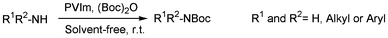Poly(N-vinylimidazole) as a halogen-free and efficient catalyst for N-Boc protection of amines under solvent-free conditions†
Nader Ghaffari
Khaligh
*
House Research of Professor Reza. Education Guilan, Rasht, District 1, 4156917139, Iran. E-mail: ngkhaligh@guilan.ac.ir; ngkhaligh@gmail.com
First published on 19th October 2012
Abstract
Poly(N-vinylimidazole) is able to promote the quantitative N-Boc protection of a variety of functionalized amines with t-butyl dicarbonate at room temperature under solvent-free conditions. This new method consistently has the advantage of providing excellent yields, while the catalyst is halogen-free and can be reused and recovered several times. Further, the reaction can be carried out even in a larger scale, making the procedure potentially useful for industrial applications.
1. Introduction
Organic bases are catalysts for a wide range of reactions.1–5 The development of heterogeneous catalysts has become a major area of research in synthetic organic chemistry due to some potential advantages of these materials over homogeneous systems, such as simplified recovery, reusability, enhanced selectivity and reactivity, ease of product isolation, and the ability for incorporation in continuous reactors and microreactors.6 It is noteworthy, that in several cases the use of solvent-free conditions is necessary for the success of the process, as the use of an organic solvent can dramatically slow the process or make it practically unfeasible.7,8In recent years, various methods have been developed for the protection of amines in their N-Boc form, either in the presence of a base (DMAP,9 aq. NaOH,10 NaHMDS11) or Lewis acid catalyst, such as Zr(ClO4)2·6H2O,12 ZrCl4,13 LiClO414 or yttria-zirconia.15 A Brønsted acid ionic liquid [(HMIm)BF4],16 sulfamic acid,17 sulfonic-acid-functionalized silica,18 β-cyclodextrine,19 thiourea,20 iodine CsF,21 H3PW12O40,22 montmorillonite K10 and KSF,23 and Indion 190 Resinare,24 were also employed for N-Boc protection. However, despite the potential utility of these catalysts, many of these methodologies are associated with several shortcomings, such as prolonged reaction times, reaction under oxidizing conditions, use of strong acids, low yields, harsh reaction conditions, difficulty in the preparation and moisture sensitivity of the catalysts, and the high cost and toxicity of the reagents. Chakraborti and Chankeshwara25 reported the catalyst-free chemoselective N-tert-butyloxycarbonylation of amines in water. This method is not reproducible because of the limited solubility of (Boc)2O in water at ambient temperature. Therefore, there is a need to develop an alternative method for the protection of amines.
Following our current interest based on the use of solid phase catalysts under solvent-free conditions,26 in this paper, we describe our work on the successful use of non-toxic, environmentally benign and inexpensive poly(N-vinylimidazole) [PVIm] as a solid nucleophilic catalyst for N-Boc protection of amines with t-butyl dicarbonate at room temperature under solvent-free conditions.
The practical applications of PVIm are numerous, ranging from dyestuffs, catalysts, corrosion inhibitors, and ion exchange resins, to their utility in quenching media and metal ion complexations.27 The novelty of this work is the first time use of PVIm for the N-Boc protection of amines with (Boc)2O. The results could be useful in finding new applications for this polymer.
2. Experimental
2.1. Materials
Unless specified, all chemicals were of analytical grade and purchased from Merck, Aldrich and Fluka Chemical Companies and used without further purification. Products were characterized by their physical constants and by comparison with authentic samples. The purity determination of the substrates and reaction monitoring were accompanied by TLC using silica gel SIL G/UV 254 plates.2.2. Instrumentation
The purity determination of the products was accomplished by GC-MS on an Agilent GC-Mass-6890 instrument under 70 eV conditions. The IR spectra were recorded on a Perkin-Elmer 781 Spectrophotometer using KBr pellets for solid and neat samples, or CCl4 for liquid samples in the range of 4000–400 cm−1. The UV spectra were recorded on an Agilent 8453 UV-vis spectrophotometer at room temperature. In all the cases the 1H NMR spectra were recorded with a Bruker Avance 400 or 300 MHz instrument using. 13C NMR data were collected on Bruker Avance 100 or 75 MHz instrument. All chemical shifts are quoted in parts per million (ppm) relative to TMS using deuterated solvent. Microanalyses were performed on a Perkin-Elmer 240-B microanalyzer. Melting points were recorded on a Büchi B-545 apparatus in open capillary tubes.2.3. Preparation of poly(N-vinylimidazole) (PVIm)
Poly(N-vinylimidazole) (PVIm) was obtained by free radical polymerization of N-vinylimidazole in benzene under a nitrogen atmosphere with azoisobutyronitrile (AIBN) as the initiator28,29 (Scheme 1). The polymer was precipitated as a white powder. The solid was separated by filtration and dried at 40 °C. PVIm was purified by dissolving in methanol and precipitating using acetone twice. Finally, the polymer was dialyzed against distilled water using dialysis tubing. PVIm was isolated by lyophilization and dried over P4O10 in vacuum at room temperature. The molecular weight of the PVIm sample was determined by viscometry using the Mark–Houwink–Sakurada equation with parameters taken from the literature.30
| [μ] = K(Mv)a |
The Mv value of PVIm was found to be 305![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1.
000 g mol−1.
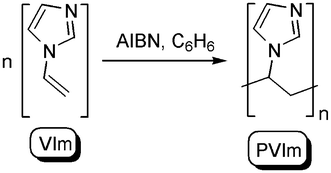 | ||
| Scheme 1 Synthesis of poly(N-vinylimidazole). | ||
2.4. General procedure for N-Boc protection of amines
Amine (1 mmol) was added to the mixture of (Boc)2O (1 mmol) and PVIm (50 mg) with constant stirring at room temperature. After completion of the reaction (monitored by TLC), ethyl acetate (3 × 5 mL) was added to the reaction mixture and the catalyst was decanted and washed with ethyl acetate (2 × 5 mL) and dried. The product was purified by column chromatography, using an ethyl acetate–petroleum ether (b.p. 40–60 °C) (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) eluent. The physical and spectral data of known compounds were in agreement with those reported in the literature.13,14,31–33
8) eluent. The physical and spectral data of known compounds were in agreement with those reported in the literature.13,14,31–33
3. Results and discussion
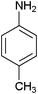
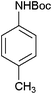
In continuation of our ongoing research program on the development of new catalysts and methods for organic transformations,34–36 here we wish to report the synthesis of poly(N-vinylimidazole) [PVIm] for the N-Boc protection of amines at room temperature under solvent-free conditions (Scheme 2).In order to standardize the reaction, different amounts of PVIm were used for the N-Boc protection of aniline with (Boc)2O at room temperature under solvent-free conditions. The results are summarized in Table 1. It was clear that the reaction could be carried out in the presence of 50 mg of PVIm and the yield was excellent. However, it was found that the reaction was not completed without catalyst (Table 1, entry 1). One of the reasons why an efficient amount of PVIm catalyst was necessary seems to due to the active sites being poisoned by molecules, such as CO2, that were produced over the course of the reaction or in the medium. Therefore, the coated surface with CO2 showed no activity for the N-Boc protection reaction. This was verified by the initial reaction of PVIm with CO2, then the produced PVIm was reacted with aniline in the presence of (Boc)2O (Table 1, entry 7). The generation of active sites requires temperature pre-treatment. It is worthwhile to mention that the same transformation was also achieved within 15 min with lesser yields using a larger amount of the catalyst (Table 1, entries 5 and 6). This may be explained to be a result of the difficulty associated with the mixing and diffusion of the reactants.
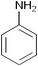
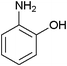
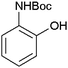
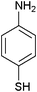
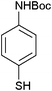
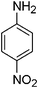
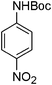
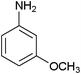
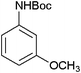
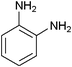
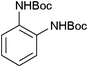
After optimization of the reaction conditions, various aromatic, aliphatic and heterocyclic amines were subjected to N-Boc protection with (Boc)2O under the selected conditions. The results are summarized in Table 2. Obviously, the present method was quite general and has been found to be very effective for the N-Boc protection of the amines. The reaction conditions were mild enough not to induce any damage to moieties, like the methoxy group (Table 2, entries 8 and 24), which often undergo cleavage in the presence of strong acids or certain Lewis acids. Aromatic amines bearing electron-withdrawing substituents, such as 4-nitroaniline (Table 2, entry 7), also a gave good yield after a long reaction time under the presented conditions. Mention must be made here that the hydroxyl groups were not protected as O-Boc in aromatic amines containing this functionality (Table 2, entries 2 and 26–28). In addition, the hindered amines (Table 2, entries 20–22 and 26) gave good yields of the target products. Moreover, the protocol could also work equally well with heterocyclic and aliphatic amines (Table 2, entries 11 and 17–23). Amines containing a double bond (Table 2, entry 19) was selectively converted to the corresponding N-Boc derivatives and the double bond remained intact under the reaction conditions.
| Entry | Substrate | Product | Time (min) | Yieldb (%) |
|---|---|---|---|---|
| a Yields refer to pure isolated products and the products were characterized by m.p., IR, 1H NMR. b Isolated yield. c 2 mmol of t-butyl dicarbonate was used. | ||||
| 1 |
|
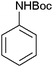
|
15 | 95 |
| 2 |
|
|
21 | 91 |
| 3 |
|
|
23 | 90 |
| 4 |
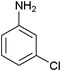
|
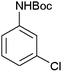
|
28 | 90 |
| 5 |
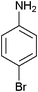
|
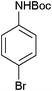
|
25 | 94 |
| 6 |
|
|
23 | 95 |
| 7 |
|
|
90 | 65 |
| 8 |
|
|
32 | 91 |
| 9 |
|
|
23 | 95 |
| 10 |
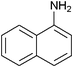
|
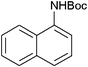
|
28 | 88 |
| 11 |
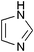
|
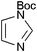
|
15 | 90 |
| 12 |
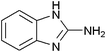
|
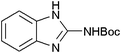
|
14 | 82 |
| 13 |
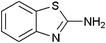
|
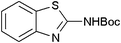
|
21 | 80 |
| 14 |
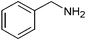
|
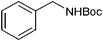
|
14 | 96 |
| 15 |
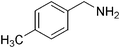
|
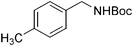
|
15 | 94 |
| 16 |
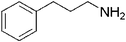
|
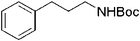
|
18 | 95 |
| 17 |
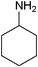
|
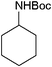
|
14 | 98 |
| 18 |
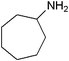
|
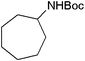
|
18 | 96 |
| 19 |
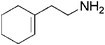
|
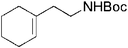
|
17 | 91 |
| 20 |
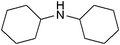
|
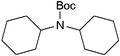
|
43 | 78 |
| 21 |
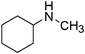
|
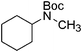
|
23 | 92 |
| 22 |
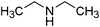
|
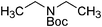
|
21 | 95 |
| 23 |
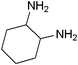 mixture of isomers (cis and trans) mixture of isomers (cis and trans) |
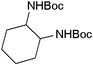 mixture of isomers (cis and trans) mixture of isomers (cis and trans) |
12 | 94c (44.97 and 55.21%) |
| 24 |
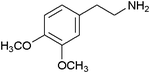
|
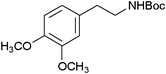
|
23 | 92 |
| 25 |
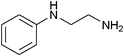
|
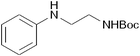
|
14 | 96 |
| 26 |
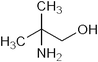
|
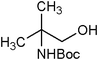
|
15 | 90 |
| 27 |
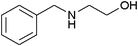
|
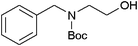
|
15 | 94 |
| 28 |

|

|
19 | 94 |
| 29 |
|
|
17 | 96 |
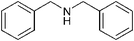
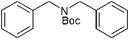
The difference in the rate of reaction of the amine groups under various electronic and steric environments encouraged us to study the selectivity of the N-Boc protection of the different amine groups. The reaction of aniline (1 mmol) and benzylamine (1 mmol) with (Boc)2O (1 mmol) afforded the corresponding N-Boc derivatives in 18% and 82% yields, respectively, after 15 min (GC-MS yields) (Scheme 3, entry 1). The cyclohexyl carbamic acid tert-butyl ester was formed exclusively when a mixture of cyclohexylamine (1 mmol) and dicyclohexylamine (1 mmol) were treated with (Boc)2O (1 mmol) for 15 min (IR, TLC comparison with authentic compounds) (Scheme 3, entries 2 and 3). The preferential N-Boc formation of cyclohexylamine over dicyclohexylamine was due to steric factors, as otherwise the latter was expected to form the N-Boc derivative selectively, because a secondary amine is more nucleophilic than a primary amine. When a mixture of benzylamine (1 mmol) and dibenzylamine (1 mmol) were treated with (Boc)2O (1 mmol) for 15 min a 78% selectivity (GC-MS yields) was observed in favor of the N-Boc of dibenzylamine. This result established that in the absence of an appreciable amount of steric effect, the N-Boc of the secondary amine was formed preferably.
 | ||
| Scheme 3 Competitive N-Boc protection of the different amine groups. | ||
We have also found that PVIm can be easily decanted, washed with ethyl acetate and then dried at 50 °C. The reusability of this reagent was exemplified by the N-Boc protection of aniline in the presence of recycled PVIm, which gave the requested product in 95, 95, 95 and 93% isolated yields after four runs. The average time for four consecutive runs was 15.5 min and 100% conversion for all, which clearly demonstrats the practical recyclability of this catalyst (Fig. 1). It seems that a slight amount of the catalyst was lost after the 4th run in the catalytic cycle (5% weight).
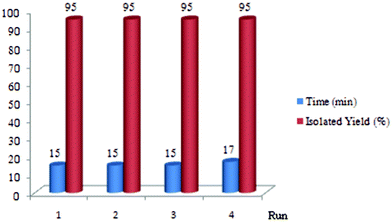 | ||
| Fig. 1 Reusability of PVIm. | ||
FTIR spectra of the fresh PVIm (bottom) and the recycled PVIm (top) are shown in Fig. 2. The PVIm spectrum contains bands of stretching vibrations for the imidazole rings (1516, 1422, 1285, and 1233 cm−1), stretching vibrations of the azole C–H (1080 cm−1), and bending vibrations of heterocycles (914, 825 and 747 cm−1).37 The imidazole units of PVIm, corresponding to the ring deformation, suffer from shifts towards lower and higher wave numbers when the catalyst is recycled for the 4th run.
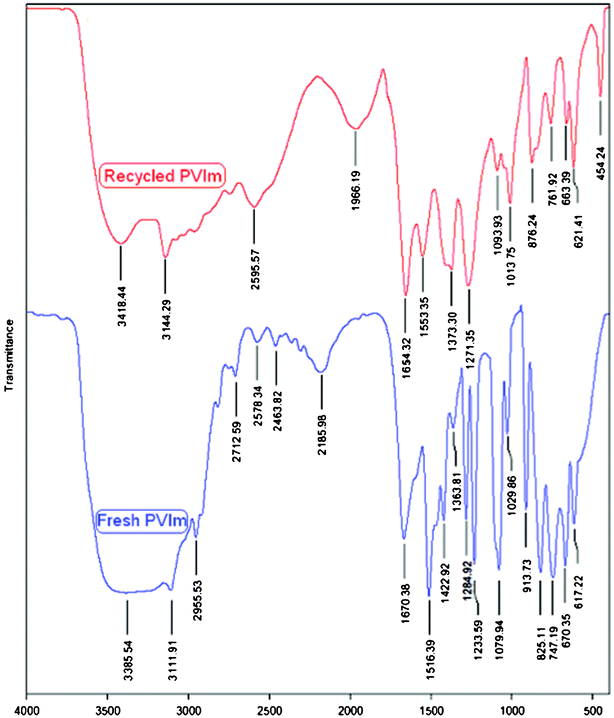 | ||
| Fig. 2 FTIR spectra for the fresh PVIm (bottom) and the recycled PVIm (top, after 4th run). | ||
Interestingly, we note that the conversion of aniline to the respective N-tert-butoxycarbonate derivative can be achieved on a larger scale without any difficulty by using only 50 mg of PVIm. The reaction of aniline (10 mmol) was investigated in Boc2O (10 mmol) in the presence of PVIm (50 mg). The reaction was complete within 24 min, and the desired product was isolated in 85% yield.
The possible mechanism for N-Boc protection of various amines in the presence of PVIm is not clear, but based on the literature,38 the mechanism, which is shown in Scheme 4, was proposed for the clarification of the catalytic role of PVIm in the reported method. This mechanism shows that PVIm undergoes nucleophilic attack on (Boc)2O and generates the tetrahedral intermediate I. Elimination of CO2 and t-BuOH from I leads to the formation of II and anion amide, that can further react with II to form the requested product and PVIm, which reenters the catalytic cycle.
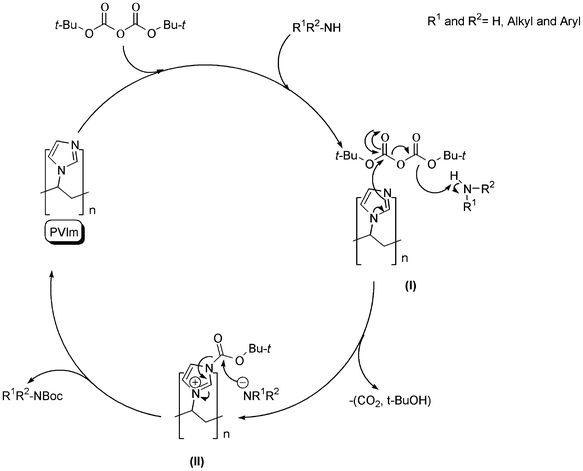 | ||
| Scheme 4 Proposed mechanism for the N-Boc protection of amines in the presence of PVIm. | ||
4. Conclusions
In conclusion, we have developed a simple, efficient and chemoselective protocol for the N-Boc protection of various amines using PVIm as a solid catalyst. The protocol is highly chemoselective, offering potentials in different applications. The methodology also has several other advantages such as: good to excellent yields, no side reactions, ease of handling of the catalyst, cost efficiency, effective reusability of the catalyst, use of an inexpensive catalyst and a simple experimental procedure. Further work to explore this novel catalyst in other organic transformations is in progress.Acknowledgements
The author is thankful to House Research of Professor Reza, Education Guilan, Rasht, District 1 for partial support of this work.References
- S. Bertelsen and K. A. Jørgensen, Chem. Soc. Rev., 2009, 38, 2178–2189 RSC.
- D. W. C. McMillan, Nature, 2008, 455, 304–308 CrossRef.
- A. Dondoni and A. Massi, Angew. Chem., Int. Ed., 2008, 47, 4638–4660 CrossRef CAS.
- P. Melchiorre, M. Merigo, A. Carlone and G. Bartoli, Angew. Chem., Int. Ed., 2008, 47, 6138–6171 CrossRef CAS.
- C. F. Barbas, Angew. Chem., Int. Ed., 2008, 47, 42–47 CrossRef CAS.
- K. Smith, Solid Supports and Catalysis in Organic Synthesis, Ellis Horwood and PTR Prentice Hall, New York, 1992 Search PubMed.
- T. Angelini, F. Fringuelli, D. Lanari, F. Pizzo and L. Vaccaro, Tetrahedron Lett., 2010, 51, 1566–1569 CrossRef CAS.
- F. Fringuelli, D. Lanari, F. Pizzo and L. Vaccaro, Curr. Org. Synth., 2009, 6, 203–208 CrossRef CAS.
- Y. Basel and A. Hasners, J. Org. Chem., 2000, 65, 6368–6380 CrossRef CAS.
- C. Lutz, V. Lutz and P. Knochel, Tetrahedron, 1998, 54, 6385–6402 CrossRef CAS.
- T. A. Kelly and D. W. McNeil, Tetrahedron Lett., 1994, 35, 9003–9006 CrossRef CAS.
- D. L. Boger and J. A. McKie, J. Org. Chem., 1995, 60, 1271–1275 CrossRef CAS.
- J. M. Muchowski and M. C. Venuti, J. Org. Chem., 1980, 45, 4798–4801 CrossRef CAS.
- A. Heydari and S. E. Hosseini, Adv. Synth. Catal., 2005, 347, 1929–1932 CrossRef CAS.
- R. K. Pandey, S. P. Dagade, R. K. Upadhaya, M. K. Dongare and P. Kumar, ARKIVOC, 2002, vii, 28–33 Search PubMed.
- S. Sunitha, S. Kanjilal, S. P. Reddy and B. N. Prasad, Tetrahedron Lett., 2008, 49, 2527–2532 CrossRef CAS.
- D. J. Upadhyaya, A. Barge, R. Stefania and G. Cravottoa, Tetrahedron Lett., 2007, 48, 8318–8322 CrossRef CAS.
- B. Das, K. Venkateswarlu, M. Krishnaiah and H. Holla, Tetrahedron Lett., 2006, 47, 7551–7556 CrossRef CAS.
- M. S. Reddy, M. Narender, Y. V. D. Nageswar and K. R. Rao, Synlett, 2006, 1110–1112 CAS.
- S. Khaksar, A. Heydari, M. Tajbakhsh and S. M. Vahdat, Tetrahedron Lett., 2008, 49, 3527–3529 CrossRef CAS.
- N. Inahashi, A. Matsumiya and T. Sato, Synlett, 2008, 294–296 CAS.
- A. Heydari, R. K. Shiroodi, H. Hamadi, M. Esfandyari and M. Pourayoubi, Tetrahedron Lett., 2007, 48, 5865–5868 CrossRef CAS.
- S. V. Chankeshwara and A. S. Chakraborti, J. Mol. Catal. A: Chem., 2006, 253, 198–202 CrossRef CAS.
- A. Chaskar, S. Yewale, B. Langi and H. Deokar, J. Korean Chem. Soc., 2009, 53, 422–426 CrossRef CAS.
- S. V. Chankeshwara and A. K. Chakraborti, Org. Lett., 2006, 8, 3259–3262 CrossRef CAS.
- F. Shirini and N. G. Khaligh, J. Iran. Chem. Soc., 2012, 9, 495–502 CrossRef.
- R. C. Sutton, L. Thai, J. M. Hewitt, C. L. Voycheck and J. S. Tan, Macromolecules, 1988, 21, 2432–2439 CrossRef CAS.
- A. Chapiro and Z. Mankowski, Eur. Polym. J., 1988, 24, 1019–1028 CrossRef CAS.
- B. Cabot, A. Deratani and A. Foissy, Colloids Surf., A, 1998, 139, 287–297 CrossRef CAS.
- J. Brandrup, E. H. Immergut and E. Grulke, Solution Properties, in Polymer Handbook, Wiley, New York, 4th edn, 1999 Search PubMed.
- T. A. Vilaivan, Tetrahedron Lett., 2006, 47, 6739–6742 CrossRef CAS.
- S. V. Chankeshwara and A. K. Chakraborti, Org. Lett., 2006, 8, 3259–3262 CrossRef CAS.
- S. V. Chankeshwara and A. K. Chakraborti, Tetrahedron Lett., 2006, 47, 1087–1091 CrossRef CAS.
- N. G. Khaligh, J. Mol. Catal. A: Chem., 2011, 349, 63–70 CrossRef CAS.
- N. G. Khaligh, Tetrahedron Lett., 2012, 53, 1637–1640 CrossRef CAS.
- N. G. Khaligh, Ultrason. Sonochem., 2012, 19, 736–739 CrossRef CAS.
- J. L. Lippert, J. A. Robertson, J. R. Havens and J. S. Tan, Macromolecules, 1985, 18, 63–67 CrossRef CAS.
- H. Ouchi, Y. Saito, Y. Yamamoto and H. Takahara, Org. Lett., 2002, 4, 585–587 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra20905e |
| This journal is © The Royal Society of Chemistry 2012 |