From magnetotactic bacteria to hollow spirilla-shaped silica containing a magnetic chain†
Jens
Baumgartner
a,
Paul
Lesevic
a,
Monika
Kumari
b,
Karin
Halbmair
ac,
Mathieu
Bennet
a,
André
Körnig
a,
Marc
Widdrat
a,
Janet
Andert
a,
Markus
Wollgarten
d,
Luca
Bertinetti
a,
Peter
Strauch
c,
Ann
Hirt
b and
Damien
Faivre
*a
aDepartment of Biomaterials, Max Planck Institute of Colloids and Interfaces, Science Park Golm, 14424, Potsdam, Germany. E-mail: damien.faivre@mpikg.mpg.de; Fax: +49 331 567 9402; Tel: +49 331 567 9405
bInstitute of Geophysics, ETH-Zürich, Zürich, Switzerland
cInstitut für Chemie, Üniversität Potsdam, Karl-Liebknecht-str. 24-25, 14476, Potsdam, Germany
dHelmholtz Zentrum Berlin für Materialien und Energie, Bereich Solarenergieforschung, Institut für Technologie, Hahn-Meitner-Platz 1, 14109, Berlin, Germany
First published on 17th July 2012
Abstract
Magnetotactic bacteria produce chains of magnetite nanoparticles, which are called magnetosomes and are used for navigational purposes. We use these cells as a biological template to prepare a hollow hybrid material based on silica and magnetite, and show that the synthetic route is nondestructive as the material conserves the cell morphology as well as the alignment of the magnetic particles. The hybrid material can be resuspended in aqueous solution, and can be shown to orient itself in an external magnetic field. We anticipate that chemical modification of the silica can be used to functionalize the material surface in order to obtain multifunctional materials with specialized applications, e.g. targeted drug delivery.
Introduction
The organization and functionalization of individual building blocks into hierarchically ordered nanomaterials has received much attention in recent years. The structuring of individual entities into hierarchical structures gives rise to function, as beautifully exemplified in biological materials.1 One-dimensional magnetic nanostructures are of particular interest in terms of both fundamental and practical aspects, e.g., their use as micromechanical sensors.2,3 The following techniques are currently used to form such materials: templating,4,5 self-assembly,6 or a mixture of both.7Research on hollow particles has intensified as their physicochemical properties differ significantly from those of bulk materials.8 Potential applications, specifically for composite or core-shell hollow spheres, include drug-delivery carriers or nanoreactors.8,9 Biological templates and specifically microorganisms represent an interesting approach for obtaining these structures.10 Coupling both, a magnetic chain within hollow particles in a facile synthesis method can open doors to new functional materials.
Complex, often hierarchially-structured, biogenic minerals have evolved from naturally optimized processes.1,11 Biopolymeric templates, such as proteins, generally control both mineralization and properties of the inorganic components, because biomineralization processes are genetically driven.11,12 However, biominerals cannot directly lead to the generation of materials with specific technical properties, therefore further treatment is required to form hybrid functional materials that can be used for a variety of applications.13
Magnetotactic bacteria are one example of a biomineralizing organism that form hybrid materials with properties that can so far not be matched by synthetic approaches. These simple cells have the ability to orient and navigate in the magnetic field of the Earth with the help of special magnetic inclusions called magnetosomes.14 Magnetosomes are membrane-enclosed, nano-sized crystals of a magnetic mineral, typically magnetite (Fe3O4), which can be assembled in chains along the cell axis. The hierarchical structuring of the magnetosome chain starts with the formation of structurally pure magnetite nanoparticles, and follows with the organization of stable single domain-sized magnetic particles in single or multiple chains or clusters; these give rise to enhanced magnetic properties.15 The organisms are therefore a paradigm of hierachical magnetic chains. However, while several bio- and nanotechnological applications have been envisaged for the isolated magnetosomes,16,17 less have been proposed for whole cells,18 partly because of possible undesirable immunogenic responses.
In this study, we have used magnetotactic bacteria (Magnetospirillum gryphiswaldense MSR-1) as a template to synthesize a composite material made up of a hierarchically-structured magnetic chain in a hollow silica particle. The bacteria are first grown, then embedded in silica following the Stöber process,19 and the material is then calcined to remove any organic content. The composite material is characterized by an integrated analytical approach, including electron microscopy (TEM and SEM), synchrotron X-ray diffraction (XRD), and magnetic measurements [ferromagnetic resonance (FMR), hysteresis loops and analysis of first order reversal curves (FORC)]. The structures obtained can be redispersed in aqueous solutions and keep their ability to orient in an external magnetic field as shown by optical microscopy.
Results and discussion
Cells of Magnetospirillum gryphiswaldense (Fig. 1c) were used as a template. The synthesis process formed silica in the shape of long hollow tubes in the order of several microns, which covered the bacteria completely (Fig. 1a). This is consistent with the typical dimensions of these bacteria that are generally 3 μm long and 0.5 μm in diameter.14 The average thickness of the silica coating was approximately 100 nm (Fig. 1b), also seen from a thin cut of the embedded material under TEM (supplementary material†). This thickness corresponds to the lower end of size obtained by the Stöber process, which typically produces tubes that vary between 50 nm to 2 μm in length. The morphology of the chain was not affected as seen from the shape of the silica shell (Fig. 1a), and a STEM image of the chain within the shell (Fig. 1b).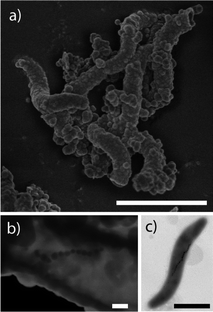 | ||
| Fig. 1 Summary of representative electron microscope images (scale bars 1μm in a and c and 100 nm in b). (a) HRSEM image of the materials, (b) STEM image of the materials, (c) TEM image of a magnetotactic bacterium used as the template. | ||
In order to verify if the nanoparticles that were observed within the hollow silica in the EM images were magnetite, XRD was performed. Fig. 2 shows an XRD diffractogram. All peaks could be assigned to magnetite, thus demonstrating that the chemical compostion was not affected in the synthesis process.
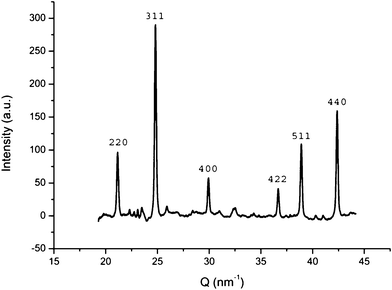 | ||
| Fig. 2 indexed X-ray diffractogram without baseline showing the presence of magnetite in the sample. | ||
The magnetic properties of the material were characterized by FMR, hysteresis loops and FORC analysis, in order to confirm that the hierarchical structure of the chain based on stable single domain size of magnetite nanoparticles was not lost during processing. FMR spectroscopy is a specific application of the classical electron spin resonance (ESR) spectroscopy in which coupled spins of the magnetically ordered phase are detected.20 FMR has been extensively used to detect single-domain magnetic particles aligned in chains in magnetotactic bacteria.21,22 The effective splitting factor (geff) and asymmetry ratio (A) parameters, which are obtained from the spectra, are used to describe the properties of a sample. A value of geff < 2.12 together with 0 < A < 1 indicate a configuration of stable single domain magnetic particles that are arranged in a chain.21 The spectrum of the material, which is typically shown as the first derivative of a spectrum, is presented in Fig. 3a, and depicts geff = 1.91 and A = 0.46. Therefore, FMR supports the presence of stable single-domain magnetic particles of magnetite together with an anisotropic signal due to the presence of chains.
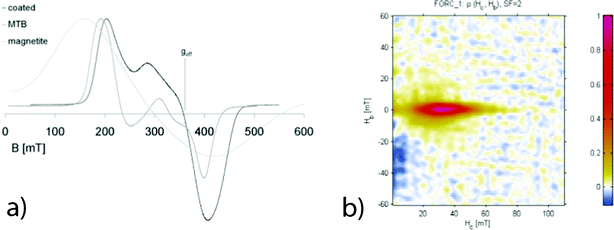 | ||
| Fig. 3 a) Comparison of first derivative FMR spectra for the synthesized material (black), magnetotactic bacteria (dark grey) and synthetic magnetite nanoparticles (light grey). The spectrum for the synthesized materials clearly resembles that of magnetotactic bacteria. b) FORC diagram with smoothing factor 2 for a sample of silica-coated bacteria indicating the presence of non-interacting single-domain particles. For details, see text. | ||
Hysteresis measurements (supplementary material†) showed an open loop with a ratio of remanent to saturation magnetization (Mr/Ms) of 0.38, while the backfield curve was saturated at around 100 mT. This indicates the presence of a low coercive, crystalline magnetic mineral, probably magnetite. The ratio between remanence coercivity and coercivity (Hcr/Hc) is 1.4. The FORC distribution was bimodal with contributions from the narrow and elongated contours, indicating the presence of non-interacting, single-domain particles and a sharp peak at the Hc = 0 axis, showing the presence of superparamagnetic particles.23,24 The FORC distribution showed coercivity ranging from 0 to 80 mT and peak coercivity at 32 mT, whereas the hysteresis loop showed an average coercivity of 23 mT. The superparamagnetic contribution can therefore be attributed to nascent magnetosomes as they typically occur at the chain ends. The FORC diagram also displayed a pronounced negative region close to Hc = 0 and in the negative Hb region indicative of non-interacting single-domain grains.25 The values of the remanence and coercivity ratios deviate slightly from those reported for magnetosomes in intact chains.26 This difference can be attributed to the presence of superparamagnetic particles. All magnetic properties were thus fully compatible with the magnetosomes with chains.
Further confirmation for the preservation of chain structure within the silica coating is seen by placing the synthesized material in an external magnetic field. Single isolated magnetosomes should have no preferred orientaion of their crystallographic axes within the silica shell, and therefore should show no preferential alignment. Chains of magnetite, however, will display a strong anisotropy that causes them to align with a field. When the material was redispersed in an aqueous solution and placed in a 50 mT field, the long axes of the chain followed the orientation of the external field (Fig. 4).
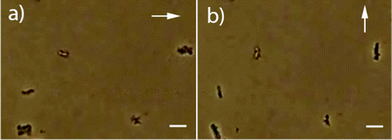 | ||
| Fig. 4 Optical microscopy images of the resuspended materials in external fields (direction indicated by the white arrows, scale bars: 5 μm). | ||
Conclusions
In summary, the present work demonstrates the one step synthesis of hybrid hollow silica/magnetic microparticles from a magnetotactic bacteria precursor. The synthetic route has a clear advantage over alternative techniques in that it can be performed in a single stage and does not require the use of an external magnetic field for assembly.27,28 In addition, the magnetic dipole consists of a chain of stable single-domain particles with a high remanent magnetization that is more responsive to external fields than superparamagnetic particles. Finally, the properties of the shell should allow for further functionalization. We thus anticipate our synthetic route to be the initial step towards multifunctional materials with applications, for example, in targeted drug delivery.Acknowledgements
The authors thank Heike Runge for technical assistance. Research for this study was supported by the Max Planck Society, the DFG (SPP 1420) and the ERC (Starting Grant 256915-MB2).References
- P. Fratzl and R. Weinkamer, Prog. Mater. Sci., 2007, 52, 1263–1334 CrossRef CAS.
- J. Yuan, Y. Xu and A. H. E. Müller, Chem. Soc. Rev., 2011, 40, 640–655 RSC.
- H. Wang, Y. Yu, Y. Sun and Q. Chen, Nano, 2011, 6, 1–17 CrossRef.
- I. A. Banerjee, L. Y. M. Shima, T. Yoshino, H. Takeyama, T. Matsunaga and H. Matsui, Adv. Mater., 2005, 17, 1128–1131 CrossRef CAS.
- X. Yan, G. Liu, F. Liu, B. Z. Tang, H. Peng, A. B. Pakhomov and C. Y. Wong, Angew. Chem., Int. Ed., 2001, 40, 3593–3596 CrossRef CAS.
- Z. Nie, A. Petukhova and E. Kumacheva, Nat. Nanotechnol., 2010, 5, 15–25 CrossRef CAS.
- J. Zhou, L. Meng, X. Feng, X. Zhang and Q. Lu, Angew. Chem. Int. Ed., 2010, n/a-n/a Search PubMed.
- J. Bertling, J. Blömer and R. Kümmel, Chem. Eng. Technol., 2004, 27, 829–837 CrossRef CAS.
- F. Caruso, M. Spasova, A. Susha, M. Giersig and R. A. Caruso, Chem. Mater., 2001, 13, 109–116 CrossRef CAS.
- T. Nomura, Y. Moritomo, H. Tokumoto and Y. Konishi, Mater. Lett., 2008, 62, 3727–3729 CrossRef CAS.
- S. Mann, Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry, Oxford University Press, Oxford, 2001 Search PubMed.
- E. Baeuerlein, The Biology of Biominerals Structure Formation, Wiley-VCH, Weinheim, 2007 Search PubMed.
- C. Sanchez, K. J. Shea and S. Kitagawa, Chem. Soc. Rev., 2011, 40, 471–472 RSC.
- D. Faivre and D. Schüler, Chem. Rev., 2008, 108, 4875–4898 CrossRef CAS.
- A. Fischer, M. Schmitz, B. Aichmayer, P. Fratzl and D. Faivre, J. R. Soc. Interface, 2011, 8, 1011–1018 CrossRef CAS.
- C. Lang, D. Schüler and D. Faivre, Macromol. Biosci., 2007, 7, 144–151 CrossRef CAS.
- T. Matsunaga, T. Suzuki, M. Tanaka and A. Arakaki, Trends Biotechnol., 2007, 25, 182–188 CrossRef CAS.
- S. Martel, M. Mohammadi, O. Felfoul, Z. Lu and P. Pouponneau, Int. J. Rob. Res., 2009, 28, 571–582 CrossRef.
- W. Stöber, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62–69 CrossRef.
- S. V. Vonsovskii, Ferromagnetic Resonance, Pergamon Press, Oxford, U.K., 1966 Search PubMed.
- B. P. Weiss, S. S. Kim, J. L. Kirschvink, R. E. Kopp, M. Sankaran, A. Kobayashi and A. Komeili, Earth Planet. Sci. Lett., 2004, 224, 73–89 CrossRef CAS.
- D. Faivre, A. Fischer, I. Garcia-Rubio, G. Mastrogiacomo and A. U. Gehring, Biophys. J., 2010, 99, 1268–1273 CrossRef CAS.
- C. Carvallo, S. Hickey, D. Faivre and N. Menguy, Earth Planets Space, 2009, 61, 143–145 CAS.
- J. H. Li, Y. X. Pan, G. J. Chen, Q. S. Liu, L. X. Tian and W. Lin, Geophys. J. Int., 2009, 177, 33–42 CrossRef CAS.
- A. J. Newell, Geochem. Geophys. Geosys., 2005, 6 Search PubMed.
- H. Fischer, G. Mastrogiacomo, J. F. Loffler, R. J. Warthmann, P. G. Weidler and A. U. Gehring, Earth Planet. Sci. Lett., 2008, 270, 200–208 CrossRef CAS.
- M. M. Ye, S. Zorba, L. He, Y. X. Hu, R. T. Maxwell, C. Farah, Q. Zhang and Y. D. Yin, J. Mater. Chem., 2010, 20, 7965–7969 RSC.
- M. Z. Wu, Y. Q. Ma, Y. M. Liu, H. Bi, Q. Q. Fang, H. L. Niu and Q. W. Chen, Mater. Res. Bull., 2008, 43, 1321–1326 CrossRef CAS.
- M. Winklhofer and G. T. Zimanyi, J. Appl. Phys., 2006, 99 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra20911j |
| This journal is © The Royal Society of Chemistry 2012 |
