (CDCl3, 400 MHz): 8.38–8.33 (m, 2H), 7.81 (s, 1H), 7.36 (dd, J = 8.8, 6.9 Hz, 1H), 7.06 (td, J = 7.3, 1.0 Hz, 1H), 4.40 (q, J = 7.1 Hz, 2H), 1.43 (t, J = 7.1 Hz, 3H); 13C NMR (CDCl3, 100 MHz): 163.3, 137.8, 126.0, 125.7, 125.2, 120.5, 115.0, 112.6, 106.1, 96.7, 60.3, 14.5; IR (NaCl): 3119, 2987, 2210, 1691, 1515, 1486, 1445, 1390; HRMS Calculated for [C12H10N2O2+Na]+: 237.0634, Found: 237.0631.3,3′-Carbonyldiindolizine-1-carboxylate (7i′)
Yellow solid; mp 223–225 °C; 1H NMR (CDCl3, 400 MHz): 9.67 (d, J = 7.0 Hz, 1H), 8.38 (d, J = 9.0 Hz, 1H), 8.00 (s, 1H), 7.38 (dd, J = 8.2, 7.4 Hz, 1H), 7.01 (t, J = 6.8 Hz, 1H), 4.43 (q, J = 7.1 Hz, 2H), 1.45 (t, J = 7.1 Hz, 3H); 13C NMR (CDCl3, 100 MHz): 174.5, 164.3, 139.6, 128.6, 126.7, 126.1, 122.9, 119.7, 114.6, 106.0, 60.1, 14.6; IR (NaCl): 2981, 2961, 2923, 2852, 1699, 1626, 1520, 1480, 1441, 1369; HRMS Calculated for [C23H20N2O5+Na]+: 427.1264, Found: 427.1263.Acknowledgements
We are grateful to Jiangsu Province NSF (No.: BK2011408), Jiangsu Province Department of Education (No.: 10KJB150002), China Postdoctoral Science Foundation funded project (No.: 2012M511645), JSKLCLDM (No.: JSKC11093), HYNU and NBRPC (No.: 2011CB933503) for their financial support.References
- (a) W. Flisch, in Comprehensive Heterocyclic Chemistry, ed. A. R. Katritzky and C. W. Rees, Pergamon, Oxford, vol. 4, 1984, pp. 476 Search PubMed; (b) J. Ewing, G. K. Hughes, E. Ritchie and W. C. Taylor, Nature, 1952, 169, 618 CrossRef CAS.
- (a) E. Reimann, Prog. Chem. Org. Nat. Prod., 2007, 88, 1 CrossRef CAS; (b) J. P. Michael, Nat. Prod. Rep., 2008, 25, 139 RSC; (c) J. P. Michael, Nat. Prod. Rep., 2003, 20, 458 RSC; (d) J. W. Daly, T. F. Spande and H. M. Garraffo, J. Nat. Prod., 2005, 68, 1556 CrossRef CAS; (e) M. Lebceuf, A. Cave, A. Ranaivo and H. Moskowitz, Can. J. Chem., 1989, 67, 947 CrossRef CAS; (f) S. A. Snyder, A. M. ElSohly and F. Kontes, Angew. Chem., Int. Ed., 2010, 49, 9693 CrossRef CAS.
- (a) A. K.Ghosh, G. Bilcer and G. Schiltz, Synthesis, 2001, 2203 CrossRef; (b) B. List and C. Castello, Synlett, 2001, 1687 CrossRef CAS; (c) P. Sonnet, P. Dallemagne, J. Guillon, C. Enguehard, S. Stiebing, J. Tanguy, R. Bureau, S. Rault, P. Auvray, S. Moslemi, P. Sourdaine and G. E. Seralini, Bioorg. Med. Chem., 2000, 8, 945 CrossRef CAS; (d) O. B. Ostby, B. Dalhus, L.-L. Gundersen, F. Rise, A. Bast and G. R. M. M. Haenen, Eur. J. Org. Chem., 2000, 3763 CrossRef CAS; (e) B. E. Maryanoff, J. L. Vaught, R. P. Shank, D. F. McComsey, M. J. Costanzo and S. O. Nortey, J. Med. Chem., 1990, 33, 2793 CrossRef CAS; (f) V. H. Lillelund, H. H. Jensen, X. Liang and M. Bols, Chem. Rev., 2002, 102, 515 CrossRef CAS; (g) J. Gubin, H. D. Vogelaer, H. Inion, C. Houben, J. Lucchetti, J. Mahaux, G. Rosseels, M. Peiren, M. Clinet, P. Polster and P. Chatelain, J. Med. Chem., 1993, 36, 1425 CrossRef CAS; (h) J. Bermudez, C. S. Fake, G. F. Joiner, K. A. Joiner, F. D. King, W. D. Miner and G. J. Sanger, J. Med. Chem., 1990, 33, 1924 CrossRef CAS.
- (a) A. Vlahovici, M. Andrei and I. Druta, J. Lumin., 2002, 96, 279 CrossRef CAS; (b) A. Vlahovici, I. Druta, M. Andrei, M. Cotlet and R. Dinica, J. Lumin., 1999, 82, 155 CrossRef CAS; (c) T. Mitsumori, M. Bendikov, O. Dautel, F. Wudl, T. Shioya, H. Sato and Y. Sato, J. Am. Chem. Soc., 2004, 126, 16793 CrossRef CAS; (d) F. D. Saeva and H. R. Luss, J. Org. Chem., 1988, 53, 1804 CrossRef CAS; (e) G. L. Fletetcher, S. L. Bender and D. H. Wadsworth. US Patent 4577024, 1986; Chem. Abstr., 1984, 100, 122772 Search PubMed; (f) H. Sonnenschein, G. Henrich, U. Resch-Genger and B. Schulz, Dyes Pigm., 2000, 46, 23 CrossRef CAS; (g) A. V. Retaru, L. D. Druta, T. Deser and T. J. Mueller, Helv. Chim. Acta, 2005, 88, 1798 CrossRef CAS; (h) F. Delattre, P. Woisel, G. Surpateanu, F. Cazier and P. Blach, Tetrahedron, 2005, 61, 3939 CrossRef CAS.
- (a) F. Amblard, J. H. Cho and R. F. Schinazi, Chem. Rev., 2009, 109, 4207 CrossRef CAS; (b) V. Nair and T. D. Suja, Tetrahedron, 2007, 63, 12247 CrossRef CAS; (c) N. A. Bokach and V. Y. Kukushkin, Russ. Chem. Bull., 2006, 55, 1869 CrossRef CAS; (d) C. Najera and J. M. Sansano, Angew. Chem., Int. Ed., 2005, 44, 6272 CrossRef CAS; (e) K. Rueck-Braun, T. H. E. Freysoldt and F. Wierschem, Chem. Soc. Rev., 2005, 34, 507 RSC; (f) K. Harju and J. Yli-Kauhaluoma, Rec. Dev. Org. Chem., 2004, 8, 111 CAS.
- (a) K. Matsumoto, Synthesis, 1976, 209 Search PubMed; (b) Y. Shang, M. Zhang, S. Yu, K. Ju, C. Wang and X. He, Tetrahedron Lett., 2009, 50, 6981 CrossRef CAS; (c) M. K. Bayazit and K. S. Coleman, J. Am. Chem. Soc., 2009, 131, 10670 CrossRef CAS; (d) B.-X. Wang, Z.-X. Xu and J. Wu, Youji Huaxue, 2006, 26, 1587 CAS; (e) B.-X. Wang, W.-W. Liu, T. He and H.-W. Hu, Chin. J. Chem., 2006, 24, 279 CrossRef CAS; (f) A. V. Retaru, I. D. Druta, T. Oeser and T. Mueller, Helv. Chim. Acta, 2005, 88, 1798 CrossRef CAS; (g) E. M. Mmutlane, J. M. Harris and A. Padwa, J. Org. Chem., 2005, 70, 8055 CrossRef CAS; (h) K. Wu and Q.-Y. Chen, Synthesis, 2003, 35 CAS; (i) R. M. Dinica, I. I. Druta and C. Pettinari, Synlett, 2000, 1013 CAS.
- V. Boekelheide and k. Fahrenholtz, J. Am. Chem. Soc., 1961, 83, 458 CrossRef CAS.
- (a) K. M. Dawood, E. A. Ragab and N. A. Khedr, J. Chin. Chem. Soc. (Taipei, Taiwan), 2009, 56, 1180 CAS; (b) E. S. Darwish, Molecules, 2008, 13, 1066 CrossRef CAS; (c) N. A. Kheder, E. S. Darwish and K. M. Dawood, Heterocycles, 2009, 78, 177 CrossRef CAS.
- (a) Y.-M. Shen, B.-X. Wang, Y.-Y. Feng, Z.-Y. Shen, J. Shen, C. Li and H.-W. Hu, Gaodeng Xuexiao Huaxue Xuebao, 2006, 27, 651 CAS; (b) N. S. Prostakov, A. V. Varlamov, N. Islam, N. Saksena and D. Nende, Khim. Geterotsikl. Soedin., 1987, 707 CAS; (c) A. Tanaka and T. Usui, Chem. Pharm. Bull., 1979, 27, 3078 CrossRef CAS; (d) B.-X. Wang and H.-W. Hu, Gaodeng Xuexiao Huaxue Xuebao, 2004, 25, 273 CAS; (e) Y. Tominaga, S. Hidaki, Y. Matsuda, G. Kobayashi and K. Sakemi, Yakugaku Zasshi, 1979, 99, 540 CAS.
- http://www.alfa.com/en/gp100w.pgm?dsstk=A16203; Data was present on 2012-3-20, which may be changed by website holder..
- Data from http://china.chemnet.com on 2012-3-20, which may be changed by website holder..
- (a) C. Han, M. Yu, W. Sun and X. Yao, Synlett, 2011, 2363 CAS; (b) X. Jia, K. Yin, C. Li, J. Li and H. Bian, Green Chem., 2011, 13, 2175 RSC; (c) C. P. Frazier, J. R. Engelking and J. R. Alaniz, J. Am. Chem. Soc., 2011, 133, 10430 CrossRef CAS; (d) R. D. Patil and S. Adimurthy, Adv. Synth. Catal., 2011, 353, 1695 CrossRef CAS; (e) C. Zhang and N. Jiao, Angew. Chem., Int. Ed., 2010, 49, 6174 CrossRef CAS; (f) D. Yang, H. Yang and H. Fu, Chem. Commun., 2011, 47, 2348 RSC; (g) L. Chu and F. Qing, J. Am. Chem. Soc., 2010, 132, 7262 CrossRef CAS.
- (a) H. Hu, K. Shi, R. Hou, Z. Zhang, Y. Zhu and J. Zhou, Synthesis, 2010, 4007 CrossRef CAS; (b) Y. Liu, H. Hu, Y. Zhang, H. Hu and J. Xu, Org. Biomol. Chem., 2010, 8, 4921 RSC; (c) Y. Liu, H. Hu, Q. Liu, H. Hu and J. Xu, Tetrahedron, 2007, 63, 2024 CrossRef CAS; (d) H. Hu, Y. Liu, H. Zhong, Y. Zhu, C. Wang and M. Ji, Chem.–Asian J., 2012, 7, 884 CrossRef CAS.
- (a) Y. Yang, L. Chen, Z. Zhang and Y. Zhang, Org. Lett., 2011, 13, 342 Search PubMed; (b) E. Georgescu, M. R. Caira, F. Georgescu, B. Draghici, M. M. Popa and F. Dumitrascu, Synlett, 2009, 1795 CAS; (c) A. Hazra, S. Mondal, A. Maity, S. Naskar, P. Saha, R. Paira, K. B. Sahu, P. Paira, S. Ghosh, C. Sinha, A. Samanta, S. Banerjee and N. B. Mondal, Eur. J. Med. Chem., 2011, 46, 2132 CrossRef CAS; (d) S. Som and A. K. Das, Orient. J. Chem., 2006, 22, 415 CAS; (e) R. A. Nugent and M. Murphy, J. Org. Chem., 1987, 52, 2206 CrossRef CAS; (f) Y. Shang, M. Zhang, S. Yu, K. Ju, C. Wang and X. He, Tetrahedron Lett., 2009, 50, 6981 CrossRef CAS; (g) I. Kim, S. G. Kim, J. Y. Kim and G. H. Lee, Tetrahedron Lett., 2007, 48, 8976 CrossRef CAS; (h) S. Recnik, J. Svete and B. Stanovnik, Eur. J. Org. Chem., 2001, 3705 CrossRef CAS; (i) C. Zhu, X. Wei, J. Hu, D. Wang and H. Hu, Chem. Res. Chin. Univ., 1994, 10, 93 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra21213g |
| This journal is © The Royal Society of Chemistry 2012 |