Accurate determination of the thiol-to-metal ratio in metalloproteins by on-line combination of UV-vis spectrophotometry with electrochemistry
Tianhan
Kai†
,
Ning
Xia†
,
Lin
Liu
and
Jianxiu
Wang
*
College of Chemistry and Chemical Engineering, Central South University, Changsha, Hunan 410083, P. R. China. E-mail: jxiuwang@csu.edu.cn
First published on 17th August 2012
Abstract
Determination of the thiol-to-metal ratio in proteins is of great biological relevance. Common methods for the characterization of thiol-containing metalloproteins are applicable to either sulfhydryl groups or metal ions, but not suitable for the simultaneous determination of both. In this study, UV-vis spectrophotometry has been combined in tandem with voltammetry for accurate determination of the thiol-to-metal ratio in metalloproteins. A protein sample and the electrophilic disulphide DTDP (4, 4′-dithiodipyridine) were introduced to the UV-vis detector for spectrophotometric thiol assay and then delivered into the electrochemical flow cell for metal ion determination. Zn7-metallothionein (Zn7MT) was used as a model system due to the specific thiol-to-Zn ratio. The feasibility of the method for analysis of formamidopyrimidine-DNA glycosylase (Fpg) containing a zinc-finger consensus sequence was also demonstrated. With this coupled technique, uncertainties that cannot be addressed by the individual techniques are largely circumvented. Such a hyphenated system is amenable to the accurate and convenient determination of both sulfhydryl groups and metal ions in metalloproteins and should be useful for physiological studies.
1. Introduction
Thiol-containing metalloproteins play a crucial role in a variety of biological processes, such as controlling metabolic pathways and scavenging free radicals.1–3 Cysteine residues in these proteins exhibit fascinating properties, such as nucleophilicity, redox activity, and metal-binding affinity.4 Metallothionein (MT) and zinc finger protein (ZFP) are two kinds of important metalloproteins. In MT, 7 divalent metal ions (e.g. zinc and cadmium) are bound to 12 terminal and 8 bridging cysteine ligands.5,6 While in ZFP, zinc ions that stabilize the protein domain are typically sequestered by histidine and cysteine residues.7,8Due to the unique dumbbell-like structure and the intriguing role of MT in environmental toxicology, identification or characterization of MT has been carried out extensively.9–17 However, the lack of aromatic amino acids and chromophore-containing functional groups in MT imposes difficulties in structural identification and functionality studies.18,19 As a result, cysteine residues and the sequestrated metal ions in MT have generally been examined. For example, atomic absorption spectroscopy (AAS), inductively coupled plasma–atomic emission spectroscopy (ICP–AES), ICP–mass spectrometry (ICP–MS), and electrochemistry have been employed to measure metal contents.9,10,17 For quantification of protein sulfhydryl groups in MT, spectrophotometric, electrochemical, and enzymatic assays have been conducted.11,14,16,20 Differing from MT, ZFP contains aromatic amino acids which can be characterized by spectrophotometric techniques.7,8 However, these methods are applicable to either sulfhydryl groups or metal ions, but are not suitable for the characterization of both. Thus, a coupled technique for accurate and convenient determination of both sulfhydryl groups and metal ions or the thiol-to-metal ratio in metalloproteins is much preferred and is of great significance in physiological studies. Since the stability of the metal–thiolate complexes is pH-dependent and the cysteine residues are easy to be oxidized,21–23 separate determination of the cysteine residues and metal ions in metalloproteins might bring about uncertainty due to the structural perturbation arising from metal or thiol loss. With this coupled technique, such ambiguities have been largely mitigated.
Among the various techniques for quantification of metal ions or metal-containing species, electrochemical techniques attract much attention due to its high sensitivity, simplicity, and rapidness.24,25 Different electrochemical methods, such as cyclic voltammetry, square wave voltammetry and differential pulse voltammetry, have been employed to investigate the redox properties of and redox-induced metal release from MT.9,11,26–29 Electrochemistry combined on-line with other techniques, such as anodic stripping voltammetry combined with ICP–MS or ICP–AES, has emerged as a powerful technique for enhanced trace metal analysis.30–33 Spectrophotometric thiol assay, despite its low sensitivity, is rapid and simple to implement. The reaction between thiols and the electrophilic disulphides, such as Ellman's reagent (5,5′-dithio-bis(2-nitrobenzoic acid), DTNB) or 4,4′-dithiodipyridine (DTDP) has been utilized to measure protein sulfhydryls.20,34–36 Previously we used a thin mercury film electrode to quantify zinc release from MT modulated by the glutathione redox couple and characterized the concomitant change of sulfhydryl groups with electrochemistry and UV-vis spectrophotometry.37
In the present study, voltammetry combined on-line with UV-vis spectrophotometry has been utilized for characterization of both sulfhydryl groups and metal ions in Zn7-metallothionein (Zn7MT) and Fpg protein containing a zinc-finger consensus sequence. Spectrophotometric thiol assay was conducted by examining the intensely colored product formed upon reaction of Zn7MT or Fpg protein with DTDP. Metal ions released were determined using a thin-layer electrochemical flow cell incorporating a Nafion-coated mercury film electrode (NCMFE). Such a hyphenated system is expected to implement exact quantification of the thiol-to-metal ratio in metalloproteins.
2. Experimental
2.1. Reagents and chemicals
Rabbit liver Zn7MT was obtained from Hunan Lugu Biotech Co., Ltd (Changsha, China). Nafion (5% w/v solution in a mixture of alcohol and water), Hg2+ and Zn2+ standard solutions were purchased from Aldrich Chemical Company, Inc. (Milwaukee, WI). L-Cysteine and 4, 4′-dithiodipyridine (DTDP) were acquired from Sigma (St. Louis, MO). Fpg protein was kindly donated by Dr. Xin Wen from California State University, Los Angeles. DTDP was dissolved in 1mM HCl solution and then diluted with 50 mM phosphate buffer (pH 7.0). All other solutions were prepared with the phosphate buffer.2.2. Instruments
Thiol determination was performed using a UV-vis spectrophotometer (Shimadzu Co., Japan). The UV-vis flow cell was purchased from Starna Cells Inc. (Atascadero, CA). The phosphate buffer was used as the carrier solution which was driven by a Genie Plus syringe pump (Kent Scientific, Torrington, CT). Voltammetric determination of Zn2+ was conducted with a CHI 660B electrochemical workstation (Austin, TX) in an electrochemical flow cell. The experimental setup of the electrochemical flow cell was designed as described.32 A thin-layer flow cell housing a 3 mm-diameter glassy carbon (GC) electrode (Bioanalytical Systems, Inc. West Lafayette, IN) was employed. A platinum electrode and a Ag/AgCl electrode, also incorporated into the setup, were used as the auxiliary and reference electrodes, respectively. A flame atomic absorption spectrophotometer (WXY-402C, Shenyang Analytical Instrument Co., LTD, China) was used to confirm the accuracy of Zn2+ by voltammetric means.2.3. Procedures
Prior to each measurement, the GC electrode was polished with alumina slurry down to 0.05 μm on a polishing cloth, followed by sonication in water and ethanol. Nafion-coated mercury film electrode (NCMFE) was prepared off-line to avoid contamination of the flow cell by Hg2+. The preparation of NCMFE has been reported elsewhere.9,32 Briefly, 5 μL of 2% Nafion solution was spread onto the GC electrode surface and then left to dry in the ambient atmosphere. Mercury was deposited into the pores of the Nafion film by holding the electrode potential at −0.4 V for 8 min in N2-degassed HNO3 solution (10 mM) containing 1 mM Hg2+.The metalloprotein (Zn7MT or Fpg) or the mixed solution of cysteine and Zn2+ was incubated with DTDP for a predetermined time. The above solution was preloaded into a 25 μL sample loop on a six-port injection valve and then delivered to the UV-vis flow cell. The mixture of cysteine and Zn2+ was used to construct the calibration curves for both thiols and metal ions. The amount of thiols in Zn7MT or Fpg was estimated by integrating the elution peak of 4-thiopyridone (4-TP) at 324 nm. 4-TP, the intensively colored product, was formed via reaction of the thiolate anion with DTDP.36 The thiol–disulphide interchange between the metalloprotein and DTDP results in breakage of the metal–thiolate bonds in Zn7MT or Fpg with the accompanying metal release.20,34,36 Upon completion of the spectrophotometric thiol assay, the solution eluted out of the UV-vis flow cell was introduced into the electrochemical flow cell (Fig. 1). By holding the electrode potential at a preset value for 180 s, Zn was electrogenerated and subsequently accumulated into the NCMFE. Anodic stripping voltammetry was used for Zn2+ quantification.
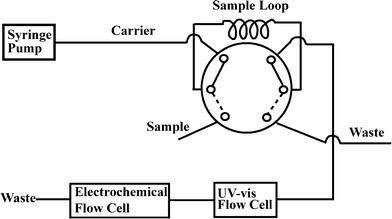 | ||
| Fig. 1 Schematic representation of the consecutive determination of thiols and metal ions in Zn7MT or Fpg by on-line combination of UV-vis spectrophotometry with electrochemistry. | ||
3. Results and discussion
3.1. Kinetic study on the reaction of Zn7MT or Fpg with DTDP
Both DTDP and DTNB are known to be electrophilic reagents for spectrophotometric measurement of protein sulfhydryl groups. The respective reaction products, 4-thiopyridone (4-TP) and 5-thio-2-nitrobenzoic acid (TNB), exhibit intense light absorption at 324 nm (ε324 = 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 M−1 cm−1, pH 3–7) and 412 nm (ε412 = 14
400 M−1 cm−1, pH 3–7) and 412 nm (ε412 = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 M−1 cm−1, pH > 7.3), respectively.36 In comparison with DTNB, DTDP is smaller, more hydrophobic, and uncharged at neutral pH. Thus, higher sensitivity was obtained using the DTDP method.36 In the present work, measurement of sulfhydryl groups in Zn7MT and Fpg was carried out with DTDP.
150 M−1 cm−1, pH > 7.3), respectively.36 In comparison with DTNB, DTDP is smaller, more hydrophobic, and uncharged at neutral pH. Thus, higher sensitivity was obtained using the DTDP method.36 In the present work, measurement of sulfhydryl groups in Zn7MT and Fpg was carried out with DTDP.
The reaction of DTDP with Zn7MT or Fpg was monitored by UV-vis spectrophotometry (Fig. 2A). In contrast to curve 1 in the case of DTDP alone, curves 2 and 3 exhibit a characteristic absorption peak of 4-TP at around 324 nm upon reaction of DTDP with Zn7MT or Fpg. Fig. 2B depicts the reaction kinetics of DTDP with cysteine (triangles), Zn7MT (squares), and Fpg (circles). The reaction of DTDP with cysteine is fast and reaches saturation after 60 s. However, due to the steric hindrance imposed by the unique structures of Zn7MT and Fpg, much slower rates were obtained and the reaction proceeds to completion after 20 min.
 | ||
| Fig. 2 (A) UV-vis absorption spectra of 250 μM DTDP in the absence (curve 1) and presence of 2 μM Zn7MT (curve 2), or Fpg with an unknown concentration (curve 3) at pH 7.0. The incubation time is maintained at 20 min. (B) Reaction kinetics of DTDP with cysteine (triangles), Zn7MT (squares) and Fpg (circles). | ||
3.2. Determination of the thiol-to-metal ratio in Zn7MT
MT adopts a characteristic cluster structure, serving as a biological donor of metal ions. In the presence of electrophilic disulfides or under redox conditions, metal ions can be released despite the high metal-binding affinity.9,17,23,30Fig. 3 depicts the time-resolved UV-vis absorption of DTDP and 4-TP monitored at 324 nm together with the DPV signals at the NCMFE (the inset). Peaks 1 and 2 were recorded by delivering the preloaded DTDP and the mixed solution of Zn7MT with DTDP to the UV-vis flow cell, respectively. As characterized by peak 2, 4-TP exhibits intense absorption, though weak absorption of DTDP was also observed (peak 1, ε324 = 150 M−1 cm−136). The reaction of Zn7MT with DTDP results in the formation of a mixed disulfide and one equivalent of 4-TP. Due to the same weak absorption of DTDP with the mixed disulfide at 324 nm, the net change in molar absorptivity equals to that of 4-TP.36 Upon completion of the UV-vis assay, the respective eluents were introduced into the electrochemical flow cell by the carrier solution. The anodic peak at about −1.2 V (solid line curve in the inset) is attributable to the stripping of Zn generated via reduction of the released Zn2+. Zinc accumulated into the NCMFE could be stripped effectively and was directed to waste. The incorporation of 4-TP and the mixed disulfide during the electrochemical determination yields negligible effect on the stripping signal of Zn (data not shown). The background signal of DTDP alone is shown as the dotted line curve in the inset and no electrochemical response was obtained. The above results demonstrate that the hyphenated technique is amenable to the consecutive determination of thiols and metal ions in MT. | ||
| Fig. 3 Time-resolved UV-vis absorption of DTDP (peak 1) and 4-TP (peak 2) monitored at 324 nm using a UV-vis flow cell. The inset shows differential pulse voltammetric (DPV) responses of DTDP (dotted line) and the released Zn2+ (solid line) via reaction of DTDP with Zn7MT at the NCMFE after completion of the UV-vis assay. The concentrations of DTDP and Zn7MT were 250 μM and 5 μM, respectively. Before easy assay, Zn7MT was incubated with DTDP for 20 min. A deposition time of 240 s at −1.35 V was used prior to the potential scans. The arrow indicates the scan direction. | ||
The feasibility of the method for determination of the thiol-to-metal ratio in metalloproteins was demonstrated. Zn7MT was used as a model system due to the specified thiol-to-Zn2+ ratio. Fig. 4 displays time-resolved UV-vis absorption (A) together with the DPV responses (B) of the mixture comprising DTDP and cysteine/Zn2+ or Zn7MT. The DPV responses in curves 1–5 in panel B were recorded after completion of the UV-vis assay of the respective solutions (curves 1–5 in panel A). The absorption peak intensity (A) and the stripping signal (ipa) are proportional to the concentrations of cysteine and Zn2+ with linear regression equations expressed as A = 0.10 + 0.0087 Ccysteine (μM) (r = 0.99) and ipa (μA) = 0.27 + 0.058 CZn2+ (μM) (r = 0.99), respectively. Based on the calibration curves, the contents of thiol and zinc ions in Zn7MT were found to be 81.4 and 27.7 μM, respectively. Thus, a thiol-to-Zn2+ ratio of 2.9 was obtained (n = 3, RSD = 8.1%). This is in accordance with the contention that 7 divalent metal ions are bound to 12 terminal and 8 bridging cysteine ligands in MT.5,6
 | ||
| Fig. 4 Time-resolved UV-vis absorption (A) and the DPV responses (B) of the mixture comprising DTDP and cysteine/Zn2+ or Zn7MT. The concentrations of cysteine in curves 1–5 in panel A are 15 μM, 30 μM, 50 μM, 100 μM, 200 μM, respectively. For curves 1–5 in panel B, the contents of Zn2+ are 5 μM, 10 μM, 16.7 μM, 33.3 μM, 66.7 μM, respectively. The insets in panels A and B show the dependence of the absorption peak intensity (A) and the anodic peak current (ipa) on the concentrations of cysteine and Zn2+, respectively. The absolute errors were deduced from at least three replicate measurements and are shown as the error bars. Other experimental conditions are the same as those in Fig. 3. | ||
We conducted off-line UV-vis spectrophotometric assay and flame AAS to verify the accuracy of the method. The amount of thiol and Zn2+ in Zn7MT, determined by the coupled technique, is in good agreement with that by off-line UV-vis spectrophotometry and AAS (data not shown), which suggests that the hyphenated technique is reliable for accurate determination of the thiol-to-metal ratio in metalloproteins.
3.3. Determination of the thiol-to-metal ratio in Fpg protein
The developed method is thus amenable to the determination of other metalloproteins, such as Fpg. It is worth noting that Fpg, unlike MT, contains aromatic amino acids which also show poor absorption at 324 nm. Thus, background-subtracted absorption of 4-TP via excluding the contribution of individual Fpg at 324 nm was recorded (data not shown). The method for Fpg analysis is reproducible, as three consecutive injections of the mixture comprising DTDP and Fpg generated nearly identical anodic peak currents and UV-vis absorption (RSD of 3.2% and 1.9%, respectively). Again, the concentration levels of thiol and Zn2+ in Fpg were determined to be 40.9 and 7.4 μM, respectively. The ratio of thiol to Zn2+ was estimated to be 5.5 (n = 3, RSD = 10.9%), being in agreement with the known value of 6 in Fpg.38 The coupled technique thus holds great promise for identification and characterization of thiol-containing metalloproteins.4. Conclusions
In this work, UV-vis spectrophotometry has been used in tandem with electrochemistry to determine the thiol-to-metal ratio in metalloproteins. The mixture comprising DTDP and Zn7MT or Fpg was introduced to the UV-vis flow cell for spectrophotometric thiol assay and then to the electrochemical flow cell for metal ion determination. A thiol-to-zinc ratio of 2.9 and 5.5 was obtained for Zn7MT and Fpg, respectively, being in excellent agreement with the reported values and those by off-line UV-vis spectrophotometric assay and flame AAS. The hyphenated UV-vis and voltammetric technique is simple, straightforward, and reliable, and thus amenable to the identification and accurate determination of thiol-containing metalloproteins.Acknowledgements
We thank Dr. Xin Wen for donating the Fpg sample. Partial support of this work by the National Natural Science Foundation of China (No. 20975114), the Specialized Research Fund for the Doctoral Program of Higher Education (No. 20100162110018), the Program for New Century Excellent Talents in University (NCET-10-0796), and the Graduate Degree Thesis Innovation Foundation of Hunan Province (CX2011B082) is gratefully acknowledged.References
- D. H. Hamer, Annu. Rev. Biochem., 1986, 55, 913 CrossRef CAS.
- M. Mrksich and G. M. Whitesides, Annu. Rev. Biophys. Biomol. Struct., 1996, 25, 55 CrossRef CAS.
- M. Valko, H. Morris and M. T. D. Cronin, Curr. Med. Chem., 2005, 12, 1161 CrossRef CAS.
- N. M. Giles, A. B. Watts, G. I. Giles, F. H. Fry, J. A. Littlechild and C. Jacob, Chem. Biol., 2003, 10, 677 CrossRef CAS.
- M. Margoshes and B. L. Vallee, J. Am. Chem. Soc., 1957, 79, 4813 CrossRef CAS.
- M. Vašák, J. Mol. Catal., 1984, 23, 293 CrossRef.
- S. Sri Krishna, I. Majumdar and N. V. Grishin, Nucleic Acids Res., 2003, 31, 532 CrossRef.
- M. Dhanasekaran, S. Negi and Y. Sugiura, Acc. Chem. Res., 2006, 39, 45 CrossRef CAS.
- Y. Fu, M. Xu, X. Li, M. Du, J. Wang and F. Zhou, Electroanalysis, 2008, 20, 888 CrossRef CAS.
- R. Lobinski, H. Chassaigne and J. Szpunar, Talanta, 1998, 46, 271 CrossRef CAS.
- V. Adam, S. Krizkova, O. Zitka, L. Trnkova, J. Petrlova, M. Beklova and R. Kizek, Electroanalysis, 2007, 19, 339 CrossRef CAS.
- M. Dabrio, A. R. Rodríguez, G. Bordin, M. J. Bebianno, M. D. Ley, I. Šestáková, M. Vašák and M. Nordberg, J. Inorg. Biochem., 2002, 88, 123 CrossRef CAS.
- S. Miyairi, S. Shibata and A. Naganuma, Anal. Biochem., 1998, 258, 168 CrossRef CAS.
- R. W. Olafson, Methods Enzymol., 1991, 205, 283 CAS.
- M. Tomschik, L. Havran, E. Palecek and M. Heyrovský, Electroanalysis, 2000, 12, 274 CrossRef CAS.
- A. Viarengo, E. Ponzano, F. Dondero and R. Fabbri, Mar. Environ. Res., 1997, 44, 69 CrossRef CAS.
- S. Zhang, J. Li, C.-C. Wang and C.-L. Tsou, FEBS Lett., 1999, 462, 383 CrossRef CAS.
- J. H. R. Kägi, Methods Enzymol., 1991, 205, 613 Search PubMed.
- M. Xu, Y. Wu, J. Wang and F. Zhou, Electroanalysis, 2006, 18, 2099 CrossRef CAS.
- B. Xing, Y. Shi and W. Tang, BioMetals, 2000, 13, 295 CrossRef CAS.
- E. H. Fischer and E. W. Davie, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 3333 CrossRef CAS.
- P. E. Hunziker, Methods Enzymol., 1991, 205, 451 CAS.
- L. J. Jiang, W. Maret and B. L. Vallee, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 3483 CrossRef CAS.
- A. J. Bard and L. R. Faulkner, Electrochemical Methods: Fundamentals and Applications, Wiley, New York, 2001 Search PubMed.
- I. Šestáková and P. Mader, Cell. Mol. Biol., 2000, 46, 257 Search PubMed.
- M. Erk and R. Biserka, Cell. Mol. Biol., 2000, 46, 269 CAS.
- C. Harlyk, O. Nieto, G. Bordin and A. R. Rodríguez, J. Electroanal. Chem., 1998, 451, 267 CrossRef CAS.
- J. Petrlova, D. Potesil, R. Mikelova, O. Blastik, V. Adam, L. Trnkova, F. Jelen, R. Prusa, J. Kukacka and R. Kizek, Electrochim. Acta, 2006, 51, 5112 CrossRef CAS.
- A. R. Rodríguez and M. Esteban, Cell. Mol. Biol., 2000, 46, 237 Search PubMed.
- A. J. Baca, Y. Garcia, A. L. Briseno and F. Zhou, J. Electroanal. Chem., 2001, 513, 25 CrossRef CAS.
- A. L. Briseno, A. J. Baca, Q. Zhou, R. Lai and F. Zhou, Anal. Chim. Acta, 2001, 441, 123 CrossRef CAS.
- G. X. Cao, O. Jimenez, M. Xu and F. Zhou, J. Am. Soc. Mass Spectrom., 2006, 17, 945 CrossRef CAS.
- J. R. Pretty, G. J. V. Berkel and D. C. Duckworth, Int. J. Mass Spectrom. Ion Processes, 1998, 178, 51 CAS.
- T.-Y. Li, D. T. Minkel, C. Shaw and D. H. Petering, Biochem. J., 1981, 193, 441 CAS.
- A. Muñoz, D. H. Petering and C. Shaw, Inorg. Chem., 1999, 38, 5655 CrossRef.
- C. K. Riener, G. Kada and H. J. Gruber, Anal. Bioanal. Chem., 2002, 373, 266 CrossRef CAS.
- L. Liu, J. Yang, N. Xia, J. Wang and F. Zhou, Electroanalysis, 2008, 20, 2253 CrossRef CAS.
- T. R. O'Connor and J. Larval, J. Biol. Chem., 1990, 265, 3916 Search PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2012 |
