DOI:
10.1039/C2RA21116E
(Paper)
RSC Adv., 2012,
2, 7681-7688
Novel syntheses of pyrrolo[2,1-a]isoquinolines via 1,3-dipolar cycloaddition between Isoquinoliniums and alkynes†
Received
4th February 2012
, Accepted 13th June 2012
First published on 13th June 2012
Abstract
A straightforward synthesis of pyrrolo[2,1-a]isoquinolines has been achieved from 1,3-dipolar cycloaddition reaction between isoquinoliniums and alkynes in the presence of K2CO3, and features with versatility, easy operation and mild reaction conditions. 22 examples were synthesized.
Introduction
Bridgehead nitrogen heterocycles show potential applications in pharmaceutical, agrochemical, and industrial areas. Among those, pyrrolo[2,1-a]isoquinoline moieties are ubiquitous structural motifs in a myriad of biologically active alkaloid natural products, such as erythrina and lamellarin. 1 Recently, these compounds have attracted extensive research interest for applications such as anti-tuberculosis agents,2 MPtpA/B phosphatases inhibitors,3 15-lipoxygenase inhibitors,4anti-inflammatory,5 antidepressant,6 anticancer,7,8 HIV-1 integrase inhibiting reagents,9 calcium entry blockers10 and potential in vitro antioxidants for lipid peroxidation inhibition.11 As a result, there is a continuous interest in the development of efficient methods for the synthesis of pyrrolo[2,1-a] isoquinolines.12
Methods available for the synthesis of these types of compounds include Schöltz,13 Tschitschibabin,14 1,3-dipolar cycloadditions,15 1,5-dipolar cyclizations,16 metal-catalyzed intramolecular cycloadditions and multicomponent reactions.17 Problems associated with these strategies include limited versatility, requirements of a transition-metal catalyst or high temperatures. Therefore, the development of new synthetic methods for the synthesis of pyrrolo[2,1-a]isoquinolines is highly desirable.
The classic 1,3-dipolar cycloaddition between pyridinium-related heteroaromatic ylides and alkynes is very attractive considering its versatility and efficiency.18,15c Based on this reaction, we recently have reported an efficient synthesis of indolizines derivatives from N-ylides (Scheme 1).19 Subsequently, we explored the possible application of this strategy for the preparation of pyrrolo[2,1-a]isoquinolines from isoquinoline and halogenated ketones. Herein, we report a facile and efficient preparation of pyrrolo[2,1-a]isoquinolines in high yields via the 1,3-dipolar cycloaddition between N-ylides 1 and alkyne 2 under mild reaction conditions (Scheme 1).
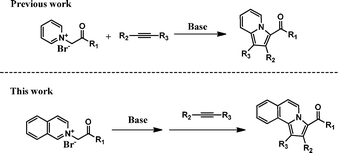 |
| | Scheme 1 Bridgehead N-heterocycles syntheses via the classic 1,3-dipolar cycloaddition between N-ylides and alkynes. | |
Results and discussion
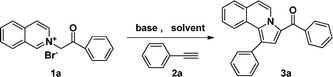
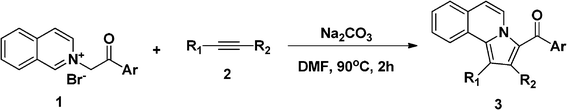
Initially, our investigation was performed by reacting 1-(2-oxo-2-phenylethyl)isoquinolinium bromide (1a) with phenylacetylene (2a) in the presence of K2CO3 in DMF at 120 °C, from which phenyl(1-phenylpyrrolo[2,1-a]isoquinolin-3-yl) methanone (3a) was obtained in 82% yield. As shown in Table 1, solvent, base and temperature were found to have an important effect for this reaction. Among those, base and its amount were found to be critical for this reaction. In comparison with triethylamine (TEA), K2CO3 gave better yields, and 1.5 equiv of base was necessary for this reaction (Table 1, entries 1, 2 and 8). Further screening of solvents showed that DMF gave the best result in comparison to other solvents such as CH3CN, THF, and H2O (Table 1, entries 4–7). In DMF, at 90 °C or refluxing conditions, this reaction smoothly generates the desired product 3a in 82% yield (Table 1, entries 3 and 4). Thus, the optimal reaction conditions were found to be in DMF at 90 °C with 1.5 equiv of K2CO3. To test the versatility of this reaction, these optimized reaction conditions were also applied to isoquinoliniums 1b–f and alkynes 2b–d, 2g, and 2h as summarized in Table 2. In most cases, the desired pyrrolo[2,1-a]isoquinolines were smoothly obtained in high yields. Among those, isoquinoliniums bearing electron-donating groups gave higher yields in comparison to those with electron-withdrawing groups (Table 2, entries 2–4, and 18), while alkynes with strong electron-donating substituents, like methoxyl groups attached on the phenyl ring gave relatively lower yields in comparison to other alkynes studied (Table 2, entries 8–10). The structure of 3d was confirmed by X-ray analysis, the result is shown in Fig. 1.20 To test the versatility of this reaction, the optimized reaction conditions were further applied to the reaction of other alkynes, such as dimethyl acetylenedicarboxylate (2g) and 1-heptyne (2h). In comparison with phenylacetylene, when activated acetylene (2g) was used for this reaction, also 83% 3u was obtained (Table 2, entry 21). To our delight, when alkyl alkyne (2h) was used, the desired product 3v was obtained in 50% yield (Table 2, entry 22), associated with the inert nature of 1-heptyne.
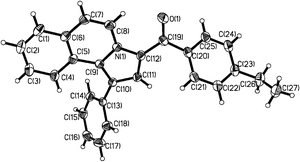 |
| | Fig. 1 The X-ray single-crystal structure of 3d. | |
Table 1 The optimal reaction conditions for the 1,3-dipolar cycloaddition reaction between isoquinolinium and phenylacetylene
| Entry |
Basea |
Solvent |
T/°C |
Time (h) |
Yieldd (%) |
|
1.5 equiv base was used.
1.0 equiv PTC was used.
1.0 equiv base was used.
Yields refer to isoquinolinium.
|
| 1 |
K2CO3 |
DMF |
120 |
2 |
82 |
| 2 |
Et3N |
DMF |
90 |
2 |
65 |
| 3 |
K2CO3 |
DMF |
90 |
2 |
82 |
| 4 |
K2CO3 |
DMF |
reflux |
2 |
82 |
| 5 |
K2CO3 |
CH3CN |
reflux |
5 |
73 |
| 6 |
K2CO3 |
THF |
reflux |
5 |
75 |
| 7b |
K2CO3 |
H2O |
90 |
5 |
46 |
| 8c |
K2CO3 |
DMF |
90 |
5 |
60 |
Table 2 Syntheses of pyrrolo[2,1-a]isoquinolines 3via the 1,3-dipolar cycloaddition reaction between isoquinoliniums 1 and alkynes 2
| Entry |
Isoquinolinium |
Alkyne |
Product |
Yield (%) |
| 1 |
 1a
1a
|
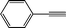 2a
2a
|
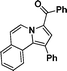 3a
3a
|
82 |
| 2 |
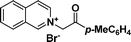 1b
1b
|
2a
|
 3b
3b
|
79 |
| 3 |
 1c
1c
|
2a
|
 3c
3c
|
80 |
| 4 |
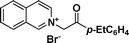 1d
1d
|
2a
|
 3d
3d
|
82 |
| 5 |
1a
|
 2b
2b
|
 3e
3e
|
81 |
| 6 |
1b
|
2b
|
 3f
3f
|
80 |
| 7 |
1c
|
2b
|
 3g
3g
|
83 |
| 8 |
1a
|
 2c
2c
|
 3h
3h
|
57 |
| 9 |
1b
|
2c
|
 3i
3i
|
61 |
| 10 |
1c
|
2c
|
 3j
3j
|
59 |
| 11 |
1a
|
 2d
2d
|
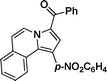 3k
3k
|
88 |
| 12 |
1b
|
2d
|
 3l
3l
|
90 |
| 13 |
1c
|
2d
|
 3m
3m
|
91 |
| 14 |
1a
|
 2e
2e
|
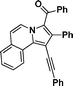 3n
3n
|
86 |
| 15 |
1b
|
2e
|
 3o
3o
|
84 |
| 16 |
1c
|
2e
|
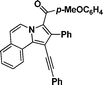 3p
3p
|
83 |
| 17 |
 1e
1e
|
2e
|
 3q
3q
|
52 |
| 18 |
1a
|
 2f
2f
|
 3r
3r
|
86 |
| 19 |
1b
|
2f
|
 3s
3s
|
82 |
| 20 |
1c
|
2f
|
 3t
3t
|
85 |
| 21 |
1a
|
 2g
2g
|
 3u
3u
|
83 |
| 22 |
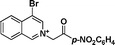 1f
1f
|
 2h
2h
|
 3v
3v
|
50 |
Interestingly, further application of our optimized reaction conditions to internal alkynes 2e and 2f provided the corresponding 1,2,3-trisubstituted pyrrolo[2,1-a]isoquinolines as major products in high yields (Table 2, entries 14, 15, 16, 18, 19 and 20). Unfortunately, when isoquinolinium 1e, which has a strong electron-withdrawing group (–NO2) on the benzene ring, was used for the reaction, a low yield was observed (Table 2, entry 17). The structure of 3t was confirmed by X-ray analysis results as shown in Fig. 2.21 This high regioselectivity might be attributed to the relatively strong electronic negativity on the C2 and C3 of 2e and 2f than those on the C1 and C4 of 2e and 2f.
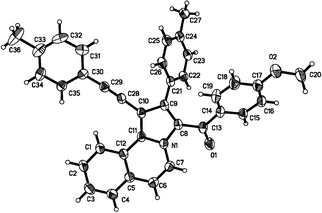 |
| | Fig. 2 The X-ray single-crystal structure of 3t. | |
We propose a plausible mechanism for the synthesis of pyrrolo[2,1-a]isoquinoline as shown in Scheme 2. The deprotonation of isoquinolinium by K2CO3 generated the corresponding N-ylide, which acted as a dipole to participate in the subsequent reaction with alkynes to generate the desired pyrrolo[2,1-a]isoquinoline via a 1,3-dipolar cycloaddition reaction.
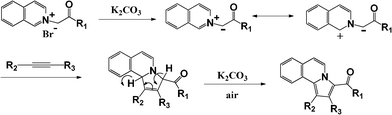 |
| | Scheme 2 Proposed mechanism for the 1,3-dipolar cycloaddition between N-ylides and alkynes. | |
Conclusion
In conclusion, we have reported a novel and efficient general method for the syntheses of pyrrolo[2,1-a]isoquinolines via a classic 1,3-dipolar cycloaddition reaction between isoquinoliniums and alkynes. Further investigation of this reaction and the electronic properties of the resultant compounds are currently under way in our laboratory.
Experimental section
General
All reactions were carried out using standard Schlenk techniques. Solvents like CH2Cl2, CH3CN and EtOAc were distilled from CaH2. 1H NMR and 13C NMR spectra were recorded at room temperature in CDCl3 on Bruker AXS-300 MHz instrument with TMS as internal standard. Coupling constants are reported in Hertz (Hz). IR spectra were taken as KBr plates, HRMS was obtained using ESI ionization. Melting points were measured with micro melting point apparatus. Single crystal X-ray diffraction data were collected in Bruker SMART APEX diffractiometers with molybdenum cathodes.
General procedure for the syntheses of 2-(2-oxo-2-phenylethyl)isoquinolin-2-ium bromide 1
The mixture of 2-bromo-1-phenylethanone (10 mmol) and isoquinoline (10 mmol) were stirred in 10 mL dry CH3CN at room temperature for a period. After reaction, the precipitate was filtered and washed with acetonitrile to get the pure product 1.
General procedure for the syntheses of polypyrrolo[2,1-a]isoquinolines 3
To a stirred mixture of 2-(2-oxo-2-phenylethyl)isoquinolin-2-ium bromide 1 (1 mmol) and K2CO3 (1.5 mmol) in DMF was added phenylacetylene 2 (1 mmol) at room temperature. The resulting mixture was heated in an oil bath at 90 °C for 2 h in a sealed reaction flask. Upon completion of this reaction, the mixture was cooled to room temperature, diluted with CH2Cl2, and washed with water. Organic layers were combined, dried over Na2SO4, filtered, and evaporated in vacuum. The residue was further purified by flash column chromatography on silica gel (PE/EtOAc = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) to afford the corresponding pyrrolo[2,1-a]isoquinolines 3.
1, v/v) to afford the corresponding pyrrolo[2,1-a]isoquinolines 3.
Phenyl(1-phenylpyrrolo[2,1-a]isoquinolin-3-yl)methanone (3a).
Yellow solid, mp 172–173 °C; 1HNMR (CDCl3, 300 MHz) δ 9.68 (d, J = 7.2 Hz, 1H), 9.29 (d, J = 6.9 Hz, 1H), 8.17 (d, J = 6.9 Hz, 1H), 7.94(d, J = 7.8 Hz, 1H), 7.85 (d, J = 6.3 Hz, 2H), 7.45–7.52 (m, 6H), 7.05–7.22 (m, 5H); 13C NMR (75 CDCl3, MHz) δ 185.5, 140.6, 136.8, 132.1, 131.3, 130.1, 130.1, 129.9, 129.7, 129.2, 128.8, 128.3, 127.8, 127.8, 127.6, 127.2, 127.0, 125.7, 125.3, 125.2, 124.2, 123.5, 123.3, 121.0, 113.9, 113.1, 103.7 ppm; IR (KBr) ν 3051, 3024, 1603, 1574, 1404, 1339, 1290, 1178, 910, 877, 792, 758, 721, 694 cm−1; HRMS (ESI) calcd for C25H17NO ([M + H]+) 348.1388, found 348.1379.
(1-Phenylpyrrolo[2,1-a]isoquinolin-3-yl)(p-tolyl)methanone (3b).
Yellow solid, mp 212–213 °C; 1H NMR (CDCl3, 300 MHz) δ 9.18 (d, J = 7.2 Hz, 1H), 8.16 (d, J = 5.7 Hz, 1H), 7.69(d, J = 3.9 Hz, 1H), 7.40–7.43(m, 5H), 7.04–7.11 (m, 6H), 6.85(s, 2H), 2.22(s, 3H); 13C NMR (CDCl3, 75 MHz) δ 185.4, 141.8, 137.8, 136.8, 130.0, 129.9, 129.3, 128.9, 128.7, 128.4, 128.2, 127.7, 127.5, 127.1, 127.0, 125.7, 125.3, 124.2, 123.4, 120.8, 113.6, 103.2, 21.6 ppm; IR (KBr) ν 3032, 2920, 1601, 1568, 1520, 1393, 1366, 1290, 1229, 912, 793, 766, 702, 664, 600 cm−1; HRMS (ESI) calcd for C26H19NO ([M + H]+) 362.1545, found 362.1542.
(4-Methoxyphenyl)(1-phenylpyrrolo[2,1-a]isoquinolin-3-yl)methanone (3c).
Yellow solid, mp 153–154 °C; 1H NMR (CDCl3, 300 MHz) δ 9.59 (d, J = 7.2 Hz, 1H), 7.88–7.96 (m, 3H), 7.67 (d, J = 8.1 Hz, 1H), 7.42–7.55 (m, 5H), 7.26 (t, J = 8.1 Hz, 3H), 7.09 (d, J = 7.8 Hz, 1H), 6.97 (d, J = 9.0 Hz, 2H), 3.88 (s, 3H); 13C NMR (CDCl3, 75 MHz) δ 184.5, 162.3, 136.9, 133.0, 132.2, 131.4, 130.1, 129.5, 128.7, 127.8, 127.6, 127.5, 127.1, 127.0, 126.9, 125.6, 125.3, 124.1, 123.5, 120.7, 113.5, 112.9, 103.2, 55.5 ppm; IR (KBr) ν 3445, 2922, 2837, 1614, 1591, 1441, 1366, 1339, 1258, 1238, 1171, 1038, 881, 839, 791, 766, 698 cm−1; HRMS (ESI) calcd for C26H19NO2 ([M + H]+) 378.1494, found 378.1489.
(4-Ethylphenyl)(1-phenylpyrrolo[2,1-a]isoquinolin-3-yl)methanone (3d).
Yellow solid, mp 174–175 °C; 1H NMR (CDCl3, 300 MHz) δ 9.58 (d, J = 8.1 Hz, 1H), 7.870 (d, J = 8.4 Hz, 1H), 7.72 (d, J = 8.4 Hz, 2H), 7.61 (d, J = 7.8 Hz, 1H), 7.34–7.47 (m, 6H), 7.17–7.24 (m, 4H), 7.04 (d, J = 7.2 Hz, 1H), 2.64 (q, J = 7.6 Hz, 2H), 1.21 (t, J = 7.5 Hz, 3H); 13C NMR (CDCl3, 75 MHz) δ 184.2, 146.8, 136.8, 135.6, 130.6, 128.8, 128.4, 128.2, 127.5, 126.5, 126.3, 126.2, 125.9, 125.7, 124.5, 124.0, 122.9, 122.2, 119.5, 112.4, 27.7, 12.2 ppm; IR (KBr) ν 3138, 2965, 2932, 1597, 1558, 1483, 1439, 1423, 1364, 1335, 1236, 1173, 972, 881, 802, 758, 702 cm−1; HRMS (ESI) calcd for C27H21NO ([M + H]+) 376.1701, found 376.1692.
Phenyl(1-(p-tolyl)pyrrolo[2,1-a]isoquinolin-3-yl)methanone (3e).
Yellow solid, mp 155–156 °C; 1H NMR (CDCl3, 300 MHz) δ 9.68 (d, J = 7.2 Hz, 1H), 7.99 (d, J = 8.1 Hz, 1H), 7.85 (d, J = 8.4 Hz, 2H), 7.69 (d, J = 7.5 Hz, 1H), 7.40–7.53 (m, 6H), 7.27 (d, J = 7.5 Hz, 3H), 7.21 (s, 1H), 7.13 (d, J = 7.2 Hz, 1H), 2.46 (s, 3H); 13C NMR (CDCl3, 75 MHz) δ 184.4, 139.5, 136.2, 132.6, 131.1, 130.1, 129.0, 128.8, 128.6, 128.4, 128.1, 127.1, 126.8, 126.7, 126.1, 125.9, 124.7, 124.3, 123.2, 122.2, 119.9, 112.7, 20.3 ppm; IR (KBr) ν 3125, 3024, 1601, 1572, 1447, 1364, 1335, 1234, 1175, 935, 876, 827, 797, 725, 698 cm−1; HRMS (ESI) calcd for C26H19NO ([M + H]+) 362.1545, found 362.1542.
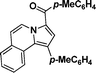
p-Tolyl(1-(p-tolyl)pyrrolo[2,1-a]isoquinolin-3-yl)methanone (3f).
Yellow solid, mp 224–225 °C; 1H NMR (CDCl3, 300 MHz) δ 9.19 (d, J = 7.2 Hz, 1H), 8.16 (d, J = 6.9 Hz, 1H), 7.68 (d, J = 5.7 Hz, 2H), 7.41–7.43 (m, 5H), 7.02–7.09 (m, 4H), 6.85 (d, J = 7.2 Hz, 2H), 2.23 (s, 6H); 13C NMR (CDCl3, 75 MHz) δ 186.3, 140.5, 136.5, 135.6, 135.0, 133.3, 131.6, 128.8, 128.7, 128.2, 128.1, 127.6, 127.5, 127.1, 126.9, 126.5, 126.4, 125.6, 124.0, 123.5, 122.2, 111.5, 102.0, 20.2, 19.8 ppm; IR (KBr) ν 3383, 3034, 2972, 2918, 2349, 2308, 1601, 1520, 1420, 1393, 1339, 1049, 912, 826, 793, 764 cm−1; HRMS (ESI) calcd for C27H21NO ([M + H]+) 376.1701, found 376.1694.
(4-Methoxyphenyl)(1-(p-tolyl)pyrrolo[2,1-a]isoquinolin-3-yl)methanone (3g).
Yellow solid, mp 147–148 °C; 1H NMR (CDCl3, 300 MHz) δ 9.52 (d, J = 7.2 Hz, 1H), 7.91 (d, J = 7.8 Hz, 1H), 7.80 (d, J = 8.4 Hz, 2H), 7.33–7.39 (m, 4H), 7.14–7.22 (m, 3H), 7.00 (d, J = 7.8 Hz, 1H), 6.89 (d, J = 8.4 Hz, 2H), 3.80 (s, 3H), 2.38 (s, 3H); 13C NMR (CDCl3, 75 MHz) δ 183.4, 161.2, 136.1, 132.8, 132.0, 130.6, 130.3, 128.8, 128.4, 126.5, 126.1, 126.0, 125.9, 124.6, 124.3, 123.1, 122.3, 119.6, 112.4, 54.4, 20.3 ppm; IR (KBr) ν 3130, 3046, 2963, 2839, 2380, 2349, 2309, 1614, 1595, 1506, 1443, 1423, 1364, 1256, 1171, 1026, 964, 880, 800, 615 cm−1; HRMS (ESI) calcd for C27H21NO2 ([M + H]+) 392.1650, found 392.1649.
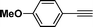
(1-(4-Methoxyphenyl)pyrrolo[2,1-a]isoquinolin-3-yl)(phenyl)methanone (3h).
Yellow solid, mp 167–168 °C; 1H NMR (CDCl3, 300 MHz) δ 9.59 (d, J = 6.9 Hz, 1H), 7.87 (d, J = 8.1 Hz, 1H), 7.77 (d, J = 6.9 Hz, 2H), 7.61 (d, J = 7.8 Hz, 1H), 7.33–7.46 (m, 5H), 7.21 (t, J = 7.8 Hz, 2H), 7.12 (s, 1H), 7.04 (d, J = 7.2 Hz, 1H), 6.92 (d, J = 7.8 Hz, 2H),3.82 (s, 3H); 13C NMR (CDCl3, 75 MHz) δ 184.2, 157.8, 139.4, 129.9, 129.9, 128.4, 127.9, 127.6, 127.0, 126.6, 126.5, 125.9, 125.7, 124.5, 124.1, 122.9, 121.9, 119.4, 112.9, 112.5, 54.2 ppm; IR (KBr) ν 3123, 3053, 2835, 2380, 2347, 2309, 1601, 1557, 1445, 1364, 1244, 1175, 1031, 966, 876, 812, 698 cm−1; HRMS (ESI) calcd for C26H19NO2 ([M + H]+) 378.1494, found 378.1482.
(1-(4-Methoxyphenyl)pyrrolo[2,1-a]isoquinolin-3-yl)(p-tolyl)methanone (3i).
Yellow solid, mp 160–161 °C; 1H NMR (CDCl3, 300 MHz) δ 9.66 (d, J = 7.5 Hz, 1H), 7.98 (d, J = 7.8 hz, 1H), 7.79 (d, J = 7.5 Hz, 2H), 7.70 (d, J = 7.8 Hz, 1H), 7.44–7.51 (m, 3H), 7.29 (d, J = 6.9, 3H), 7.23 (s, 1H), 7.12 (d, J = 7.2 Hz, 1H), 7.02 (d, J = 7.5 Hz, 2H),3.92 (s, 3H), 2.46 (s, 3H); 13C NMR (CDCl3, 75 MHz) δ 185.3, 159.0, 141.7, 137.8, 132.0, 131.1, 129.6, 129.3, 129.0, 128.9, 127.6, 127.1, 126.9, 125.7, 125.4, 124.1, 123.3, 120.4, 114.1, 113.5, 55.4, 21.6 ppm; IR (KBr) ν 3117, 3005, 2936, 2378, 2309, 1612, 1557, 1445, 1423, 1337, 1246, 1180, 1032, 880, 837, 797, 750 cm−1; HRMS (ESI) calcd for C27H21NO2 ([M + H]+) 392.1650, found 392.1641.
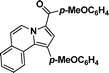
(4-Methoxyphenyl)(1-(4-methoxyphenyl)pyrrolo[2,1-a]isoquinolin-3-yl)methanone (3j).
Yellow solid, mp 173–174 °C; 1H NMR (CDCl3, 300 MHz) δ 9.61 (d, J = 7.2 Hz, 1H), 7.89–7.99 (m, 3H), 7.70 (d, J = 7.5 Hz, 1H), 7.48 (t, J = 9.0 Hz, 3H), 7.28–7.32 (m, 3H), 7.11 (d, J = 7.2 Hz, 1H), 7.02 (t, J = 9.0 Hz, 3 H), 3.92 (s, 3H), 3.91 (s, 3H); 13C NMR (CDCl3, 75 MHz) δ 184.5, 162.3, 159.0, 133.1, 131.4, 131.1, 129.5, 129.0, 127.5, 127.1, 127.1, 126.9, 125.7, 125.4, 124.1, 123.3, 120.3, 114.1, 113.5, 113.4, 55.5, 55.4 ppm; IR (KBr) ν 3132, 3009, 2967, 2934, 2839, 2349, 2309, 1607, 1557, 1439, 1364, 1260, 1182, 1171, 1028, 966, 878, 833, 822, 752, 615 cm−1; HRMS (ESI) calcd for C26H21NO3 ([M + H]+) 408.1600, found 408.1602.
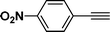
(1-(4-Nitrophenyl)pyrrolo[2,1-a]isoquinolin-3-yl)(phenyl)methanone (3k).
Yellow solid, mp 185–186 °C; 1H NMR (CDCl3, 300 MHz) δ 9.66 (d, J = 7.5 Hz, 1H), 8.32 (d, J = 8.7 Hz, 2H), 7.84–7.91 (m, 3H), 7.74 (t, J = 9.0 Hz, 3H), 7.48–7.57 (m, 3H), 7.34 (t, J = 7.2 Hz, 1H), 7.19–7.23 (m, 3H); 13C NMR (CDCl3, 75 MHz) δ 184.6, 146.0, 143.0, 139.2, 130.5, 129.6, 128.8, 128.0, 127.3, 126.4, 126.3, 126.1, 124.6, 123.6, 123.0, 122.8, 117.2, 113.2 ppm; IR (KBr) ν 3127, 3055, 2361, 1614, 1597, 1574, 1512, 1443, 1346, 1233, 1179, 876, 795, 700 cm−1; HRMS (ESI) calcd for C25H16N2O3 ([M + H]+) 393.1239, found 393.1234.
(1-(4-Nitrophenyl)pyrrolo[2,1-a]isoquinolin-3-yl)(p-tolyl)methanone (3l).
Yellow solid, mp 177–178 °C; 1H NMR (CDCl3, 300 MHz) δ 9.63 (d, J = 7.2 Hz, 1H), 8.32 (d, J = 8.1 Hz, 2H), 7.89 (d, J = 8.1 Hz, 1H), 7.77 (q, J = 7.9 Hz, 4H), 7.53 (t, J = 6.9 Hz, 2H), 7.17–7.36 (m, 5H), 2.45 (s, 3H); 13C NMR (CDCl3, 75 MHz) δ 184.5, 143.1, 141.1, 136.4, 129.6, 128.8, 128.2, 128.0, 127.2, 126.3, 126.3, 125.9, 124.5, 123.6, 122.9, 122.8, 117.1, 113.1, 20.5 ppm; IR (KBr) ν 3132, 3055, 2920, 1595, 1510, 1443, 1344, 1234, 1179, 880, 804, 745, 698 cm−1; HRMS (ESI) calcd for C26H18N2O3 ([M + H]+) 407.1396, found 407.1397.
(4-Methoxyphenyl)(1-(4-nitrophenyl)pyrrolo[2,1-a] isoquinolin-3-yl)methanone (3m).
Yellow solid, mp 189–190 °C; 1H NMR (CDCl3, 300 MHz) δ 9.57 (d, J = 7.2 Hz, 1H), 8.32 (d, J = 8.4 Hz, 2H), 7.87–7.91 (m, 3H), 7.72 (d, J = 8.1 Hz, 3H), 7.52 (t, J = 7.2 Hz, 1H), 7.33 (t, J = 7.2 Hz, 1H), 7.23 (d, J = 8.7 Hz, 1H), 7.15(d, J = 7.2 Hz, 1H), 6.99(d, J = 8.4 Hz, 2H), 3.89 (s, 3H); 13C NMR (CDCl3, 75 MHz) δ 183.5, 161.5, 145.9, 143.1, 131.6, 130.3, 129.6, 128.7, 127.1, 126.3, 125.4, 124.5, 123.7, 123.2, 122.9, 122.7, 117.0, 113.0, 112.6, 54.4 ppm; IR (KBr) ν 3065, 2920, 2843, 1309, 1626, 1589, 1501, 1335, 1254, 1177, 1105, 1230, 964, 934, 881, 839, 799, 754, 700 cm−1; HRMS (ESI) calcd for C26H18N2O4 ([M + H]+) 423.1345, found 423.1354.
Phenyl(2-phenyl-1-(phenylethynyl)pyrrolo[2,1-a]isoquinolin-3-yl)methanone (3n).
Yellow solid, mp 192–193 °C; 1H NMR (CDCl3, 300 MHz) δ 9.40 (d, J = 7.8 Hz, 1H), 9.12 (d, J = 7.5 Hz,1H), 7.65 (d, J = 6.9 Hz, 2H), 7.50–7.58 (m, 3H), 7.43 (d, J = 7.2 Hz, 2H), 7.35 (d, J = 5.7 Hz, 3H), 7.12–7.17 (m , 7H), 6.98–7.09 (m, 2H); 13C NMR (CDCl3, 75 MHz) δ 187.8, 139.0, 133.4, 131.6, 130.9, 129.8, 128.4, 127.9, 127.6, 127.3, 127.2, 126.8, 124.8, 124.3, 121.5, 114.1, 94.8, 85.3 ppm; IR (KBr) ν 3443, 3059, 2202, 1620, 1527, 1491, 1447, 1398, 1375, 1217, 895, 800, 758, 719, 692 cm−1; HRMS (ESI) calcd for C33H21NO ([M + H]+) 448.1701, found 448.1707.
(2-Phenyl-1-(phenylethynyl)pyrrolo[2,1-a]isoquinolin-3-yl)(p-tolyl)methanone (3o).
Yellow solid, mp 248–249 °C; 1H NMR (CDCl3, 300 MHz) δ 9.46 (d, J = 7.5 Hz, 1H), 9.10 (d, J = 7.2 Hz,1H), 7.72 (d, J = 6.3 Hz, 2H), 7.62 (t, J = 9.0 Hz, 3H), 7.25–7.44 (m, 8H), 7.09(s, 2 H), 6.84 (d, J = 7.2 Hz, 2 H), 2.21(s, 3H); 13C NMR (CDCl3, 75 MHz) δ 186.9, 142.3, 137.5, 135.0, 131.2, 131.0, 130.2, 128.7, 128.5, 127.9, 127.8, 127.3, 127.0, 125.0, 114.1, 98.4, 83.6, 21.6 ppm; IR (KBr) ν 3055, 3022, 2207, 1614, 1493, 1396, 1375, 1358, 1251, 1173, 968, 895, 787, 760, 694 cm−1; HRMS (ESI) calcd for C34H23NO ([M + H]+) 462.1858, found 462.1869.
(4-Methoxyphenyl)(2-phenyl-1-(phenylethynyl)pyrrolo[2,1-a]isoquinolin-3-yl)methanone (3p).
Yellow solid, mp 203–204 °C; 1H NMR (CDCl3, 300 MHz) δ 9.47 (d, J = 8.1 Hz, 1H), 9.03 (d, J = 7.8 Hz, 1H), 7.72 (d, J = 7.5 Hz, 2H), 7.61–7.66 (m, 5H), 7.46–7.48 (m, 5H), 7.07–7.15 (m, 4H), 6.57 (d, J = 8.1 Hz, 2H), 3.74(s, 3H); 13C NMR (CDCl3, 75 MHz) δ 185.5, 161.5, 131.1, 130.1, 129.8, 127.5, 127.3, 126.7, 126.6, 126.0, 125.7, 123.6, 123.1, 112.6, 112.2, 111.9, 93.8, 84.5, 54.3 ppm; IR (KBr) ν 3055, 2953, 2926, 1843, 2309, 2206, 1599, 1375, 1360, 1258, 1215, 1165, 1028, 968, 895, 845, 785, 694 cm−1; HRMS (ESI) calcd for C34H23NO2 ([M + H]+) 478.1807, found 478.1807.
(4-Nitrophenyl)(2-phenyl-1-(phenylethynyl)pyrrolo[2,1-a]isoquinolin-3-yl)methanone (3q).
Red solid, mp 227–228 °C; 1H NMR (CDCl3, 300 MHz) δ 9.50 (d, J = 6.3 Hz, 1H), 9.40 (d, J = 8.1 Hz,1H), 7.85 (d, J = 8.4 Hz, 2H), 7.78 (d, J = 6.6 Hz, 2H), 7.56–7.68 (m, 6H), 7.21–7.40 (m, 6H), 7.09 (s, 2H); 13C NMR (CDCl3, 75 MHz) δ 185.1, 148.7, 145.0, 140.7, 133.1, 131.2, 130.9, 130.2, 129.0, 128.4, 128.1, 127.7, 126.9, 124.8, 124.5, 122.6, 114.8, 95.3, 84.6 ppm; IR (KBr) ν 3140, 3051, 2309, 2212, 1614, 1593, 1519, 1408, 1377, 1364, 1346, 1213, 1186, 970, 895, 851, 793 cm−1; HRMS (ESI) calcd for C33H20N2O3 ([M + H]+) 493.1552, found 493.1538.
Phenyl(2-(p-tolyl)-1-(p-tolylethynyl)pyrrolo[2,1-a]isoquinolin-3-yl)methanone (3r).
Yellow solid, mp 187–188 °C; 1H NMR (CDCl3, 300 MHz) δ 9.40 (d, J = 8.1 Hz, 1H), 9.10 (d, J = 7.5 Hz, 1H), 7.63 (d, J = 7.5 Hz, 2H), 7.56 (q, J = 7.8 Hz, 3H), 7.43 (d, J = 7.2 Hz, 2H), 7.28 (d, J = 7.8 Hz, 2H), 6.95–7.17 (m, 6H), 6.79(d, J = 7.5 Hz, 2H), 2.29(s, 3H),2.14(s, 3H); 13C NMR (CDCl3, 75 MHz) δ 186.8, 138.1, 136.9, 135.7, 133.1, 130.3, 129.8, 129.3, 128.8, 128.6, 128.1, 127.2, 127.0, 126.6, 126.5, 125.7, 124.5, 123.7, 123.2, 120.9, 119.9, 112.8, 98.3, 93.9, 83.6, 20.5, 20.2 ppm; IR (KBr) ν 3132, 3055, 3021, 2916, 2349, 2309, 2207, 1605, 1574, 1504, 1375, 1215, 970, 895, 812, 791, 735 cm−1; HRMS (ESI) calcd for C35H25NO ([M + H]+) 476.2014, found 476.2021.
p-Tolyl(2-(p-tolyl)-1-(p-tolylethynyl)pyrrolo[2,1-a]isoquinolin-3-yl)methanone (3s).
Yellow solid, mp 207–208 °C; 1H NMR (CDCl3, 300 MHz) δ 9.47 (d, J = 7.2 Hz, 1H), 9.09 (d, J = 7.2 Hz, 1H), 7.71(d, J = 6.9 Hz, 2H), 7.60 (t, J = 9.0 Hz, 2H), 7.41 (q, J = 7.4 Hz, 4H), 7.14–7.26 (m, 5H), 7.06 (d, J = 7.2 Hz, 1H), 6.88 (t, J = 9.0 Hz, 2H); 13C NMR (CDCl3, 75 MHz) δ 186.6, 141.1, 136.8, 135.6, 131.1, 129.9, 129.8, 128.9, 128.1, 127.1, 127.1, 126.9, 126.5, 125.6, 123.7, 123.2, 112.6, 111.9, 98.1, 93.8, 83.7, 20.4, 20.4, 20.1 ppm; IR (KBr) ν 3021, 2918, 2855, 2349, 2308, 2208, 2199, 1605, 1504, 1377, 1360, 1252, 1177, 970, 895, 810, 785 cm−1; HRMS (ESI) calcd for C36H27NO ([M + H]+) 490.2171, found 490.2186.
(4-Methoxyphenyl)(2-(p-tolyl)-1-(p-tolylethynyl)pyrrolo[2,1-a]isoquinolin-3-yl)methanone (3t).
Yellow solid, mp 190–191 °C; 1H NMR (CDCl3, 300 MHz) δ 9.46 (d, J = 6.9 Hz, 1H), 8.99 (d, J = 7.2 Hz, 1H), 7.69 (d, J = 6.3 Hz, 2H), 7.56 (q, J = 9.0 Hz, 3H), 7.15–7.39 (m, 5H), 7.03 (d, J = 7.2 Hz, 2H), 6.92 (d, J = 6.3 Hz, 2H), 6.56 (d, J = 7.2 Hz, 2H), 3.73 (s, 3H), 2.37 (s, 3H), 2.25 (s, 3H); 13C NMR (CDCl3, 75 MHz) δ 185.6, 161.5, 136.8, 135.6, 132.7, 131.1, 129.9, 129.8, 128.1, 127.1, 127.0, 126.5, 125.6, 123.6, 123.1, 121.1, 120.0, 112.5, 111.9, 97.8, 93.9, 83.8, 54.3, 20.5, 20.1 ppm; IR (KBr) ν 3132, 3009, 2967, 2934, 2839, 2349, 2309, 1607, 1557, 1439, 1425, 1364, 1337, 1260, 1244, 1182, 1171, 1028, 966, 878, 833, 822, 752, 619 cm−1; HRMS (ESI) calcd for C36H27NO2 ([M + H]+) 506.2120, found 506.2117.
Dimethyl 3-benzoylpyrrolo[2,1-a]isoquinoline-1,2-dicarboxy-late (3u). 15f.
1H NMR (CDCl3, 300 MHz) δ 8.78 (q, J = 6.9 Hz, 2H), 7.65 (m, 3H), 7.53 (m, 3H), 7.39 (d, J = 7.2 Hz, 2H), 7.08 (d, J = 7.8 Hz, 1H), 3.86 (s, 3H), 3.16 (s, 3H); 13C NMR (CDCl3, 75 MHz) δ 186.2, 164.9, 163.6, 138.7, 131.6, 128.5, 128.1, 127.9, 127.4, 127.3, 126.2, 124.6, 123.2, 122.6, 114.9, 51.6, 50.9.
(4-nitrophenyl)(1-pentylpyrrolo[2,1-a]isoquinolin-3-yl)metha-none (3v).
Yellow solid, mp 175–176 °C; 1H NMR (CDCl3, 300 MHz) δ 9.72 (d, J = 8.1 Hz, 1H), 8.43 (d, J = 8.1 Hz, 2H), 8.21 (d, J = 7.8 Hz, 1H), 8.01 (d, J = 8.4 Hz, 2H), 7.72–7.84 (m, 3H), 7.64 (s, 1H), 2.95 (t, J = 7.2 Hz, 2H), 1.74–1.79 (s, 2H), 1.38–1.43 (s, 2H), 1.20–1.25 (s, 2H), 0.95 (t, J = 7.1 Hz, 3H); 13C NMR (CDCl3, 75 MHz) δ 195.8, 148.9, 144.0, 130.0, 129.0, 128.5, 128.3, 128.1, 127.4, 125.7, 124.9, 123.8, 122.9, 119.2, 112.3, 40.7, 30.1, 26.1, 21.5. 13.2; IR (KBr) ν 3126, 3084, 2964, 2951, 2927, 2872, 1680, 1622, 1595, 1519, 1438, 1400, 1350, 1332, 1234, 1201, 1163, 1043, 927, 854, 813, 756, 709 cm−1; HRMS (ESI) calcd for C24H22BrN2O3 ([M + H]+) 465.0814, found 465.0819.
Acknowledgements
The work was partially supported by the National Natural Science Foundation of China (nos. 20872001, 21172001), and the Program for the NCET (NCET-10-0004) for their financial support.
References
-
(a) For a general review on pyrrolo[2-a]isoquinolines, see: H. Adams, T. M. Elsunaki, I. Ojea-Jiménez, S. Jones and A. J. H. M. Meijer, J. Org. Chem., 2010, 75, 6252 CrossRef CAS;
(b) A. K. Verma, T. Kesharwani, J. Singh, V. Tandon and R. C. Larock, Angew. Chem., Int. Ed., 2009, 48, 1138 CrossRef;
(c) J. Liang, J. Chen, J. Liu, L. Li and H. Zhang, Chem. Commun., 2010, 46, 3666 RSC;
(d) J. L. García Ruano, A. Fraile, M. R. Martín, G. González, C. Fajardo and A. M. Martín-Castro, J. Org. Chem., 2011, 76, 3296 CrossRef.
- L. L. Gundersen, C. Charnock, A. H. Negussie, F. Rise and S. Teklu, Eur. J. Pharm. Sci., 2007, 30, 26 CrossRef CAS.
- T. Weide, L. Arve, H. Prinz, H. Waldmann and H. Kessler, Bioorg. Med. Chem. Lett., 2006, 16, 59 CrossRef CAS.
- S. Teklu, L. L. Gundersen, T. Larsen, K. E. Malterud and F. Rise, Bioorg. Med. Chem., 2005, 13, 3127 CrossRef CAS.
-
C. J. Cavallito, A. P. Gray, Fr. Pat., 2135297, 1973 Search PubMed.
- B. E. Maryanoff, D. F. McComsey, J. F. Gardocki, R. P. Shank, M. J. Costanzo, S. O. Nortey, C. R. Schneider and P. E. Setler, J. Med. Chem., 1987, 30, 1433 CrossRef CAS.
- E. Marco, W. Laine, C. Tardy, A. Lansiaux, M. Iwao, F. Ishibashi, C. Bailly and F. Gago, J. Med. Chem., 2005, 48, 3796 CrossRef CAS.
- J. Kluza, M. A. Gallego, A. Loyens, J. C. Beauvillain, J. M. Fernandez Sousa-Faro, C. Cuevas, P. Marchetti and C. Bailly, Cancer Res., 2006, 66, 3177 CrossRef CAS.
- M. V. R. Reddy, M. R. Rao, D. Rhodes, M. S. T. Hansen, K. Rubins, F. D. Bushman, Y. Venkateswarlu and D. J. Faulkner, J. Med. Chem., 1999, 42, 1901 CrossRef CAS.
-
(a) S. P. Gupta, A. N. Mathur, A. N. Nagappa, D. Kumar and S. Kumaran, Eur. J. Med. Chem., 2003, 38, 867 CrossRef CAS;
(b) C. Poty, V. Gibon, G. Evrard, B. Norberg, D. P. Vercauteren, J. Gubin, P. Chatelain and F. Durant, Eur. J. Med. Chem., 1994, 29, 911 CrossRef CAS.
-
(a) O. B. Østby, B. Dalhus, L. L. Gundersen, F. Rise, A. Bast and G. R. M. M. Haenen, Eur. J. Org. Chem., 2000, 3763 CrossRef;
(b) L. L. Gundersen, K. E. Malterud, A. H. Negussie, F. Rise, S. Teklu and O. B. Ostby, Bioorg. Med. Chem., 2003, 11, 5409 CrossRef CAS.
-
(a) See for examples Y. Liu, Y. Zhang, Y. M. Shen, H. W. Hu and J. H. Xu, Org. Biomol. Chem., 2010, 8, 2449 RSC;
(b) I. Yavari, M. Piltan and L. Moradi, Tetrahedron, 2009, 65, 2067 CrossRef CAS;
(c) A. Alizadeh and N. Zohreh, Synthesis, 2008, 429 CrossRef CAS;
(d) S. Su and Jr. J. A. Porco, J. Am. Chem. Soc., 2007, 129, 7744 CrossRef CAS;
(e) D. J. St. Cyr, N. Martin and B. A. Arndtsen, Org. Lett., 2007, 9, 449 CrossRef;
(f) I. V. Seregin and V. Gevorgyan, J. Am. Chem. Soc., 2006, 128, 12050 CrossRef CAS;
(g) P. Ploypradith, T. Petchmanee, P. Sahakitpichan, N. D. Litvinas and S. Ruchirawat, J. Org. Chem., 2006, 71, 9440 CrossRef CAS;
(h) D. Pla, D. Marchal, C. A. Olsen, A. Francesch, C. Cuevas, F. Albericio and M. Alvarez, J. Med. Chem., 2006, 49, 3257 CrossRef CAS;
(i) T. Yamaguchi, T. Fukuda, F. Ishibashi and M. Iwao, Tetrahedron Lett., 2006, 47, 3755 CrossRef CAS;
(j) I. Yavari, Z. Hossaini and M. Sabbaghan, Tetrahedron Lett., 2006, 47, 6037 CrossRef CAS.
-
(a) M. Ber. Dtsch. Scholtz, Ber. Dtsch. Chem. Ges., 1912, 45, 734 CrossRef CAS;
(b) V. Boekelheide and R. J. Jr. Windgassen, J. Am. Chem. Soc., 1959, 81, 1456 CrossRef CAS.
-
(a) A. E. Ber. Dtsch. Tschitschibabin, Ber. Dtsch. Chem. Ges., 1927, 60, 1607 CrossRef;
(b) J. Hurst, T. Melton and D. G. Wibberley, J. Chem. Soc., 1965, 2948 RSC;
(c) For a one-pot reaction, see U. Bora, A. Saikia and R. C. Boruah, Org. Lett., 2003, 5, 435 CrossRef CAS.
-
(a) Y. Miki, H. Hachiken, S. Takemura and M. Ikeda, Heterocycles, 1984, 22, 701 CrossRef CAS;
(b) G. Poissonnet, M. H. Theret-Bettiol and R. H. Dodd, J. Org. Chem., 1996, 61, 2273 CrossRef CAS;
(c) A. R. Katritzky, G. Qiu, B. Yang and H. Y. He, J. Org. Chem., 1999, 64, 7618 CrossRef CAS;
(d) X. Fang, Y. M. Wu, J. Deng and S. W. Wang, Tetrahedron, 2004, 60, 5487 CrossRef CAS;
(e) Y. C. Shen, Y. M. Zhang and J. Sun, J. Fluorine Chem., 2002, 116, 157 CrossRef CAS;
(f) A. Hazra, S. Mondal, A. Maity, S. Naskar, P. Saha, R. Paira, K. B. Sahu, P. Paira, S. Ghosh, C. Sinha, A. Samanta, S. Banerjee and N. B. Mondal, Eur. J. Med. Chem., 2011, 46, 2132 CrossRef CAS;
(g) E. Kianmehr, H. Estiri and A. Bahreman, J. Heterocycl. Chem., 2009, 46, 1203 CrossRef CAS;
(h) F. Dumitrascu, M. R. Caira, E. Georgescu, F. Georgescu, C. Draghici and M. M. Popa, Heteroat. Chem., 2011, 22, 723 CrossRef CAS.
-
(a) T. Sasaki, K. Kanematsu, A. Kakehi and G. Ito, Tetrahedron, 1972, 28, 4947 CrossRef CAS;
(b) E. Pohjala, Tetrahedron Lett., 1972, 13, 2585 CrossRef.
-
(a) C. G. Yu, Y. N. Zhang, S. L. Zhang, H. Li and W. Wang, Chem. Commun., 2011, 47, 1036 RSC;
(b) Y. Han, H. Hou, Q. Fu and C. G. Yan, Tetrahedron, 2011, 67, 2313 CrossRef CAS;
(c) Y. X. Jia, J. Zhong, S. F. Zhu, C. M. Zhang and Q. L. Zhou, Angew. Chem., Int. Ed., 2007, 46, 5565 CrossRef CAS.
- O. Tsuge, S. Kanemasa, S. Kuraoka and S. Takenaka, Chem. Lett., 1984, 13, 279 CrossRef.
- Y. J. Shang, M. Zhang, S. Y. Yu, K. Ju, C. E. Wang and X. W. He, Tetrahedron Lett., 2009, 50, 6981 CrossRef CAS.
- Crystallographic data for 3d: space group P
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 9.1922(11) Å, b = 10.0794(12) Å, c = 11.4797(13) Å, α = 105.7280(10)°, β = 97.6090(10)°, γ = 99.7730(10)°, V = 990.6(2) Å3, T = 293(2)K, Z = 2. Crystallographic data for compound 3d (CCDC 847772) reported in this paper can be found in the ESI†.
, a = 9.1922(11) Å, b = 10.0794(12) Å, c = 11.4797(13) Å, α = 105.7280(10)°, β = 97.6090(10)°, γ = 99.7730(10)°, V = 990.6(2) Å3, T = 293(2)K, Z = 2. Crystallographic data for compound 3d (CCDC 847772) reported in this paper can be found in the ESI†.
- Crystallographic data for 3t: space group P21/c, a = 12.4387(9) Å, b = 18.3179(14) Å, c = 11.7836(9) Å, α = 90°, β = 94.4610(10)°, γ = 90°, V = 2676.8(3) Å3, T = 293(2)K, Z = 4. Crystallographic data for compound 3t (CCDC 847773) reported in this paper can be found in the ESI†.
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here. 

 1a
1a
 2a
2a
 3a
3a
 1b
1b
 3b
3b
 1c
1c
 3c
3c
 1d
1d
 3d
3d
 2b
2b
 3e
3e
 3f
3f
 3g
3g
 2c
2c
 3h
3h
 3i
3i
 3j
3j
 2d
2d
 3k
3k
 3l
3l
 3m
3m
 2e
2e
 3n
3n
 3o
3o
 3p
3p
 1e
1e
 3q
3q
 2f
2f
 3r
3r
 3s
3s
 3t
3t
 2g
2g
 3u
3u
 1f
1f
 2h
2h
 3v
3v


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) to afford the corresponding pyrrolo[2,1-a]isoquinolines 3.
1, v/v) to afford the corresponding pyrrolo[2,1-a]isoquinolines 3.
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 9.1922(11) Å, b = 10.0794(12) Å, c = 11.4797(13) Å, α = 105.7280(10)°, β = 97.6090(10)°, γ = 99.7730(10)°, V = 990.6(2) Å3, T = 293(2)K, Z = 2. Crystallographic data for compound 3d (CCDC 847772) reported in this paper can be found in the ESI†.
, a = 9.1922(11) Å, b = 10.0794(12) Å, c = 11.4797(13) Å, α = 105.7280(10)°, β = 97.6090(10)°, γ = 99.7730(10)°, V = 990.6(2) Å3, T = 293(2)K, Z = 2. Crystallographic data for compound 3d (CCDC 847772) reported in this paper can be found in the ESI†.