One-pot synthesis of luminescent hydrophilic silicon nanocrystals
Dezhi
Tan
a,
Beibei
Xu
a,
Ping
Chen
a,
Ye
Dai
b,
Shifeng
Zhou
a,
Guohong
Ma
b and
Jianrong
Qiu
*ac
aState Key Laboratory of Silicon Materials, Zhejiang University, Hangzhou, Zhejiang, 310027, P. R. China
bDepartment of Physics, Shanghai University, Shanghai, 200444, P. R. China
cState Key Laboratory of Luminescence Physics and Chemistry and the Institute of Optical Communication Materials, South China University of Technology, Guangzhou, Guangdong 510640, P. R. China. E-mail: qjr@zju.edu.cn
First published on 17th July 2012
Abstract
A facile one-pot method of femtosecond laser ablation in solution to synthesize hydrophilic silicon nanocrystals is presented in this communication. These silicon nanoscrystals emit colorful photoluminescence when excited at different wavelengths. This simple method has potential applications in the preparation of luminescent silicon nanocrystals passivated with different organic molecules.
Silicon nanocrystals (SiNCs) have attracted great attention due to their size-dependent optical and electronic properties, high photostability, brightness, suitability for biological imaging and the wide spectrum of promising applications in optoelectronic devices in the past decades.1–6 The quantum confinement effect in SiNCs, with sizes ranging from 1–5 nm, can cause a partial relaxation of the selection rules. These SiNCs behave more like direct-gap semiconductors with high luminescent efficiency and short photoluminescence lifetimes (in the order of nanoseconds), which are very important for practical applications.5 Compared with organic dye molecules, the better stability of photoluminescence against photobleaching makes the luminescent SiNCs a better choice for bio-applications.5,6 Due to the lower inherent toxicity and better biocompatibility of SiNCs over that of cadmium-containing quantum dots, such as CdSe/ZnS, SiNCs provide a better alternative for a technological union between biochemistry and electronics.6,7 Hence, the synthesis of luminescent SiNCs is currently an area of intense research.
Usually, surface passivation through the attachment of organic molecules to the surface of SiNCs is utilized to improve the stability of the photoluminescence and enhance their solubility in solvents. Recently, a variety of techniques have been developed to fabricate passivated luminescent SiNCs.5,6,8–14 SiNCs are usually synthesized through either the laser pyrolysis of silane, electrochemical etching with an HF-containing solution, thermal processing of silsesquioxanes followed by HF etching, or solution reductions in inverse micelles.5,6,8–13 Following this, Pt catalysis or UV/thermal hydrosilylation are adopted to functionalize the obtained SiNC cores with organic molecules.5,6,8–13 However, most of these methods require multiple steps and are time consuming. Some one-step methods have been reported to prepare surface passivated SiNCs.14–17 Unfortunately, all these procedures were carried out under oxygen-free conditions and high temperatures are also needed in some cases.16,17 In addition, some of the required materials are complicated, highly reactive or corrosive and even toxic. Moreover, many of these prepared SiNCs are hydrophobic. While hydrophilicity is necessary for the bio-applications.5,6 It is still a challenge to develop a versatile and simple route to produce nontoxic hydrophilic SiNCs.
In this work, we develop a one-step method utilizing femtosecond (fs) laser ablation in solution to fabricate hydrophilic luminescent SiNCs under ambient conditions. No complicated processes, such as oxygen-free conditions, or corrosive and toxic materials, such as HF− and Pt2+-containing solutions, are required. The absence of ions, such as F− and Pt2+, which may be hard to removed completely and are toxic to cells, is very important.
As a versatile and cheap method, pulsed laser ablation in solution has been of great interest for the synthesis of many kinds of nanostructures.15,18–23 As the full width at half maximum of the laser pulse is usually very short, the energy can be released out to the local environment between the interface of the target and the solution in a short time, especially when a fs laser is used as the irradiation source. A plasma plume with a high temperature and pressure is produced, as shown in Fig. 1(A, B).18,19 Under these far-from-equilibrium conditions, the species from the target and the solution are very active and many new bonds are formed.18–21 Si–C bonds can be generated as shown in Fig. 1(C). Therefore, the silicon core synthesis and surface passivation can be done in one step. Furthermore, this investigation implies that fs laser ablation in solution may be a general way to fabricate SiNCs functionalized with different organic molecules and these SiNCs show different optical properties. The control of the surface passivation and optical properties will be helpful for theoretical and application investigations.
 | ||
| Fig. 1 Schematic illustration of fs laser ablation in solution and the synthesized surface passivated SiNCs. | ||
The overall synthetic procedure of fs laser ablation in solution is illustrated in Fig. 1. A bulk silicon plate was placed in a glass vessel with a solution of acrylic acid/ethanol (1/1, v/v). Fs pulses (half width at maximum: 120 fs; wavelength: 800 nm; repetition: 1 KHz and pulse energy: 0.5 mJ pulse−1), provided by a Ti/sapphire laser system (Hurricane, Spectra Physics Lasers), was focused on the target for 1 h. The detailed and main formation mechanism of carbon functionalization on the surface of SiNCs has been discussed in our previous investigation.21
Transmission electron microscopy (TEM) and high resolution transmission electron microscopy (HRTEM) were performed on a Tecnai G2 F30 S-Twin high resolution transmission electron microscope at 300 kV and applied to investigate the structure and size distributions of the SiCNs. A typical TEM image of SiNCs passivated with acrylic acid is shown in Fig. 2. The TEM image shows that the SiNCs synthesized by fs laser ablation in an acrylic acid/ethanol solution are monodispersed, which may result from the good surface passivation. The inset (left) illustrates that these silicon nanocrystals have a size distribution of 1.90 ± 0.44 nm, determined from the TEM images. Clear crystal lattice planes can be seen in the HRTEM image of one silicon nanocrystal (inset in Fig. 2 (right)). The lattice spacing of 0.21 nm can be assigned to the plane of cubic crystalline silicon.13,15
 | ||
| Fig. 2 Typical TEM image of acrylic acid passivated SiNCs synthesized by fs laser ablation. Inset: size distribution (left); HRTEM image of a silicon nanocrystal (right). | ||
The FTIR spectrum was analyzed to investigate the surface state of the SiNCs. Fig. 3 shows the FTIR spectrum of surface passivated SiNCs. The strong absorption peak at 1724 cm−1 can be assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration mode.11 The peak at 1411 cm−1 is due to the C–O stretch coupled with O–H bending vibration modes.11,24 The C–O stretching vibration mode absorption can also be observed at 1193 cm−1.11 Two peaks observed at 1460 and 1265 cm−1 are attributed to the scissoring and stretching vibration modes of Si–C bonds, which indicate that the surface of the SiNCs are passivated by carbon.10,13,15,23 Si–O vibration mode absorption at 812, 986, 1063 cm−1 can be observed, implying that the SiNCs are partially oxidized.12,14,15,23
O stretching vibration mode.11 The peak at 1411 cm−1 is due to the C–O stretch coupled with O–H bending vibration modes.11,24 The C–O stretching vibration mode absorption can also be observed at 1193 cm−1.11 Two peaks observed at 1460 and 1265 cm−1 are attributed to the scissoring and stretching vibration modes of Si–C bonds, which indicate that the surface of the SiNCs are passivated by carbon.10,13,15,23 Si–O vibration mode absorption at 812, 986, 1063 cm−1 can be observed, implying that the SiNCs are partially oxidized.12,14,15,23
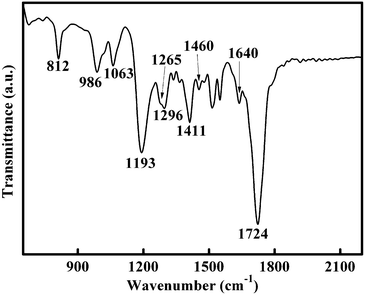 | ||
| Fig. 3 FTIR spectrum of SiNCs passivated by acrylic acid. | ||
In order to determine the surface state of SiNCs further, Si2p (a) and C1s (b) XPS spectra were recorded, as shown in Fig. S1.† Si2p XPS spectra show two dominant peaks at 103.4 and 102.3 eV, as illustrated in Fig. S1(a). The peak at 103.4 eV can be assigned to Si in the Si–O bonds.10,25 The 102.3 eV peak is due to the Si–(C,O) environment23,25 or Si–C bonds10,26 on the surface of the SiNCs, which suggests that surface passivation occurs on the SiNC surface. Three peaks can be observed in the C1s XPS spectrum after Gauss fitting at 284.8, 285.9, 289.2 eV in Fig. S1(b). The peak of 289.2 eV is characteristic of the carboxylic acid groups.6,26 The peak at 285.9 eV can be attributed to Si–C bonds21 and the peak at 284.8 eV can be ascribed to carbon bonded to carbon or hydrogen.10,26
The FTIR and XPS spectra show that SiNCs prepared by fs laser ablation in an acrylic acid/ethanol solution are functionalized by acrylic acid. The end-group of acrylic acid (carboxyl) makes these SiNCs water soluble, which is very important for their practical applications.5,6 Compared with our previous investigation, our further work here indicates that fs laser ablation in solution can be a general procedure to prepare luminescent SiNCs functionalized with different organic molecules.21
The UV–vis absorption spectrum and emission spectra of SiNCs re-dispersed in water under various excitation wavelengths ranging from 320 to 560 nm are shown in Fig. 4. The emission spectra were recorded on an FLS920 fluorescence spectrophotometer. A broad continuous and featureless absorption between 300–700 nm can be observed in the absorption spectrum. The emission spectra illustrate that the maximum intensity of photoluminescence shifts to longer wavelengths with an increase of the excitation wavelength. The maximal emission center at 474 nm can be obtained when the excitation wavelength is 380 nm. As water soluble SiNCs have a wide size distribution, longer wavelengths will excite the larger silicon nanocrystals and give rise to a red-shift of the emission as the excitation wavelength is increased, which is consistent with our observation from the emission spectra (Fig. 4). Theoretical and experimental investigations suggested that hydrogen or carbon surface terminated SiNCs with sizes in the range of 1–2 nm can emit blue photoluminescence through direct band gap transitions, which are agree with the results in this case.5,27 The photoluminescence of larger SiNCs shifts to green or even the yellow region, as shown in Fig. 4. Then, though the mechanism of the SiNC photoluminescence is complicated, the broad photoluminescence here may mainly originate from the quantum confinement effect. Bright and colorful photoluminescence can be observed when the particles are excited by lasers with different wavelengths, as seen in the inset of Fig. 4. The photoluminescence is stable for at least 6 months.
 | ||
| Fig. 4 Absorption and emission spectra, with excitation wavelengths ranging from 320 to 560 nm, of SiNCs re-dispersed in water. Inset: photographs of SiNCs re-dispersed in water and excited at 375, 405, 457, 532 nm (respectively, from left to right). | ||
The time-resolved photoluminescence decay is shown in Fig. 5. The photoluminescence decay requires a two-exponential fit with an overall lifetime of 7.8 ns. As an indirect band gap semiconductor, silicon usually shows slow recombination, while the strong quantum confinement effect can increase the possibility of direct band gap transitions and reduce indirect band gap transitions, which leads to a shorter lifetime, even to the nanosecond scale.5,27,28 Here, we propose that the photoluminescence of silicon nanocrystals originates from dipole-allowed radiative recombinations through direct band gap transitions, determined by quantum confinement effects.
 | ||
| Fig. 5 Time-resolved photoluminescence decay of acrylic acid passivated SiNCs in water recorded at an emission wavelength of 474 nm with an excitation wavelength of 380 nm. | ||
Conclusions
In summary, we developed a new and facile method, fs laser ablation in solution, that does not require harsh conditions, for the synthesis of luminescent hydrophilic SiNCs. These passivated hydrophilic SiNCs show colourful photoluminescence when excited at different wavelengths. We suggest that this size-dependent photoluminescence originates from the quantum confinement effect. This technique provides a more environmentally friendly and easier route than other methods to achieve the synthesis and surface passivation of hydrophilic SiNCs in one step. No harsh experimental conditions, such as an oxygen-free environment, toxic chemicals or even the assistance of hydrogen-terminated intermediate, are required. Furthermore, fs laser ablation in solution holds potential applications in the preparation of luminescent SiNCs passivated with different organic molecules. The luminescent hydrophilic SiNCs prepared through this simple procedure can find numerous uses in many fields, such as biological sensing, biological imaging, etc.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (grant No. 50872123, 50802083, 51072054 and 51102209), the National Basic Research Program of China (2011CB808100). Y. Dai acknowledges financial support from the National Natural Science Foundation of China (grant No. 60908007).References
- L. Pavesi, L. Dal Negro, C. Mazzoleni, G. Franz and F. Priolo, Nature, 2000, 408, 440 CrossRef CAS.
- D. P. Puzzo, E. J. Henderson, M. G. Helander, Z. B Wang, G. A. Ozin and Z. H. Lu, Nano Lett., 2011, 11, 1585 CrossRef CAS.
- C. Y. Liu, Z. C. Holman and U. R. Kortshagen, Nano Lett., 2009, 9, 449 CrossRef CAS.
- R. J. Walters, G. I. Bourianoff and H. A. Atwater, Nat. Mater., 2005, 4, 143 CrossRef CAS.
- J. H. Warner, A. Hoshino, K. Yamamoto and R. D. Tilley, Angew. Chem. Int. Ed., 2005, 117, 4626 CrossRef.
- Z. F. Li and E. Ruckenstein, Nano Lett., 2004, 4, 1463 CrossRef CAS.
- A. M. Derfus, W. C. W. Chan and S. N. Bhatia, Nano Lett., 2004, 4, 11 CrossRef CAS.
- F. Huisken, G. Ledoux, O. Guillois and C. Reynaud, Adv. Mater., 2002, 14, 1861 CrossRef CAS.
- F. Erogbogbo, K. T. Yong, I. Roy, R. Hu, W. C. Law, W. W. Zhao, H. Ding, F. Wu, R. Kumar, M. T. Swihart and P. N. Prasad, ACS Nano, 2011, 5, 413 CrossRef CAS.
- C. M. Hessel, M. R. Rasch, J. L. Hueso, B. W. Goodfellow, V. A. Akhavan, P. Puvanakrishnan, J. W. Tunnel and B. A. Korgel, Small, 2010, 6, 2026 CrossRef CAS.
- S. Sato and M. T. Swihart, Chem. Mater., 2006, 18, 4083 CrossRef CAS.
- K. Kůsová, O. Cibulka, K. Dohnalová, I. Pelant, J. Valenta, A. Fucŭíková, K. Žídek, J. Lang, J. Englich, P. Matějka, P. Štěpánek and S. Bakardjieva, ACS Nano, 2010, 4, 4495 CrossRef.
- R. D. Tilley, J. H. Warner, K. Yamamoto, I. Matsui and H. Fujimori, Chem. Commun., 2005, 1833 RSC.
- A. S. Heintz, M. J. Fink and B. S. Mitchell, Adv. Mater., 2007, 19, 3984 CrossRef CAS.
- N. Shirahata, M. R. Linford, S. Furumi, L. Pei, Y. Sakka, R. J. Gates and M. C. Asplund, Chem. Commun., 2009, 4684 RSC.
- B. A. Manhat, A. L. Brown, L. A. Black, J. B. A. Ross, K. Fichter, T. Vu, E. Richman and A. M. Goforth, Chem. Mater., 2011, 23, 2407 CrossRef CAS.
- Y. He, Y. L. Zhong, F. Peng, X. P. Wei, Y.Y. Su, Y. M. Lu, S. Su, W. Gu, L. S. Liao and S. T. Lee, J. Am. Chem. Soc., 2011, 133, 14192 CrossRef CAS.
- D. Z. Tan, G. Lin, Y. Liu, Y. Teng, Y. X. Zhuang, B. Zhu, Q. Z. Zhao and J. R. Qiu, J. Nanopart. Res., 2011, 13, 1183 CrossRef CAS.
- G. W. Yang, Prog. Mater. Sci., 2007, 52, 648 CrossRef CAS.
- P. Liu, Y. Liang, X. Z. Lin, C. X. Wang and G. W. Yang, ACS Nano, 2011, 5, 4748 CrossRef CAS.
- D. Z. Tan, Z. J. Ma, B. B. Xu, Y. Dai, G. H. Ma, M. He, Z. M. Jin and J. R. Qiu, Phys. Chem. Chem. Phys., 2011, 13, 20255 RSC.
- S. L. Hu, Y. G. Dong, J. L. Yang, J. Liu and S. R. Cao, J. Mater. Chem., 2012, 22, 1957 RSC.
- H. B. Zeng, X. W. Du, S. C. Singh, S. A. Kulinich, S. K. Yang, J. P. He and W. P. Cai, Adv. Funct. Mater., 2012, 22, 1333 CrossRef CAS.
- J. Dong, Y. Ozaki, K. Nakashima and J. Polym, Sci. Pol. Phys., 1998, 35, 507 Search PubMed.
- M. J. L. Portolés, F. R. Nieto, D. B. Soria, J. I. Amalvy, P. J. Peruzzo, D.O. Mártire, M. Kotler, O. Holub and M. C. Gonzalez, J. Phys. Chem. C, 2009, 113, 13694 Search PubMed.
- Q. Wang, H. J. Ni, A. Pietzsch, F. Hennies, Y. P. Bao and Y. M. Chao, J. Nanopart. Res., 2011, 13, 405 CrossRef CAS.
- Z. Y. Zhou, L. Brus and R. Friesner, Nano Lett., 2003, 3, 163 CrossRef CAS.
- D. S. English, L. E. Pell, Z. H. Yu, P. F. Barbara and B. A. Korgel, Nano Lett., 2002, 2, 681 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra21044d/. |
| This journal is © The Royal Society of Chemistry 2012 |
