DOI:
10.1039/C2RA20842C
(Paper)
RSC Adv., 2012,
2, 6970-6980
One-pot build-up procedure for the synthesis of variously substituted purine derivatives†
Received
2nd May 2012
, Accepted 23rd May 2012
First published on 2nd July 2012
Abstract
In this article, we report a one-pot build-up procedure leading to 6-chloro- or 2-amino-6-chloropurines bearing various alkyl or aryl substituents in position N-9. This reaction is simple, fast and effective with up to 96% yields depending on the starting amine. This reaction may be easily combined with further nucleophilic displacement of the C-6 chlorine atom using various reagents, making this procedure very attractive in the field of medicinal chemistry pertaining to compounds based on a purine scaffold.
Introduction
Many compounds containing a purine nucleobase substituted at position N-9 (especially nucleosides, nucleotides, their carbocyclic and acyclic analogues but also many more) are biologically active. In the last several decades, it has been gradually discovered that purine-based structures have wide applicability as antivirals,1 antibiotics,2 antimycobacterials3 and cytostatics.4 They have also been found to be inhibitors of cell kinases,5 a rapidly expanding research area of medicinal chemistry. The appearance of purines in biologically active compounds, both naturally occuring6 and synthetic,7 has been nicely reviewed in recent years.
The synthesis of purine-containing compounds involves in one of its steps the introduction of the nucleobase. This can be achieved in several ways—the direct alkylation of the nucleobase,8 a Mitsunobu reaction,9 a Vorbrüggen reaction,10 a Michael addition,11 a palladium-catalyzed reaction of the nucleobase with an acylated hydroxy group in an allylic position,12 chemical or enzymatic transglycosylation,13 and finally the construction of a purine nucleobase on an amino group. This can be performed either by closing the pyrimidine ring on an imidazole intermediate14 or vice versa by closing the imidazole ring on a pyrimidine intermediate—originally the Traube synthesis.15 A common target for the latter build-up procedure is a 6-chloropurine or 2-amino-6-chloropurine because of the reactivity and the ease of modification of the resulting 6-chloropurine derivatives. Modifications of the Traube synthesis have been used in the synthesis of a plethora of variously substituted purines, especially due to an easy access to diverse amine precursors and strict N-7/N-9 regioselectivity.
The standard procedure for 6-chloropurine construction is the reaction of a substrate containing a primary amino group with 4,6-dichloro-5-aminopyrimidine16 or 4,6-dichloro-5-formamidopyrimidine17 with a subsequent imidazole-ring closure performed with a diethoxy methylacetate or triethyl orthoformate under acidic conditions. Analogously, a 2-amino-6-chloropurine build-up is performed using a pyrimidine precursor with an additional amino group (free18 or formylated19) in position C-2.
Although these methodologies allow the synthesis of purine derivatives from amine precursors, they suffer from numerous drawbacks. The crucial disadvantages are the laboriousness of the whole reaction sequence (two or three separate steps including purification of each intermediate), and low yields scarcely exceeding 70 per cent, with these numbers decreasing significantly upon the introduction of less reactive amines. Another setback is the fact that the imidazole-ring closure step usually requires acidic conditions, which prevents eventual nucleophilic C-6 chlorine atom displacement without preceding purification of the 6-chloropurine or 2-amino-6-chloropurine derivative. In addition, rather lengthy reaction times present a significant problem—ranging from several tens of hours with simple and activated amines (i.e. in the benzylic position) to as long as a week for sterically hindered or otherwise unreactive amines, such as 1-adamantylamine. If the formylated pyrimidine precursors are employed, the reaction times are shortened, however rarely below ten hours.
Since the use of both triethyl orthoformate and diethoxy methylacetate practically means the introduction of a masked formyl group, we decided to further explore the reaction of the above mentioned formylated pyrimidine species under various conditions in order to determine whether this formyl group can be used to close the imidazole ring directly, without the use of any additional reagents.
Results and discussion
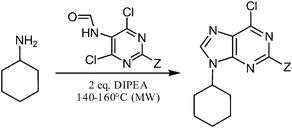
We observed that a mere increase of reaction temperature and use of a sealed reactor (both microwave and conventionally heated) not only provide the 6-chloropurine or 2-amino-6-chloropurine derivative directly but also accelerate the process significantly. We have performed a series of optimization experiments to determine the most suitable solvent, non-nucleophilic base and temperature in order to reach the best yields possible in a reasonable time. For these optimizations, we have chosen cyclohexylamine as a suitable model substrate.
Firstly, both reactions leading to 6-chloro-9-cyclohexyl-9H-purine and 6-chloro-9-cyclohexyl-9H-purin-2-amine respectively, were performed in various solvents including nonpolar, polar aprotic, polar protic and dipolar aprotic. The composition of the reaction mixtures was determined using HPLC-MS analysis, and the best results for each solvent are listed in Table 1. In general, the results of these reactions cannot be directly linked to the solvent polarity—the reactions can be carried out in all kinds of solvents except for DMF, where a 6-dimethylamino derivative is obtained.20
| Entry |
Solvent |
Z |
Time |
Temp. |
Yield [%]a |
Isolated yields. The yields in parentheses are HPLC-determined.
Isolated yield of 6-dimethylamino derivative.
Mixture 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v). 1 (v/v).
|
| 1 |
Toluene |
H |
2 h |
140 °C |
80 (83) |
| 2 |
Dioxane |
H |
2 h |
160 °C |
86 (90) |
| 3 |
DMF |
H |
1 h |
160 °C |
72b |
| 4 |
MeCN |
H |
2 h |
140 °C |
(50) |
| 5 |
n-BuOH |
H |
2 h |
140 °C |
73 (78) |
| 6 |
i-PrOH |
H |
2 h |
140 °C |
61 (62) |
| 7 |
EtOH |
H |
2 h |
140 °C |
58 (60) |
| 8 |
EtOH/H2Oc |
H |
2 h |
140 °C |
(25) |
| 9 |
Toluene |
NH2 |
2 h |
160 °C |
58 (68) |
| 10 |
Dioxane |
NH2 |
2 h |
160 °C |
(66) |
| 11 |
DMF |
NH2 |
1 h |
160 °C |
69b |
| 12 |
MeCN |
NH2 |
2 h |
160 °C |
(62) |
| 13 |
n-BuOH |
NH2 |
2 h |
160 °C |
64 (69) |
| 14 |
i-PrOH |
NH2 |
2 h |
155 °C |
(56) |
| 15 |
EtOH |
NH2 |
2 h |
145 °C |
(54) |
| 16 |
EtOH/H2Oc |
NH2 |
1 h |
140 °C |
64 (71) |
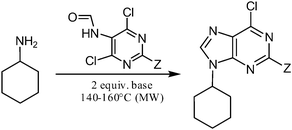
Subsequently, the three best solvents were chosen for both products and the non-nucleophilic base influence was evaluated using five organic and two inorganic bases. The use of stronger bases such as DBU and K2CO3 led either to the loss of the N-5 formyl or to complicated mixtures of products, whereas the use of less sterically hindered organic bases (proton sponge®, 2,6-lutidine), led to the partial or complete displacement of the C-6 chlorine atom. DIPEA therefore remains the base of choice for this reaction, although TEA afforded comparable results (Table 2). Despite the fact that the yields of the reactions carried out in toluene are generally good, the low solubility of the products in toluene makes manipulation with the reaction mixture unpleasant, and toluene was therefore not used in further experiments.
Table 2 Non-nucleophilic base selection study
| |
|
Toluene, Z = H |
Dioxane, Z = H |
n-BuOH, Z = H |
Toluene, Z = NH2 |
n-BuOH, Z = NH2 |
EtOH/H2Oa, Z = NH2 |
Mixture 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v).
Sealed microwave reactor. Conditions A) 140 °C/2 h; B) 160 °C/2 h; C) 140 °C/1 h; D) 120 °C/1 h.
Isolated yields. In parentheses are HPLC-determined yields.
Complicated reaction mixture.
Reaction time had to be prolonged to 4 h in order to reach the full conversion. Yields are diminished due to occurrence of 6-alkoxy derivatives (more significant at temperatures > 120 °C).
Nucleophilic adduct at the position C-6 with the base. 1 (v/v).
Sealed microwave reactor. Conditions A) 140 °C/2 h; B) 160 °C/2 h; C) 140 °C/1 h; D) 120 °C/1 h.
Isolated yields. In parentheses are HPLC-determined yields.
Complicated reaction mixture.
Reaction time had to be prolonged to 4 h in order to reach the full conversion. Yields are diminished due to occurrence of 6-alkoxy derivatives (more significant at temperatures > 120 °C).
Nucleophilic adduct at the position C-6 with the base.
|
| Ent. |
Base |
Cond.b |
Yieldc [%] |
Cond.b |
Yieldc [%] |
Cond.b |
Yieldc [%] |
Cond.b |
Yieldc [%] |
Cond.b |
Yieldc [%] |
Cond.b |
Yieldc [%] |
| 1 |
DIPEA |
A |
80 (83) |
B |
86 (90) |
C |
73 (78) |
B |
58 (68) |
B |
64 (69) |
C |
64 (71) |
| 2 |
Et3N |
A |
80 (84) |
B |
83 (86) |
C |
71 (74) |
B |
(65) |
B |
(68) |
C |
(67) |
| 4 |
K3PO4 |
B |
(41) |
C |
0d |
D |
0d |
D |
0d |
D |
0d |
D |
0d |
| 5 |
K2CO3 |
B |
(50) |
C |
0d |
D |
0d |
D |
0d |
D |
0d |
D |
0d |
| 6 |
2,6-Lutidine |
C |
76 (81) |
B |
(50) |
A |
61 (64) |
A |
54 (62) |
D |
(35)e |
D |
(54)e |
| 7 |
Proton Sponge |
A |
(30)f |
B |
(38)f |
A |
(40)f |
D |
(30)f |
A |
(37)f |
A |
58 (66) |
In order to prove the robustness and versatility of this method, we employed 15 amine substrates and performed this procedure in two different solvents—dioxane and n-butanol for the 6-chloropurine derivatives and n-butanol and a water–ethanol mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) for the 2-amino-6-chloropurine derivatives. The results listed in Table 3 indicate that both polar and non-polar substrates undergo this reaction with yields ranging from good to near quantitative. The phosphonate diester substrates afforded monoester products in both cases (Entries 15 and 16).
1 v/v) for the 2-amino-6-chloropurine derivatives. The results listed in Table 3 indicate that both polar and non-polar substrates undergo this reaction with yields ranging from good to near quantitative. The phosphonate diester substrates afforded monoester products in both cases (Entries 15 and 16).
Table 3 Purine build-up procedure employing various amine substrates
| Entry |
R
|
Z = H |
Z = NH2 |
Entry |
R
|
Z = H |
Z = NH2 |
| Cond.a |
Yield [%]b |
Cond.a |
Yield [%]b |
Cond.a |
Yield [%]b |
Cond.a |
Yield [%]b |
A: dioxane, 160 °C/2 h; B: n-BuOH, 140 °C/2 h; C: n-BuOH, 160 °C/2h; D: EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), 140 °C/1 h.
Isolated yields of MW heated experiments. The isolated yields of conventionally heated experiments are shown in parentheses. The yields of conventionally heated experiments determined with HPLC are in brackets.
Under these conditions, a 6-phenylamino or 6-p-methoxyphenylamino derivative is formed.
An amine hydrochloride was employed as a substrate.
A bipurine derivative is formed using 2.4 mmol of pyrimidine reagent.
The yield was less than 10% (HPLC based), the product was not isolated.
Isolated yield of phosphonate monoester. 1, v/v), 140 °C/1 h.
Isolated yields of MW heated experiments. The isolated yields of conventionally heated experiments are shown in parentheses. The yields of conventionally heated experiments determined with HPLC are in brackets.
Under these conditions, a 6-phenylamino or 6-p-methoxyphenylamino derivative is formed.
An amine hydrochloride was employed as a substrate.
A bipurine derivative is formed using 2.4 mmol of pyrimidine reagent.
The yield was less than 10% (HPLC based), the product was not isolated.
Isolated yield of phosphonate monoester.
|
| 1 |
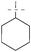
|
A |
86 (86) |
C |
69 (76) |
9 |
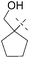
|
A |
61 [65] |
C |
66 (55) |
| B |
78 (74) |
D |
71 (70) |
B |
51 (52) |
D |
62 (60) |
| 2 |

|
A |
75 (80) |
C |
62 (73) |
10 |

|
A |
66 (66) |
C |
78 (76) |
| B |
77 [85] |
D |
0 (0)c |
B |
70 (68) |
D |
80 (75) |
| 3 |

|
A |
90 (77) |
C |
73 (80) |
11 |

|
A |
90 (82) |
C |
82 (74) |
| B |
84 [87] |
D |
0 (0)c |
B |
88 [86] |
D |
77 [79] |
| 4 |

|
A |
93 (94) |
C |
93 (92) |
12 |
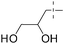
|
A |
61 [63] |
C |
70 (82) |
| B |
86 [94] |
D |
55 (49) |
B |
66 [65] |
D |
45 [52] |
| 5d |

|
A |
55 (47) |
C |
52 (66) |
13e |

|
A |
88 (83) |
C |
86 (80) |
| B |
56 (35) |
D |
57 (30) |
B |
85 (80) |
D |
72 (58) |
| 6 |

|
A |
88 (61) |
C |
96 (97) |
14 |
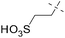
|
A |
87 (83) |
C |
71 (72) |
| B |
93 [96] |
D |
86 (87) |
B |
70 (68) |
D |
58 [70] |
| 7 |

|
A |
92 (79) |
C |
87 (82) |
15 |

|
A |
NDf |
C |
NDf |
| B |
84 (94) |
D |
90 (87) |
B |
63 (80)g |
D |
62 (73)g |
| 8d |
|
A |
79 [82] |
C |
82 (75) |
16 |

|
A |
NDf |
C |
NDf |
| B |
79 (85) |
D |
75 (70) |
B |
50 (57)g |
D |
42 (43)g |
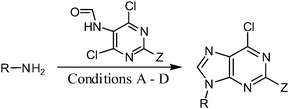
Since the modification of the C-6 position is one of the most common derivatizations of the purine nucleobase,21 the compatibility of this reaction with a subsequent one-pot nucleophilic displacement of the chlorine atom was evaluated. By heating the crude reaction mixture with an excess of a suitable nucleophilic reagent, variously C-6 substituted purines or 2-aminopurines can be easily prepared while omitting one purification procedure and maintaining very good to excellent yields (Table 4).
Table 4 Subsequent one-pot reactions of tryptamine derivatives (Table 3, entry 6) with various nucleophiles under microwave irradiation
| Entry |
Reagent |
Nu |
Z |
Conditions |
Yield [%]a |
|
Isolated yields.
3.5 M solution in ethanol.
The reaction could not be performed under MW irradiation,23 the reaction mixture was heated in an oil bath.
1 M solution in methanol.
6-alkoxy derivatives are formed if the reaction is performed in alcohols.
2 M solution in water.
|
| 1 |
Ammoniab |
NH2 |
H |
140 °C/20 min, 10 eq, n-BuOH |
86 |
| 2 |
Cyclopropyl-amine |

|
H |
140 °C/20 min 5 eq, n-BuOH |
91 |
| 3 |
Morpholine |

|
H |
140 °C/20 min 5 eq, n-BuOH |
87 |
| 4 |
Thioureac |
SH |
H |
100 °C/4 h 2 eq, n-BuOH |
80 |
| 5 |
MeONad |
OMe |
H |
80 °C/10 min 5 eq, Dioxanee |
82 |
| 6 |
NaOHf |
OH |
H |
100 °C/10 min 10 eq, Dioxanee |
79 |
| 7 |
Ammoniab |
NH2 |
NH2 |
140 °C/30 min 10 eq, n-BuOH |
92 |
| 8 |
Cyclopropyl-amine |
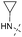
|
NH2 |
140 °C/10 min 5 eq, n-BuOH |
82 |
| 9 |
Morpholine |
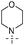
|
NH2 |
140 °C/10 min 5 eq, n-BuOH |
83 |
| 10 |
Thioureac |
SH |
NH2 |
100 °C/4 h 2 eq, n-BuOH |
84 |
| 11 |
MeONad |
OMe |
NH2 |
100 °C/10 min 5 eq, Dioxanee |
93 |
| 12 |
NaOHf |
OH |
NH2 |
100 °C/10 min 10 eq, Dioxanee |
88 |
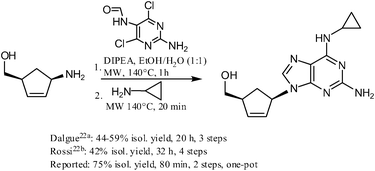
An alternative route to two clinically used antiviral drugs—Adefovir and Abacavir—was explored using this methodology, and the synthesis of the latter compound (Scheme 1), in particular, proved very successful in comparison with the existing literature procedures.22
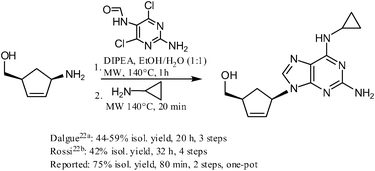 |
| | Scheme 1 Synthesis of Abacavir—a comparison with literature procedures. | |
Conclusion
We have developed a convenient and significantly simplified route to 6-chloropurine and 2-amino-6-chloropurine derivatives using commercially available pyrimidine precursors. This is the first technique leading to 9-substituted purine derivatives from amine precursors carried out in a single step, which makes it a method of choice for such transformations. The absence of acidic conditions during the imidazole-ring closure allows the conversion of these compounds to other, differently 6-substituted purines by reaction with various nucleophiles performed in one pot. We proved the usefulness of this methodology on the synthesis of a commercially successful antiviral drug Abacavir and demonstrated its potential on a number of structurally diverse substrates.
Experimental details
General information
Microwave syntheses were carried out in a CEM Discover instrument with a single-mode cavity and focused microwave heating (microwave power supply 0–300 W, 1 W increments, IR temperature sensor, sealed vessel mode, pressure range 0–20 bar, 10 or 60 mL vials). Ramping never exceeded 2 min. Conventionally heated experiments were carried out in Ace pressure tubes® with back seal bushing heated in an aluminium block (Sigma-Aldrich Co.). Column chromatography was performed on a 40–60 μm silicagel using ISCO flash chromatography system.
4,6-Dichloro-5-formamidopyrimidine was prepared by formylating 4,6-dichloro-5-aminopyrimidine (AK Scientific, Inc.; Union City, CA, USA) according to a literature procedure,19a although it is also commercially available. 2-Amino-4,6-dichloro-5-formamidopyrimidine was purchased from Tokyo Chemical Industry, Co., Ltd., Tokyo, Japan but may also be simply synthesized from readily available 2,5-diamino-4,6-dihydroxypyrimidine.22c
Solvents and reagents as well as amine substrates in Table 3, except for entries 10, 15 and 16, were purchased from Sigma-Aldrich Co.; Entry 10 amine was prepared in three simple steps22c from available (1R)-(−)-2-azabicyclo[2.2.1]hept-5-en-3-one (Sigma-Aldrich Co.). Entry 15 amine was prepared according to a literature procedure24 and entry 16 amine was prepared according to a procedure described by Franchetti.25
Analytical information
1H and 13C NMR spectra were recorded on a Bruker Avance instrument at 25 °C at 500 MHz and 125.8 MHz, respectively, using DMSO-d6 as a solvent and using its signal as a reference. Chemical shifts (δ) and coupling constants (J) were expressed in ppm and Hz, respectively. All structures were confirmed and 1H and 13C signals were assigned by a combination of 1D and 2D NMR (H,H-COSY, H,C-HSQC, H,C-HMBC) techniques. Standard pulse programs from the library of the spectrometer were used; gradient selection was used in the 2D experiments.
Analyses of products in reaction mixtures were carried out by HPLC (Waters 600 controller) on RPC18 column (4.6 × 100 mm, 3 μm) and analytes were identified by UV (Waters 2998) and MS (Waters 3100). Elution was carried out with aqueous acetonitrile gradient. A = 25 mM NH4Ac, B = 25 mM NH4Ac in 50% MeCN, C = 100% MeCN; t0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min 95% A-5% B, t6
min 95% A-5% B, t6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min 100% B, t16
min 100% B, t16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min 100% C; flow 1 mL min−1. Concentration of products in reaction mixtures was determined by calibration on an external standard on the same column, analytes were identified by UV (Waters 2996). Elution was carried out with aqueous acetonitrile gradient. A = 100 mM TEAA, B = 100 mM TEAA in 50% MeCN, C = 100% MeCN. t0
min 100% C; flow 1 mL min−1. Concentration of products in reaction mixtures was determined by calibration on an external standard on the same column, analytes were identified by UV (Waters 2996). Elution was carried out with aqueous acetonitrile gradient. A = 100 mM TEAA, B = 100 mM TEAA in 50% MeCN, C = 100% MeCN. t0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min 95% A-5% B, t6
min 95% A-5% B, t6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min 100% B, t16
min 100% B, t16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min 100% C; flow 1 mL min−1.
min 100% C; flow 1 mL min−1.
GCMS analyses of known compounds (state of purity) were measured on a 6890 N gas chromatograph (Agilent, Santa Clara, CA, USA) equipped with a Phenomenex ZB-5 HT capillary column (30 m × 0.25 mm, film thickness 0.25 mm); temperature: 60 °C (2 min), then 10 °C min−1 to 320 °C (10 min); coupled to a 5975 B quadrupole mass spectrometer. The 70 eV spectra were recorded in the 25–800 m/z range. HRMS analysis of pure compounds was conducted on an LTQ Orbitrap XL instrument (Thermo Fisher Scientific). Purity of all prepared compounds was higher than 98%.
Melting points were measured on a Büchi apparatus and are uncorrected.
General reaction procedure for the construction of a 6-chloropurine or a 2-amino-6-chloropurine nucleobase
To a solution of the amine substrate (1 mmol) in a suitable solvent (5 mL), 4,6-dichloro-5-formamidopyrimidine (230 mg, 1.2 mmol) or 2-amino-4,6-dichloro-5-formamidopyrimidine (250 mg, 1.2 mmol) and DIPEA (348 μL, 2 mmol when starting from a free amine substrate, 523 μL, 3 mmol when starting from a hydrochloride, substrate containing free acid group or phosphonate diester) was added and the reaction mixture was heated in a sealed vessel on corresponding temperature for the specified time (Table 3). Purification was performed either by crystallization (precipitation of unsoluble product from the reaction mixture), flash chromatography on silica gel (hexane–ethyl acetate or ethyl acetate–methanol gradient) or HPLC (water–acetonitrile or 50 mM TEAB–acetonitrile gradient). Chromatographically obtained products were further crystalized if possible.
9-Cyclohexyl-N,N-dimethyl-9H-purin-6-amin hydrochloride (Table 1, Entry 3, Z=H).
Mobile phase: 30–50% ethyl acetate in hexanes. Crystallization from ethanol–diethylether. White crystals, m.p. 238–239 °C. Found: C, 55.38; H, 7.21; N, 25.00; Cl, 12.44. Calc. for C13H20ClN5: C, 55.41; H, 7.15; N, 24.85; Cl, 12.58%. 1H NMR: δ 1.25 (1H, m, H-4′ax), 1.44 (2H, m, H-3′ax), 1.70 (1H, m, H-4′eq), 1.82–1.93 (4H, m, H-2′ax, H-3′eq), 2.00 (2H, m, H-2′eq), 3.96 (6H, bs, CH3), 4.47 (1H, tt, J1,2′ax = 12.0, J1′,2′eq = 3.9, H-1′), 8.40 (1H, s, H-2), 8.56 (1H, s, H-8). 13C NMR: δ 24.83 (C4′), 25.17 (C-3′), 32.39 (C-2′), 54.62 (C-1′), 119.00 (C-5), 140.34 (C-8), 146.06 (C-2), 147.60 (C-4), 149.97 (C-6). MS (ESI) m/z: 246.2 (MH+, 100%); HRMS (ESI) calc. for C13H20N5: 246.17132; found: 246.17119.
6-Chloro-9-(4-methoxyphenyl)-9H-purine (Table 3, Entry 3, Z = H).
Mobile phase: 50–70% ethyl acetate in hexanes. Crystallization from toluene. White crystals, m.p. 202–203 °C (decomp.). Found: C, 55.48; H, 3.49; N, 21.30; Cl, 13.56. Calc. for C12H9N4OCl: C, 55.29; H, 3.48; N, 21.49; Cl, 13.60%. 1H NMR: δ 3.84 (3H, s, CH3), 7.18 (2H, m, H-3′), 7.76 (2H, m, H-2′), 8.81 (1H, s, H-2), 9.00 (1H, s, H-8). 13C NMR: δ 55.77 (CH3), 114.91 (C-3′), 125.67 (C-2′), 126.99 (C-1′), 131.41 (C-5), 146.78 (C-8), 149.62 (C-6), 151.81 (C-4), 152.22 (C-2), 159.36 (C-4′). MS (ESI) m/z: 276.3 (MH+, 100%); 298.3 (MNa+, 77); HRMS (ESI) calc. for C12H11ON5Cl: 276.06466; found: 276.06483.
6-Chloro-9-[(1R)-1-(4-fluorophenyl)ethyl]-9H-purine (Table 3, Entry 4, Z = H).
Mobile phase: 30–50% ethyl acetate in hexanes. Pale yellow oil. Found: C, 56.59; H, 3.72; N, 20.40; F, 6.55; Cl, 12.69. Calc. for C13H10N4FCl: C, 56.43; H, 3.64; N, 20.25; F, 6.87; Cl, 12.81%. 1H NMR: δ 2.00 (3H, d, JCH3–CH = 7.2, CH3), 6.00 (1H, q, JCH–CH3 = 7.2, CH–CH3), 7.18 (2H, m, H-3′), 7.47 (2H, m, H-2′), 8.76 (1H, s, H-2), 8.97 (1H, s, H-8). 13C NMR: δ 20.42 (CH3), 54.00 (CH–CH3), 115.69 (d, J3′,F = 21.5, C-3′), 128.77 (d, J2′,F = 8.4, C-2′), 131.29 (C-5), 136.99 (d, J1′,F = 3.1, C-1′), 146.07 (C-8), 149.35 (C-6), 151.65 (C-2 and C-4), 161.81 (d, J4′,F = 244.3, C-4′). MS (ESI) m/z: 277.0 (MH+, 7%); 299.0 (MNa+, 100); HRMS (ESI) calc. for C13H11N4ClF: 277.08508; found: 277.08514.
6-Chloro-9-[2-(1H-indol-3-yl)ethyl]-9H-purin (Table 3, Entry 6, Z = H).
Mobile phase: 40-60% ethyl acetate in hexanes. Crystallization from cyclohexane. White crystals, m.p. 187–189 °C. Found: C, 60.50; H, 4.01; N, 23.68; Cl, 11.87. Calc. for C15H12N5Cl: C, 60.51; H, 4.06; N, 23.52; Cl, 11.91%. 1H NMR: δ 3.31 (2H, m, 3′-CH2), 4.58 (2H, m, N-CH2), 6.96 (1H, ddd, J5′,4′ = 7.9, J5′,6′ = 7.0, J5′,7′ = 1.1, H-5′), 7.04–7.08 (2H, m, H-2′, H-6′), 7.33 (1H, dm, J7′,6′ = 8.1, H-7′), 7.50 (1H, dm, J4′,5′ = 7.9, H-4′), 8.54 (1H, s, H-8), 8.79 (1H, s, H-2), 10.86 (1H, bs, NH). 13C NMR: δ 25.24 (3′–CH2), 44.74 (N–CH2), 110.03 (C-3′), 111.63 (C-7′), 118.18 (C-4′), 118.58 (C-5′), 121.26 (C-6′), 123.43 (C-2′), 127.01 (C-3′a), 130.97 (C-5), 136.35 (C-7′a), 147.64 (C-8), 149.05 (C-6), 151.58 (C-2), 152.10 (C-4). MS (ESI) m/z: 298.1 (MH+, 32%); 320.1 (MNa+, 100); HRMS (ESI) calc. for C15H13N5Cl: 298.08540; found: 298.08540.
2-(6-Chloro-9H-purin-9-yl)ethanol (Table 3, Entry 7, Z = H).
Mobile phase: 70–90% ethyl acetate in hexanes. Crystallization from toluene. White crystals, m.p. 159 °C. Found: C, 42.51; H, 3.69; N, 28.33; Cl, 17.82. Calc. for C7H7N4OCl: C, 42.33; H, 3.55; N, 28.21; Cl, 17.85%. 1H NMR: δ 3.79 (2H, q, J2′,OH = J2′,1′ = 5.4, H-2′), 4.35 (2H, t, J1′,2′ = 5.4, H-1′), 5.01 (1H, t, JOH,2′ = 5.6, OH), 8.65 (1H, s, H-8), 8.77 (1H, s, H-2). 13C NMR: δ 46.77 (C-1′), 59.04 (C-2′), 131.03 (C-5), 148.15 (C-8), 148.97 (C-6), 151.51 (C-2), 152.28 (C-4). MS (ESI) m/z: 199.1 (MH+, 79%); 221.1 (MNa+, 100); HRMS (ESI) calc. for C7H8ON4Cl: 199.03812; found: 199.03809.
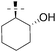
(1R*,2R*)-2-(6-Chloro-9H-purin-9-yl)cyclohexanol (Table 3, Entry 8, Z = H).
Mobile phase: 70–90% ethyl acetate in hexanes. Crystallization from toluene–cyclohexane. White crystals, m.p. 238–239 °C. Found: C, 52.33; H, 5.22; N, 22.40; Cl, 13.89. Calc. for C11H13N4ClO: C, 52.28; H, 5.19; N, 22.17; Cl, 14.03%. 1H NMR: δ 1.31–1.42 (3H, m, H-4ax, H-5ax, H-6ax), 1.74–1.79 (2H, m, H-4eq, H-5eq), 1.95–2.04 (2H, m, H-3eq, H-6eq), 2.14 (1H, m, H-3ax), 4.01 (1H, m, H-1), 4.26 (1H, ddd, J2,3ax = 12.5, J2,1 = 10.0, J2,3eq = 4.3, H-2), 4.94 (1H, d, JOH,1 = 5.3, OH), 8.74 and 8.75 (2H, s, H-2′, H-8′). 13C NMR: δ 24.13 and 24.85 (C-4, C-5), 30.78 (C-3), 34.85 (C-6), 62.14 (C-2), 70.28 (C-1), 131.39 (C-5′), 147.14 (C-8′), 148.97 (C-6′), 151.18 (C-2′), 152.36 (C-4′). MS (ESI) m/z: 253.1 (MH+, 22%); 275.1 (MNa+, 100); HRMS (ESI) calc. for C11H13ON4ClNa: 275.06701; found: 275.06708.
(1-(6-Chloro-9H-purin-9-yl)cyclopentyl)methanol (Table 3, Entry 9, Z = H).
Mobile phase: 1–2% methanol in ethyl acetate. Crystallization from hexane-ethyl acetate. White crystals, m.p. 177.5 °C. Found: C, 52.30; H, 5.23; N, 22.05; Cl. 14.20. Calc. for C11H13N4ClO: C, 52.28; H, 5.19; N, 22.17; Cl, 14.03%. 1H NMR: δ 1.69–1.74 (4H, m, H-3′), 2.27 and 2.39 (2H, m, H-2′), 3.71 (2H, d, JCH2,OH = 5.9, CH2O), 5.01 (1H, t, JOH,CH2 = 5.9, OH), 8.64 (1H, s, H-8), 8.74 (1H, s, H-2). 13C NMR: δ 22.70 (C-3′), 33.55 (C-2′), 64.15 (CH2O), 71.99 (C-1′), 132.01 (C-5), 147.49 (C-8), 149.11 (C-6), 150.77 (C-2), 152.32 (C-4). MS (ESI) m/z: 253.1 (MH+, 5%); 275.1 (MNa+, 100); HRMS (ESI) calc. for C11H14ON4Cl: 253.08507; found: 253.08507.
2-(6-Chloro-9H-purin-9-yl)propan-1,3-diol (Table 3, Entry 11, Z = H).
Mobile phase: 5–15% methanol in ethyl acetate. Crystallization from ethyl acetate. Brownish crystals, m.p. 186.5–187 °C. Found: C, 42.17; H, 4.03; N, 24.24; Cl, 15.42. Calc. for C8H9N4O2Cl: C, 42.03; H, 3.97; N, 24.50; Cl, 15.51%. 1H NMR: δ 3.84 (2H, ddd, Jgem = 11.6, J2′b,OH = 5.6, J2′b,1′ = 4.9, H-2′b), 3.93 (2H, ddd, Jgem = 11.6, J2′a,1′ = 7.5, J2′b,OH = 5.6, H-2′a), 4.69 (1H, m, H-1′), 5.06 (2H, t, JOH,2′ = 5.6, OH), 8.70 (1H, s, H-8), 8.76 (1H, s, H-2). 13C NMR: δ 59.82 (C-2′), 60.57 (C-1′), 131.20 (C-5), 147.24 (C-8), 149.02 (C-6), 151.41 (C-2), 152.67 (C-4). MS (ESI) m/z: 229.1 (MH+, 50%); 251.1 (MNa+, 100); HRMS (ESI) calc. for C8H10O2N4Cl: 229.04868; found: 229.04862.
1,3-Bis(6-chloro-9H-purin-9-yl)propan-2-ol (Table 3, Entry 13, Z = H).
Mobile phase: 5–15% methanol in ethyl acetate. Crystallization from ethyl acetate. White crystals, m.p. 246–247 °C (decomp.). Found: C, 42.64; H, 2.73; N, 30.89; Cl, 19.55. Calc. for C13H10N8Cl2O: C, 42.76; H, 2.76; N, 30.68; Cl, 19.42%. 1H NMR: δ 4.30 (2H, dd, Jgem = 14.1, J1′b,2′ = 7.9, H-1′b), 4.43 (1H, m, H-2′), 4.52 (2H, dd, Jgem = 14.1, J1′a,2′ = 3.7, H-1′a), 5.65 (1H, d, JOH,2′ = 5.6, OH), 8.63 (2H, s, H-8), 8.78 (2H, s, H-2). 13C NMR: δ 47.52 (C-1′), 66.82 (C-2′), 130.94 (C-5), 148.29 (C-8), 149.06 (C-6), 151.62 (C-2), 152.40 (C-4). MS (ESI) m/z: 365.0 (MH+, 11%); 387.0 (MNa+, 100); HRMS (ESI) calc. for C13H11ON8Cl2: 365.04274; found: 365.04273.
2-(6-Chloro-9H-purin-9-yl)ethanesulfonic acid, TEA salt (Table 3, Entry 14, Z = H).
Purification on HPLC (50 mM TEAB-methanol). Pale orange foam (methanol). Found: C, 42.80; H, 6.23; N, 19.17; Cl, 9.67; S, 8.96. Calc. for C13H22N5ClSO3: C, 42.91; H, 6.09; N, 19.25; Cl, 9.74; S, 8.81%. 1H NMR: δ 1.17 (9H, t, JCH3,CH2 = 7.3, CH3), 3.03 (2H, m, H-2′), 3.09 (6H, q, JCH2,CH3 = 7.3, CH2CH3), 4.54 (2H, m, H-1′), 8.67 (1H, s, H-8), 8.76 (1H, s, H-2), 8.99 (1H, bs, NH). 13C NMR: δ 8.79 (CH3), 41.22 (C-1′), 45.90 (CH2CH3), 49.84 (C-2′), 131.00 (C-5), 148.35 (C-8), 148.78 (C-6), 151.39 (C-2), 152.09 (C-4). MS (negESI) m/z: 261.0 (MH−, 100%); 522.6 (2 MH−, 8); HRMS (negESI) calc. for C7H6O3N4ClS: 260.98546; found: 260.98652.
Isopropyl (6-chloro-9H-purin-9-yl)methylphosphonate, DIPEA salt (Table 3, Entry 15, Z = H).
Purification on HPLC (water-methanol). Yellow oil. Found: C, 48.38; H, 7.20; N, 16.81; Cl, 8.69; P, 7.29. Calc. for C17H31N5ClO3P: C, 48.63; H, 7.44; N, 16.68; Cl, 8.44; P, 7.38%. 1H NMR: δ 0.98 (6H, d, JCH3,CH = 6.2, P–O–CH–CH3), 1.20–1.24 (15H, m, N–CH–CH3, N–CH2–CH3), 3.05 (2H, m, N–CH2–CH3), 3.52 (2H, m, N–CH–CH3), 4.18–4.26 (3H, m, P–CH2, P–O–CH–CH3), 8.67 (1H, s, H-8), 8.76 (1H, s, H-2), 9.61 (1H, bs, NH). 13C NMR: δ 12.37 (N–CH2–CH3), 16.81 and 18.10 (N–CH–CH3), 24.42 (d, JC,C,O,P = 3.6, P–O–CH–CH3), 41.50 (d, JC,P = 137.8, CH2P), 41.65 (N–CH2–CH3), 53.29 (N–CH–CH3), 67.09 (d, JC–O–P = 5.8, CH2P), 130.39 (C-5), 147.91 (C-8), 148.84 (C-6), 151.46 (C-2), 152.17 (C-4). MS (negESI) m/z: 289.0 (MH−, 100%); HRMS (negESI) calc. for C9H11O3N4ClP: 289.02628; found: 289.02611.
Isopropyl hydrogen (2-(6-chloro-9H-purin-9-yl)ethoxy)ethylphosphonate, TEA salt (Table 3, Entry 16, Z = H).
Purification performed on HPLC (50 mM TEAB-methanol). White foam (methanol). Found: C, 46.57; H, 7.01; N, 16.38; Cl, 8.33; P, 7.03. Calc. for C17H31N5ClO4P: C, 46.84; H, 7.17; N, 16.07; Cl, 8.13; P, 7.11%. 1H NMR: δ 0.97 (6H, d, JCH3,CH = 6.2, P–O–CH–CH3), 1.14 (9H, t, JCH3,CH2 = 7.3, N–CH2–CH3), 2.95 (6H, q, JCH2,CH3 = 7.3, N–CH2–CH3), 3.45 (2H, d, JH,C,P = 8.3, P–CH2), 3.91 (2H, m, H-2′), 4.17 (1H, dsept, JH,C,O,P = 8.2, JCH,CH3 = 6.2, CHi–Pr), 4.47 (2H, m, H-1′), 8.77 (1H, s, H-2), 8.78 (1H, s, H-8). 13C NMR: δ 8.51 (N–CH2–CH3), 24.35(d, JC,C,O,P = 3.8, P–O–CH–CH3), 43.67 (C-1), 45.16 (N–CH2–CH3), 66.74 (d, JC,O,P = 5.3, P–O–CH–CH3), 67.38 (d, JC,P = 155.6, CH2P), 69.42 (d, J2′,P = 9.8, C-2′), 130.86 (C-5), 148.17 (C-8), 148.98 (C-6), 151.53 (C-2), 152.14 (C-4). MS (negESI) m/z: 333.1 (MH−, 100%); HRMS (negESI) calc. for C11H15ClO4N4P: 333.05249; found: 333.05252.
9-Cyclohexyl-N6,N6-dimethyl-9H-purine-2,6-diamine (Table 1, Entry 11).
Mobile phase: 50–70% ethyl acetate in hexanes. Crystallization from toluene-cyclohexane. White crystals, m.p. 173.5–174 °C. Found: C, 59.76; H, 7.71; N, 32.50. Calc. for C13H20N6: C, 59.98; H, 7.74; N, 32.28%. 1H NMR: δ 1.23 (1H, m, H-4′ax), 1.37 (2H, m, H-3′ax), 1.67 (1H, m, H-4′eq), 1.75 (2H, m, H-2′ax), 1.82 (2H, m, H-3′eq), 1.92 (2H, m, H-2′eq), 4.16 (1H, tt, J1,2′ax = 12.0, J1′,2′eq = 3.9, H-1′), 5.77 (2H, bs, NH2), 7.80 (1H, s, H-8). 13C NMR: δ 25.00 (C-4′), 25.42 (C-3′), 32.57 (C-2′), 52.49 (C-1′), 113.96 (C-5), 134.38 (C-8), 152.35 (C-4), 154.90 (C-6), 159.44 (C-2). MS (ESI) m/z: 261.1 (MH+, 100%); 283.1 (MNa+, 2); HRMS (ESI) calc. for C13H21N6: 261.18222; found: 261.18213.
6-Chloro-9-(4-methoxyphenyl)-9H-purin-2-amine (Table 3, Entry 3, Z = NH2).
Mobile phase: 80–100% ethyl acetate in hexanes. Crystallization from ethyl acetate. Off-white crystals, m.p. 250–251 °C. Found: C, 52.41; H, 3.68; N, 25.28; Cl, 12.95. Calc. for C12H10N5ClO: C, 52.28; H, 3.66; N, 25.40; Cl, 12.86%. 1H NMR: δ 3.82 (3H, s, CH3), 6.98 (2H, bs, NH2), 7.12 (2H, m, H-3′), 7.67 (2H, m, H-2′), 8.40 (1H, s, H-8). 13C NMR: δ 55.73 (CH3), 114.75 (C-3′), 123.69 (C-5), 125.57 (C-2′), 127.58 (C-1′), 142.45 (C-8), 149.98 (C-6), 154.02 (C-4), 158.94 (C-4′), 160.34 (C-2). MS (ESI) m/z: 276.3 (MH+, 100%); 298.3 (MNa+, 77); HRMS (ESI) calc. for C12H11ON5Cl: 276.06466; found: 276.06483.
6-Chloro-9-[(1R)-1-(4-fluorophenyl)ethyl]-9H-purin-2-amine (Table 3, Entry 4, Z = NH2).
Mobile phase: 50–80% ethyl acetate in hexanes. White foam on rapid evaporation from chloroform. Found: C, 53.41; H, 3.87; N, 23.99; F, 6.59; Cl, 12.00. Calc. for C13H11N5FCl: C, 53.53; H, 3.80; N, 24.01; F, 6.51; Cl, 12.15%. 1H NMR: δ 1.90 (3H, d, JCH3,CH = 7.2, CH3), 5.71 (1H, q, JCH–CH3 = 7.2, CH–CH3), 6.89 (2H, bs, NH2), 7.18 (2H, m, H-3′), 7.36 (2H, m, H-2′), 8.36 (1H, s, H-8). 13C NMR: δ 20.56 (CH3), 52.77 (CH–CH3), 115.63 (d, J3′,F = 21.5, C-3′), 123.67 (C-5), 128.48 (d, J2′,F = 8.4, C-2′), 137.53 (d, J1′,F = 3.1, C-1′), 141.60 (C-8), 149.66 (C-6), 153.87 (C-4), 159.86 (C-2), 161.68 (d, J4′,F = 244.1, C-4′). MS (ESI) m/z: 292.1 (MH+, 4%); 314.1 (MNa+, 100); HRMS (ESI) calc. for C13H11N5ClFNa: 314.05792; found: 314.05795.
6-Chloro-9-(1-adamantyl)-9H-purin-2-amine (Table 3, Entry 5, Z = NH2).
Mobile phase: 50–80% ethyl acetate in hexanes. Crystallization from toluene. White crystals, m.p. 252 °C. Found: C, 59.02; H, 6.10; N, 23.22; Cl, 11.60. Calc. for C15H18N5Cl: C, 59.30; H, 5.97; N, 23.05; Cl, 11.67%. 1H NMR: δ 1.73 (6H, m, H-4′), 2.17 (3H, m, H-3′), 2.35 (6H, m, H-2′), 6.77 (2H, bs, NH2), 8.11 (1H, s, H-8). 13C NMR: δ 29.06 (C3′), 35.60 (C-4′), 40.47 (C-2′), 57.63 (C-1′), 124.72 (C-5), 140.62 (C-8), 149.80 (C-6), 154.17 (C-4), 158.91 (C-2). MS (ESI) m/z: 304.2 (MH+, 100%); 326.1 (MNa+, 53); HRMS (ESI) calc. for C15H19N5Cl: 304.13235; found: 304.13234.
6-Chloro-9-[2-(1H-indol-3-yl)ethyl]-9H-purin-2-amine (Table 3, Entry 6, Z = NH2).
Mobile phase: 50–70% ethyl acetate in hexanes. Crystallization from toluene. Pale yellow crystals, m.p. 188 °C. Found: C, 57.79; H, 4.18; N, 26.92; Cl, 11.28. Calc. for C15H13N6Cl: C, 57.60; H, 4.19; N, 26.87; Cl, 11.34%. 1H NMR: δ 3.22 (2H, m, 3′–CH2), 4.34 (2H, m, N–CH2), 6.91 (2H, bs, NH2), 6.98 (1H, ddd, J5′,4′ = 7.9, J5′,6′ = 7.0, J5′,7′ = 1.0, H-5′), 7.05–7.09 (2H, m, H-2′, H-6′), 7.34 (1H, dm, J7′,6′ = 8.1, H-7′), 7.53 (1H, dm, J4′,5′ = 7.9, H-4′), 7.95 (1H, s, H-8), 10.86 (1H, bs, NH). 13C NMR: δ 25.08 (3′–CH2), 43.82 (N–CH2), 110.34 (C-3′), 111.62 (C-7′), 118.36 (C-4′), 118.59 (C-5′), 121.26 (C-6′), 123.22 (C-2′), 123.53 (C-5), 127.07 (C-3′a), 136.35 (C-7′a), 143.39 (C-8), 149.41 (C-6), 154.24 (C-4), 159.91 (C-2). MS (ESI) m/z: 313.1 (MH+, 100%); 335.1 (MNa+, 94); HRMS (ESI) calc. for C15H14N6Cl: 313.09630; found: 313.09633.
2-(2-Amino-6-chloro-9H-purin-9-yl)ethanol (Table 3, Entry 7, Z = NH2).
Mobile phase: 1–3% methanol in ethyl acetate. Crystallization from acetone. Off-white crystals, m.p. 232 °C. Found: C, 39.42; H, 3.85; N, 32.91; Cl, 16.40. Calc. for C7H8N5OCl: C, 39.36; H, 3.77; N, 32.78; Cl, 16.60%. 1H NMR: δ 3.71 (2H, q, J2′,OH = J2′,1′ = 5.4, H-2′), 4.09 (2H, t, J1′,2′ = 5.5, H-1′), 5.00 (1H, t, JOH,2′ = 5.4, OH), 6.86 (2H, bs, NH2), 8.06 (1H, s, H-8). 13C NMR: δ 45.95 (C-1′), 58.99 (C-2′), 123.55 (C-5), 143.96 (C-8), 149.35 (C-6), 154.36 (C-4), 159.86 (C-2). MS (ESI) m/z: 214.1 (MH+, 43%); 236.1 (MNa+, 100); HRMS (ESI) calc. for C7H9ON5Cl: 214.04901; found: 214.04906.
(1-(2-Amino-6-chloro-9H-purin-9-yl)cyclopentyl)methanol (Table 3, Entry 9, Z = NH2).
Mobile phase: 1–5% methanol in ethyl acetate. Crystallization from ethyl acetate-acetone. Off-white crystals, m.p. 199 °C. Found: C, 59.43; H, 5.29; N, 26.33; Cl, 13.36. Calc. for C11H14N5ClO: C, 49.35; H, 5.27; N, 26.16; Cl, 13.24%. 1H NMR: δ 1.65–1.71 (4H, m, H-3′), 2.13 and 2.33 (2H, m, H-2′), 3.68 (2H, d, JCH2,OH = 5.8, CH2O), 4.98 (1H, t, JOH,CH2 = 5.9, OH), 6.74 (1H, bs, NH2), 8.06 (1H, s, H-8). 13C NMR: δ 22.64 (C-3′), 33.35 (C-2′), 63.73 (CH2O), 70.99 (C-1′), 124.57 (C-5), 143.20 (C-8), 149.44 (C-6), 154.38 (C-4), 159.13 (C-2). MS (ESI) m/z: 268.1 (MH+, 19%); 290.1 (MNa+, 100); HRMS (ESI) calc. for C11H15ON5Cl: 268.09596; found: 268.09593.
2-(2-Amino-6-chloro-9H-purin-9-yl)propan-1,3-diol (Table 3, Entry 11, Z = NH2).
Mobile phase: 5–10% methanol in ethyl acetate. Crystallization from ethyl acetate. White crystals, m.p. 224 °C (decomp.). Found: C, 39.35; H, 4.11; N, 28.80; Cl, 14.67. Calc. for C8H10N5O2Cl: C, 39.44; H, 4.14; N, 28.74; Cl, 14.55%. 1H NMR: δ 3.76 (2H, dt, Jgem = 11.4, J2′b,1′ = J2′b,OH = 5.3, H-2′b), 3.84 (2H, ddd, Jgem = 11.4, J2′a,1′ = 7.0, J2′b,OH = 5.5, H-2′a), 4.42 (1H, m, H-1′), 5.01 (2H, t, JOH,2′ = 5.5, OH), 6.83 (2H, bs, NH2), 8.11 (1H, s, H-8). 13C NMR: δ 59.21 (C-1′), 59.74 (C-2′), 123.65 (C-5), 142.85 (C-8), 149.28 (C-6), 154.64 (C-4), 159.66 (C-2). MS (ESI) m/z: 244.1 (MH+, 39%); 266.1 (MNa+, 100); HRMS (ESI) calc. for C8H11O2N5Cl: 244.05958; found: 244.05949.
1,3-Bis(2-amino-6-chloro-9H-purin-9-yl)propan-2-ol (Table 3, Entry 13, Z = NH2).
Poorly soluble product was filtered off directly from the reaction mixture boiled in methanol (20 mL) and collected. Orange solid, m.p. > 350 °C. Found: C, 39.66; H, 3.10; N, 35.17; Cl, 18.02. Calc. for C13H12N10Cl2O: C, 39.51; H, 3.06; N, 35.44; Cl, 17.94%. 1H NMR: δ 4.00 (2H, dd, Jgem = 14.3, J1′b,2′ = 8.1, H-1′b), 4.19 (2H, dd, Jgem = 14.2, J1′a,2′ = 3.5, H-1′a), 4.27 (1H, m, H-2′), 5.60 (1H, d, JOH,2′ = 5.6, OH), 6.88 (4H, bs, NH2), 8.05 (2H, s, H-8). 13C NMR: δ 47.06 (C-1′); 66.73 (C-2′); 123.43 (C-5); 144.10 (C-8); 149.43 (C-6); 154.50 (C-4); 159.90 (C-2). MS (ESI) m/z: 395.1 (MH+, 11%); 417.1 (MNa+, 100); HRMS (ESI) calc. for C13H13ON10Cl2: 395.06454; found: 395.06444.
2-(2-Amino-6-chloro-9H-purin-9-yl)ethanesulfonic acid, TEA salt (Table 3, Entry 14, Z = NH2).
Purification on HPLC (50 mM TEAB-methanol). Light orange foam (methanol). Found: C, 41.44; H, 6.26; N, 22.40; Cl, 9.20; S, 8.33. Calc. for C13H23N6ClSO3: C, 41.21; H, 6.12; N, 22.18; Cl, 9.36; S, 8.46%. 1H NMR: δ 1.17 (9H, t, JCH3,CH2 = 7.3, CH3), 2.94 (2H, m, H-2′), 3.09 (6H, q, JCH2,CH3 = 7.3, CH2CH3), 4.29 (2H, m, H-1′), 6.91 (2H, bs, NH2), 8.12 (1H, s, H-8), 8.94 (1H, bs, NH). 13C NMR: δ 8.79 (CH3), 40.42 (C-1′), 45.92 (CH2CH3), 50.19 (C-2′), 123.49 (C-5), 143.96 (C-8), 149.24 (C-6), 154.17 (C-4), 159.84 (C-2). MS (ESI) m/z: 278.0 (MH+, 45%); 379.2 (MEt3N+, 100); HRMS (ESI) calc. for C7H9O3N5ClS: 278.01091; found: 278.01087.
Propan-2-yl hydrogen [(2-amino-6-chloro-9H-purin-9-yl)methyl]phosphonate, DIPEA salt (Table 3, Entry 15, Z = NH2).
Purification on HPLC (water-methanol). Orange wax. Found: C, 46.80; H, 7.38; N, 19.53; Cl, 7.59; P, 7.05. Calc. for C17H32N6ClO3P: C, 46.95; H, 7.42; N, 19.32; Cl, 7.42; P, 7.12%. 1H NMR: δ 0.97 (6H, bs, P–O–CH–CH3), 1.23–1.27 (15H, m, N–CH–CH3, N–CH2–CH3), 3.07 (2H, m, N–CH2–CH3), 3.54 (2H, m, N–CH–CH3), 3.97 (2H, bs, P–CH2), 4.21 (1H, m, P–O–CH–CH3), 6.80 (2H, bs, NH2), 8.21 (1H, bs, H–8), 9.66 (1H, bs, NH). 13C NMR: δ 12.32 (N–CH2–CH3), 16.84 and 18.10 (N–CH–CH3), 24.44 (P–O–CH–CH3), 41.61 (N–CH2–CH3), 53.29 (N–CH–CH3), 122.97 (C-5), 143.85 (C-8), 149.07 (C-6), 154.45 (C-4), 159.78 (C-2). MS (negESI) m/z: 304.0 (MH−, 100%); HRMS (negESI) calc. for C9H12ClN5O3P: 304.03718; found: 304.03731.
Isopropyl (2-(2-amino-6-chloro-9H-purin-9-yl)ethoxy)methylphosphonate TEA salt (Table 3, Entry 16, Z = NH2).
Purification on HPLC (50 mM TEAB-methanol). Orange wax. Found: C, 44.92; H, 6.97; N, 18.91; Cl, 8.00; P, 6.82. Calc. for C17H32N6ClO4P: C, 45.28; H, 7.15; N, 18.64; Cl, 7.86; P, 6.87%. 1H NMR: δ 1.02 (6H, d, JCH3,CH = 6.2, P–O–CH–CH3), 1.13 (9H, t, JCH3,CH2 = 7.3, N–CH2–CH3), 2.93 (6H, q, JCH2,CH3 = 7.3, N–CH2–CH3), 3.43 (2H, d, JH,C,P = 8.3, P–CH2), 3.82 (2H, m, H-2′), 4.20 (2H, m, H-1′), 4.23 (1H, dsept, JH,C,O,P = 8.2, JCH,CH3 = 6.2, P–O–CH–CH3), 6.88 (2H, bs, NH2), 8.16 (1H, s, H-8). 13C NMR: δ 8.48 (N–CH2–CH3), 24.45(d, JC,C,O,P = 3.7, P–O–CH–CH3), 42.92 (C-1), 45.11 (N–CH2–CH3), 66.73 (d, JC,O,P = 5.6, P–O–CH–CH3), 67.58 (d, JC,P = 156.0, CH2P), 69.54 (d, J2′,P = 10.1, C-2′), 123.39 (C-5), 143.85 (C-8), 149.34 (C-6), 154.27 (C-4), 159.89 (C-2). MS (negESI) m/z: 289.0 (MH−, 100%); HRMS (negESI) calc. for C9H11O3N4ClP: 289.02628; found: 289.02611.
General reaction procedure for the subsequent nucleophilic displacement of C-6 chlorine atom of tryptamine derivatives
To a solution of tryptamine (160 mg, 1 mmol) in a suitable solvent (5 mL), 4,6-dichloro-5-formamidopyrimidine (230 mg, 1.2 mmol) or 2-amino-4,6-dichloro-5-formamidopyrimidine (248 mg, 1.2 mmol) and DIPEA (348 μL, 2 mmol) were added and the reaction mixture was heated in a sealed vessel on the corresponding temperature for the specified time (Table 3, Entry 6). Nucleophilic reagent (Table 4) was added either neat or as a solution to the crude reaction mixture from the purine nucleobase construction and the resulting mixture was heated in a sealed vessel under microwave irradiation (with the exception of reaction with thiourea, which was heated conventionally) on the corresponding temperature for the specified time. Completion of the reaction was determined by TLC (hexanes–ethyl acetate = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9).
9).
9-(2-(1H-Indol-3-yl)ethyl)-9H-purin-6-amine (Table 4, Entry 1).
Ethanolic ammonia was added as a 3.5 M solution (2.9 mL, 10 mmol). Mobile phase: 5–15% methanol in ethyl acetate. Crystallization from water-methanol. Yellow crystals, m.p. 235 °C (decomp.). Found: C, 64.53; H, 5.00; N, 30.41. Calc. for C15H14N6: C, 64.73; H, 5.07; N, 30.20%. 1H NMR: δ 3.25 (2H, m, 3′–CH2), 4.42 (2H, m, N–CH2), 6.98 (1H, ddd, J5′,4′ = 7.9, J5′,6′ = 7.0, J5′,7′ = 1.1, H-5′), 7.05–7.08 (2H, m, H-2′, H-6′), 7.15 (2H, bs, NH2), 7.33 (1H, dm, J7′,6′ = 8.1, H-7′), 7.55 (1H, dm, J4′,5′ = 7.9, H-4′), 7.99 (1H, s, H-8), 8.18 (1H, s, H-2), 10.84 (1H, bs, H-1′). 13C NMR: δ 25.63 (3′–CH2), 43.80 (N–CH2), 110.54 (C-3′), 111.60 (C-7′), 118.36 (C-4′), 118.56 (C-5′), 118.94 (C-5), 121.22 (C-6′), 123.20 (C-2′), 127.14 (C-3′a), 136.36 (C-7′a), 140.96 (C-8), 149.70 (C-4), 152.54 (C-2), 156.10 (C-6). MS (ESI) m/z: 279.4 (MH+, 100%); 301.3 (MNa+, 39); HRMS (ESI) calc. for C15H15N5: 279.13527; found: 279.13539.
9-(2-(1H-Indol-3-yl)ethyl)-N6-cyclopropyl-9H-purin-6-amine (Table 4, Entry 2).
Cyclopropylamine was added neat (346 μL, 5 mmol). Mobile phase: 5–10% methanol in ethyl acetate. Crystallization from toluene-cyclohexane. Pale brown crystals, m.p. 180–181 °C. Found: C, 67.97; H, 5.72; N, 26.35. Calc. for C18H18N6: C, 67.90; H, 5.70; N, 26.40%%. 1H NMR: δ 0.61 and 0.71 (4H, m, CH2-cyclop), 3.05 (1H, bs, CH-cyclop), 3.25 (2H, m, 3′–CH2), 4.43 (2H, m, N–CH2), 6.98 (1H, ddd, J5′,4′ = 7.9, J5′,6′ = 7.0, J5′,7′ = 1.1, H-5′), 7.05–7.09 (2H, m, H-2′, H-6′), 7.33 (1H, dm, J7′,6′ = 8.1, H-7′), 7.56 (1H, dm, J4′,5′ = 7.9, H-4′), 7.80 (1H, m, 6-NH), 8.00 (1H, s, H-8), 8.28 (1H, bs, H-2), 10.84 (1H, bs, H-1′). 13C NMR: δ 6.59 (CH2-cyclop), 24.2 (CH-cyclop), 25.64 (3′–CH2), 43.77 (N–CH2), 110.52 (C-3′), 111.60 (C-7′), 118.36 (C-4′), 118.56 (C-5′), 119.29 (C-5), 121.23 (C-6′), 123.20 (C-2′), 127.14 (C-3′a), 136.36 (C-7′a), 140.78 (C-8), 149.3 (C-4), 152.45 (C-2), 155.67 (C-6). MS (ESI) m/z: 319.4 (MH+, 100%); 341.4 (MNa+, 4); HRMS (ESI) calc. for C18H19N6: 319.16657; found: 319.16674.
9-(2-(1H-Indol-3-yl)ethyl)-6-morpholino-9H-purine (Table 4, Entry 3).
Morpholine was added neat (437 μL, 5 mmol). Mobile phase: 1–2% methanol in ethyl acetate. Crystallization from toluene. Pale yellow crystals, m.p. 194–197 °C. Found: C, 65.44; H, 5.81; N, 24.33. Calc. for C19H20N6O: C, 65.50; H, 5.79; N, 24.12%. 1H NMR: δ 3.25 (2H, m, 3′–CH2), 3.71 (4H, m, O–CH2), 4.19 (4H, bs, O–CH2–CH2), 4.45 (2H, m, 9–CH2), 6.98 (1H, ddd, J5′,4′ = 7.9, J5′,6′ = 7.0, J5′,7′ = 1.1, H-5′), 7.06–7.09 (2H, m, H-2′, H-6′), 7.33 (1H, dm, J7′,6′ = 8.1, H-7′), 7.56 (1H, dm, J4′,5′ = 7.9, H-4′), 8.06 (1H, s, H-8), 8.30 (1H, s, H-2), 10.84 (1H, bs, H-1′). 13C NMR: δ 25.48 (3′–CH2), 43.80 (N–CH2), 66.32 (O–CH2), 110.41 (C-3′), 111.57 (C-7′), 118.31 (C-4′), 118.53 (C-5′), 119.31 (C-5), 121.20 (C-6′), 123.21 (C-2′), 127.11 (C-3′a), 136.35 (C-7′a), 140.29 (C-8), 150.82 (C-4), 151.85 (C-2), 153.39 (C-6). MS (ESI) m/z: 349.4 (MH+, 100%); 371.4 (MNa+, 26); HRMS (ESI) calc. for C19H20ON6Na: 371.15908; found: 371.15913.
9-(2-(1H-Indol-3-yl)ethyl)-1H-purin-6(9H)-thione (Table 4, Entry 4).
Thiourea was added neat (152 mg, 2 mmol). Mobile phase: 1–10% methanol in ethyl acetate. Crystallization from ethanol. White crystals, m.p. 305 °C (decomp.). Found: C, 61.14; H, 4.51; N, 23.92; S, 10.66. Calc. for C15H13N5S: C, 61.00; H, 4.44; N, 23.71; S, 10.86%. 1H NMR: δ 3.25 (2H, m, 3′–CH2), 4.44 (2H, m, N–CH2), 6.98 (1H, ddd, J5′,4′ = 7.9, J5′,6′ = 6.9, J5′,7′ = 1.1, H-5′), 7.05–7.08 (2H, m,H-2′, H-6′), 7.33 (1H, dm, J7′,6′ = 8.1, H-7′), 7.55 (1H, dm, J4′,5′ = 7.8, H-4′), 8.14 (1H, s, H-8), 8.20 (1H, s, H-2), 10.84 (1H, bs, H-1′), 13.66 (1H, bs, H-1). 13C NMR: δ 25.58 (3′–CH2), 44.25 (N–CH2), 110.11 (C-3′), 111.59 (C-7′), 118.23 (C-4′), 118.55 (C-5′), 121.21 (C-6′), 123.31 (C-2′), 127.01 (C-3′a), 135.11 (C-5), 136.34 (C-7′a), 143.13 (C-8), 144.21 (C-4), 144.91 (C-2), 175.87 (C-6). MS (ESI) m/z: 296.2 (MH+, 100%); 318.2 (MNa+, 69); HRMS (ESI) calc. for C15H14N5S: 296.09644; found: 296.09650.
9-(2-(1H-Indol-3-yl)ethyl)-6-methoxy-9H-purine (Table 4, Entry 5).
Sodium methoxide was added as a 1 M solution in water (5 mL, 5 mmol). Mobile phase: 1–2% methanol in ethyl acetate. Crystallization from toluene. White crystals, m.p. 173–174 °C. Found: C, 65.29; H, 5.11; N, 24.11. Calc. for C16H15N5O: C, 65.52; H, 5.15; N, 23.88%. 1H NMR: δ 3.28 (2H, m, 3′–CH2), 4.09 (3H, s, CH3), 4.52 (2H, m, N–CH2), 6.97 (1H, ddd, J5′,4′ = 7.9, J5′,6′ = 7.0, J5′,7′ = 1.0, H-5′), 7.05–7.08 (2H, m, H-2′, H-6′), 7.33 (1H, dm, J7′,6′ = 8.1, H-7′), 7.53 (1H, dm, J4′,5′ = 7.9, H-4′), 8.23 (1H, s, H-8), 8.55 (1H, s, H-2), 10.84 (1H, bs, H-1′). 13C NMR: δ 25.47 (3′–CH2), 44.27 (N–CH2), 53.98 (O–CH3), 110.27 (C-3′), 111.61 (C-7′), 118.27 (C-4′), 118.58 (C-5′), 120.74 (C-5), 121.24 (C-6′), 123.29 (C-2′), 127.08 (C-3′a), 136.35 (C-7′a), 143.94 (C-8), 151.55 (C-2), 152.22 (C-4), 160.36 (C-6). MS (ESI) m/z: 294.1 (MH+, 71%); 316.1 (MNa+, 100); HRMS (ESI) calc. for C16H15ON5Na: 316.11688; found: 316.11689.
9-(2-(1H-Indol-3-yl)ethyl)-1H-purin-6(9H)-one (Table 4, Entry 6).
Sodium hydroxide was added as a 2 M solution in water (5 mL, 10 mmol). Mobile phase: 10–25% methanol in ethyl acetate. Crystallization from ethylacetate-ethanol. White crystals, m.p. 214 °C. Found: C, 64.66; H, 4.78; N, 24.79. Calc. for C15H13N5O: C, 64.51; H, 4.69; N, 25.07%. 1H NMR: δ 3.26 (2H, m, 3′–CH2), 4.47 (2H, m, N–CH2), 6.97 (1H, ddd, J5′,4′ = 7.9, J5′,6′ = 7.0, J5′,7′ = 1.1, H-5′), 7.07 (1H, ddd, J6′,7′ = 8.1, J6′,5′ = 7.0, J6′,4′ = 1.2, H-6′), 7.09 (1H, dm, J2′,1′ = 2.4, H-2′), 7.34 (1H, dm, J7′,6′ = 8.1, H-7′), 7.52 (1H, dm, J4′,5′ = 7.9, H-4′), 8.18 (1H, s, H-2), 8.47 (1H, s, H-8), 10.92 (1H, bs, H-1′), 12.67 (1H, bs, H-1). 13C NMR: δ 25.54 (3′–CH2), 44.97 (N–CH2), 109.91 (C-3′), 111.67 (C-7′), 118.18 (C-4′), 118.61 (C-5′), 121.20 (C-5), 121.28 (C-6′), 123.45 (C-2′), 127.04 (C-3′a), 136.37 (C-7′a), 140.21 (C-8), 146.93 (C-2), 148.01 (C-4), 155.59 (C-6). MS (ESI) m/z: 280.2 (MH+, 2%); 302.1 (MNa+, 100); HRMS (ESI) calc. for C15H13ON5Na: 302.10123; found: 302.10115.
9-[2-(1H-Indol-3-yl)ethyl]-9H-purin-2,6-diamine (Table 4, Entry 7).
Ethanolic ammonia was added as a 3.5 M solution (2.9 mL, 10 mmol). Poorly soluble product was filtered off directly from the reaction mixture and washed successively with water (3 × 5 mL) and methanol (3 × 5mL). Pale red solid, m.p. 318 °C (decomp.). Found: C, 61.30; H, 5.17; N, 33.70. Calc. for C15H15N7: C, 61.42; H, 5.15; N, 33.43%. 1H NMR: δ 3.18 (2H, m, 3′–CH2), 4.24 (2H, m, N–CH2), 5.80 (2H, bs, NH2), 6.62 (2H, bs, 6–NH2), 6.98 (1H, ddd, J5′,4′ = 7.9, J5′,6′ = 7.0, J5′,7′ = 1.1, H-5′), 7.06–7.09 (2H, m, H-2′, H-6′), 7.34 (1H, dm, J7′,6′ = 8.1, H-7′), 7.56 (1H, s, H-8), 7.56 (1H, dm, J4′,5′ = 7.9, H-4′), 10.84 (1H, bs, H-1′). 13C NMR: δ 25.59 (3′–CH2), 43.15 (N–CH2), 110.82 (C-3′), 111.58 (C-7′), 113.42 (C-5), 118.48 (C-4′), 118.56 (C-5′), 121.22 (C-6′), 123.05 (C-2′), 127.18 (C-3′a), 136.36 (C-7′a), 137.64 (C-8), 151.94 (C-4), 156.26 (C-6), 160.41 (C-2). MS (ESI) m/z: 294.1 (MH+, 100%); 316.1 (MNa+, 63); HRMS (ESI) calc. for C15H16N7: 294.14617; found: 294.14619.
9-(2-(1H-Indol-3-yl)ethyl)-N6-cyclopropyl-9H-purin-2,6-diamine (Table 4, Entry 8).
Cyclopropylamine was added neat (346 μL, 5 mmol). Mobile phase: 5–15% methanol in ethyl acetate. Crystallization from small amount of ethyl acetate. Off-white crystals, m.p. 203 °C (decomp.). Found: C, 64.62; H, 5.79; N, 29.65. Calc. for C18H19N7: C, 64.85; H, 5.74; N, 29.41%. 1H NMR: δ 0.58 and 0.65 (4H, m, CH2-cyclop), 3.04 (1H, bs, CH-cyclop), 3.17 (2H, m, 3′–CH2), 4.25 (2H, m, N–CH2), 5.84 (2H, bs, NH2), 6.98 (1H, ddd, J5′,4′ = 7.9, J5′,6′ = 7.0, J5′,7′ = 1.0, H-5′), 7.06–7.09 (2H, m, H-2′, H-6′), 7.21 (1H, m, NH), 7.33 (1H, dm, J7′,6′ = 8.1, H-7′), 7.55 (1H, s, H-8), 7.56 (1H, dm, J4′,5′ = 7.9, H-4′), 10.83 (1H, bs, H-1′). 13C NMR: δ 6.59 (CH2-cyclop), 25.60 (3′–CH2), 43.08 (N–CH2), 110.81 (C-3′), 111.56 (C-7′), 113.64 (C-5), 118.48 (C-4′), 118.55 (C-5′), 121.21 (C-6′), 123.04 (C-2′), 127.17 (C-3′a), 136.35 (C-7′a), 137.34 (C-8), 151.5 (C-4), 156.04 (C-6), 160.33 (C-2). MS (ESI) m/z: 334.2 (MH+, 100%); 356.2 (MNa+, 16); HRMS (ESI) calc. for C18H20N7: 334.17747; found: 334.17748.
9-(2-(1H-Indol-3-yl)ethyl)-6-morpholino-9H-purin-2-amine (Table 4, Entry 9).
Morpholine was added neat (437 μL, 5 mmol). Mobile phase: 1–5% methanol in ethyl acetate. Crystallization from small amount of toluene. White crystals, m.p. 200–201 °C. Found: C, 62.73; H, 5.86; N, 26.70. Calc. for C19H21N7O: C, 62.79; H, 5.82; N, 26.98%. 1H NMR: δ 3.17 (2H, m, 3′–CH2), 3.67 (4H, m, O–CH2), 4.10 (4H, bs, O–CH2–CH2), 4.27 (2H, m, 9–CH2), 5.93 (2H, bs, NH2), 6.98 (1H, ddd, J5′,4′ = 7.9, J5′,6′ = 6.9, J5′,7′ = 1.0, H-5′), 7.06–7.09 (2H, m, H-2′, H-6′), 7.34 (1H, dm, J7′,6′ = 8.1, H-7′), 7.56 (1H, dm, J4′,5′ = 7.9, H-4′), 7.61 (1H, s, H-8), 10.84 (1H, bs, H-1′). 13C NMR: δ 25.45 (3′–CH2), 43.12 (9–CH2), 45.12 (O–CH2–CH2), 66.42 (O–CH2), 110.72 (C-3′), 111.57 (C-7′), 113.64 (C-5), 118.48 (C-4′), 118.55 (C-5′), 121.22 (C-6′), 123.08 (C-2′), 127.16 (C-3′a), 136.35 (C-7′a), 137.07 (C-8), 153.28 (C-4), 153.84 (C-6), 159.69 (C-2). MS (ESI) m/z: 364.2 (MH+, 100%); 386.2 (MNa+, 10); HRMS (ESI) calc. for C19H22ON7: 364.18803; found: 364.18799.
9-(2-(1H-Indol-3-yl)ethyl)-2-amino-1H-purin-6(9H)-thione (Table 4, Entry 10).
Thiourea was added neat (152 mg, 2 mmol). Mobile phase: 2–8% methanol in ethyl acetate. Crystallization from small amount of ethanol. White crystals, m.p. 325 °C (decomp.). Found: C, 58.47; H, 4.52; N, 26.95; S, 10.52. Calc. for C15H14N6S: C, 58.38; H, 4.55; N, 27.08; S, 10.33%. 1H NMR: δ 3.18 (2H, m, 3′–CH2), 4.25 (2H, m, N–CH2), 6.80 (2H, bs, NH2), 6.99 (1H, ddd, J5′,4′ = 7.9, J5′,6′ = 6.9, J5′,7′ = 1.0, H-5′), 7.06–7.09 (2H, m, H-2′, H-6′), 7.34 (1H, dm, J7′,6′ = 8.1, H-7′), 7.56 (1H, dm, J4′,5′ = 7.8, H-4′), 7.74 (1H, s, H-8), 10.85 (1H, bs, H-1′), 11.86 (1H, bs, H-1). 13C NMR: δ 25.33 (3′–CH2), 43.51 (N–CH2), 110.37 (C-3′), 111.59 (C-7′), 118.37 (C-4′), 118.57 (C-5′), 121.23 (C-6′), 123.17 (C-2′), 127.05 (C-3′a), 128.34 (C-5), 136.35 (C-7′a), 140.65 (C-8), 147.94 (C-4), 153.08 (C-2), 174.91 (C-6). MS (ESI) m/z: 311.1 (MH+, 62%); 333.1 (MNa+, 100); HRMS (ESI) calc. for C15H14N6NaS: 333.08929; found: 333.08928.
9-(2-(1H-Indol-3-yl)ethyl)-6-methoxy-9H-purin-2-amine (Table 4, Entry 11).
Sodium methoxide was added as a 1 M solution in water (5 mL, 5 mmol). Mobile phase: 2–5% methanol in ethyl acetate. Crystallization from toluene. White crystals, m.p. 178–179 °C. Found: C, 62.46; H, 5.12; N, 27.19. Calc. for C16H16N6O: C, 62.32; H, 5.23; N, 27.26%. 1H NMR: δ 3.19 (2H, m, 3′–CH2), 3.95 (3H, s, CH3), 4.30 (2H, m, N–CH2), 6.42 (2H, bs, NH2), 6.98 (1H, ddd, J5′,4′ = 7.9, J5′,6′ = 7.0, J5′,7′ = 1.1, H-5′), 7.05–7.09 (2H, m, H-2′, H-6′), 7.33 (1H, dm, J7′,6′ = 8.1, H-7′), 7.55 (1H, dm, J4′,5′ = 7.9, H-4′), 7.69 (1H, s, H-8), 10.84 (1H, bs, H-1′). 13C NMR: δ 25.38 (3′–CH2), 43.45 (N–CH2), 53.24 (O–CH3), 110.59 (C-3′), 111.58 (C-7′), 113.97 (C-5), 118.42 (C-4′), 118.57 (C-5′), 121.23 (C-6′), 123.10 (C-2′), 127.13 (C-3′a), 136.34 (C-7′a), 139.92 (C-8), 154.30 (C-4), 159.92 (C-2), 160.77 (C-6). MS (ESI) m/z: 309.2 (MH+, 66%); 331.1 (MNa+, 100); HRMS (ESI) calc. for C16H17ON6: 309.14584; found: 309.14583.
9-(2-(1H-Indol-3-yl)ethyl)-2-amino-1H-purin-6(9H)-on (Table 4, Entry 12).
Sodium hydroxide was added as a 2 M solution in water (5 mL, 10 mmol). Poorly soluble product was filtered off, boiled with methanol-water mixture (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and collected again. Pale red solid, m.p. 354 °C (decomp.). Found: C, 58.47; H, 4.52; N, 26.95; S, 10.52. Calc. for C15H14N6S: C, 58.38; H, 4.55; N, 27.08; S, 10.33%. 1H NMR: δ 3.18 (2H, m, 3′–CH2), 4.25 (2H, m, N–CH2), 6.80 (2H, bs, NH2), 6.99 (1H, ddd, J5′,4′ = 7.9, J5′,6′ = 6.9, J5′,7′ = 1.0, H-5′), 7.06–7.09 (2H, m, H-2′, H-6′), 7.34 (1H, dm, J7′,6′ = 8.1, H-7′), 7.56 (1H, dm, J4′,5′ = 7.8, H-4′), 7.74 (1H, s, H-8), 10.85 (1H, bs, H-1′), 11.86 (1H, bs, H-1). 13C NMR: δ 25.33 (3′-CH2), 43.51 (N-CH2), 110.37 (C-3′), 111.59 (C-7′), 118.37 (C-4′), 118.57 (C-5′), 121.23 (C-6′), 123.17 (C-2′), 127.05 (C-3′a), 128.34 (C-5), 136.35 (C-7′a), 140.65 (C-8), 147.94 (C-4), 153.08 (C-2), 174.91 (C-6). MS (ESI) m/z: 311.1 (MH+, 62%); 333.1 (MNa+, 100); HRMS (ESI) calc. for C15H14N6NaS: 333.08929; found: 333.08928.
1) and collected again. Pale red solid, m.p. 354 °C (decomp.). Found: C, 58.47; H, 4.52; N, 26.95; S, 10.52. Calc. for C15H14N6S: C, 58.38; H, 4.55; N, 27.08; S, 10.33%. 1H NMR: δ 3.18 (2H, m, 3′–CH2), 4.25 (2H, m, N–CH2), 6.80 (2H, bs, NH2), 6.99 (1H, ddd, J5′,4′ = 7.9, J5′,6′ = 6.9, J5′,7′ = 1.0, H-5′), 7.06–7.09 (2H, m, H-2′, H-6′), 7.34 (1H, dm, J7′,6′ = 8.1, H-7′), 7.56 (1H, dm, J4′,5′ = 7.8, H-4′), 7.74 (1H, s, H-8), 10.85 (1H, bs, H-1′), 11.86 (1H, bs, H-1). 13C NMR: δ 25.33 (3′-CH2), 43.51 (N-CH2), 110.37 (C-3′), 111.59 (C-7′), 118.37 (C-4′), 118.57 (C-5′), 121.23 (C-6′), 123.17 (C-2′), 127.05 (C-3′a), 128.34 (C-5), 136.35 (C-7′a), 140.65 (C-8), 147.94 (C-4), 153.08 (C-2), 174.91 (C-6). MS (ESI) m/z: 311.1 (MH+, 62%); 333.1 (MNa+, 100); HRMS (ESI) calc. for C15H14N6NaS: 333.08929; found: 333.08928.
((1S,4R)-4-(2-Amino-6-(cyclopropylamino)-9H-purin-9-yl)cyclopent-2-enyl)methanol (Scheme 1).
To a solution of ((1S,4R)-4-aminocyclopent-2-enyl)methanol (113 mg, 1 mmol) in a water–methanol mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 5 mL), 2-amino-4,6-dichloro-5-formamidopyrimidine (248 mg, 1.2 mmol) and DIPEA (348 μL, 2 mmol) were added and the reaction mixture was heated in a microwave reactor on 140 °C for 60 min (Table 3, Entry 10, Z = NH2). Cyclopropylamine was added afterwards (346 μL, 5 mmol) and the reaction mixture was heated in a microwave reactor on 140 °C for further 20 min. Flash chromatography (5–15% methanol in ethyl acetate) and crystallization from a small amount of ethyl acetate afforded product (215 mg, 75%) as slightly red crystals. Spectral characteristics match those described in literature.22c
1 v/v, 5 mL), 2-amino-4,6-dichloro-5-formamidopyrimidine (248 mg, 1.2 mmol) and DIPEA (348 μL, 2 mmol) were added and the reaction mixture was heated in a microwave reactor on 140 °C for 60 min (Table 3, Entry 10, Z = NH2). Cyclopropylamine was added afterwards (346 μL, 5 mmol) and the reaction mixture was heated in a microwave reactor on 140 °C for further 20 min. Flash chromatography (5–15% methanol in ethyl acetate) and crystallization from a small amount of ethyl acetate afforded product (215 mg, 75%) as slightly red crystals. Spectral characteristics match those described in literature.22c
Acknowledgements
This study was supported by the Ministry of the Interior of the Czech Republic (VG20102015046) and Gilead Sciences, Inc. (Foster City, CA, USA). Subvention for development of research organization (RVO: 61388963) is also acknowledged. We are obliged to Petr Jansa, PhD. (Gilead Sciences, Inc.) for his helpful suggestions. We thank Mr. David Mařák for providing the amine substrate 16 and Ms. Jaroslava Sklenářová for her excellent technical asistence.
References
-
(a) R. Vince and M. Hua, J. Med. Chem., 1990, 33, 17 CrossRef CAS;
(b) E. De Clercq, Clin. Microbiol. Rev., 1997, 10, 674 CAS.
-
(a) S. Rachakonda and L. Cartee, Curr. Med. Chem., 2004, 11, 775 CrossRef CAS;
(b) C. Balg, J. L. Huot, J. Lapointe and R. Chenevert, J. Am. Chem. Soc., 2008, 130, 2364 CrossRef.
-
(a) A. K. Bakkestuen, L. Gundersen, G. Langli, F. Liu and J. M. J. Nolsøe, Bioorg. Med. Chem. Lett., 2000, 10, 1207 CrossRef CAS;
(b) L. Gundersen, J. Nissen-Meyer and B. Spilsberg, J. Med. Chem., 2002, 45, 1383 CrossRef CAS.
-
(a) Y. Chang, S. M. Wignall, G. R. Rosania, N. S. Gray, S. R. Hanson, A. I. Su, J. Merlie, Jr., H. Moon, S. B. Sangankar, O. Perez, R. Heald and P. G. Schultz, J. Med. Chem., 2001, 44, 4497 CrossRef CAS;
(b) N. Lamanna and M. Weiss, Adv. Pharmacol., 2004, 51, 107 CrossRef CAS.
-
(a) M. Knockaert, P. Greengard and L. Meijer, Trends Pharmacol. Sci., 2002, 23, 417 CrossRef CAS;
(b) Z. Wang, B. J. Canagarajah, J. C. Boehm, S. Kassisà, M. H. Cobb, P. R. Young, S. Abdel-Meguid, J. L. Adams and E. J. Goldsmith, Structure, 1998, 6, 1117 CrossRef CAS.
- H. Rosemayer, Chem. Biodiversity, 2004, 1, 361 Search PubMed.
- M. Legraverend, Tetrahedron, 2008, 64, 8585 CrossRef CAS.
-
(a) S. Vrbková, M. Dračínský and A. Holý, Tetrahedron: Asymmetry, 2007, 18, 2233 CrossRef;
(b) A. Rustullet, R. Alibés, P. de March, M. Figueredo and J. Font, Org. Lett., 2007, 9, 2827 CrossRef CAS;
(c) C. Lambertucci, I. Antonini, M. Buccioni, D. Dal Ben, D. D. Kachare, R. Volpini, K. Klotz and G. Cristalli, Bioorg. Med. Chem., 2009, 17, 2812 CrossRef CAS.
-
(a) K. Lee, C. Cass and K. A. Jacobson, Org. Lett., 2001, 3, 597 CrossRef CAS;
(b) M. Šála, A. M. De Palma, H. Hřebabecký, R. Nencka, M. Dračínský, P. Leyssen, J. Neyts and A. Holý, Bioorg. Med. Chem., 2010, 18, 4374 CrossRef.
-
(a) D. Enders, I. Breuer and E. Drosdow, Synthesis, 2005, 19, 3239 CrossRef;
(b) R. Alibés, A. Alvárez-Larena, P. de March, M. Figueredo, J. Font, T. Parella and A. Rustullet, Org. Lett., 2006, 8, 491 CrossRef.
-
(a) S. Guillarme, S. Legoupy, A. Aubertin, C. Olicard, N. Bourgougnon and F. Huet, Tetrahedron, 2003, 59, 2177 CrossRef CAS;
(b) H. Guo, T. Yuan, H. Niu, J. Liu, R. Mao, D. Li and G. Qu, Chem.–Eur. J., 2011, 17, 4095 CrossRef CAS.
-
(a) M. R. Peel, D. D. Sternbach and M. R. Johnson, J. Org. Chem., 1991, 56, 4990 CrossRef CAS;
(b) L. A. Agrofoglio, F. Amblard, S. P. Nolan, S. Charamon, I. Gillaizeau, T. A. Zevaco and P. Guenot, Tetrahedron, 2004, 60, 8397 CrossRef CAS.
-
(a) D. Ubiali, S. Rocchietti, F. Scarmozzino, M. Terreni, A. M. Albertini, R. Fernandéz-Lafuente, J. M. Guisán and M. Pregnolato, Adv. Synth. Catal., 2004, 346, 1361 CrossRef CAS;
(b) I. A. Mikhailopulo, Curr. Org. Chem., 2007, 11, 317 CrossRef CAS;
(c) J. Boryski, Curr. Org. Chem., 2008, 12, 309 CrossRef CAS.
-
(a) A. Yamazaki, I. Kumashiro and T. Takenishi, J. Org. Chem., 1967, 32, 3258 CrossRef CAS;
(b) I. A. Zavialov, V. H. Dahanukar, H. Nguyen, C. Orr and D. R. Andrews, Org. Lett., 2004, 6, 2237 CrossRef CAS.
- W. Traube, Ber. Dtsch. Chem. Ges., 1900, 33, 1371 CrossRef.
-
(a) M. Šála, H. Hřebabecký, M. Dračínský, M. Masojídková, A. M. De Palma, J. Neyts and A. Holý, Tetrahedron, 2009, 65, 9291 CrossRef;
(b) H. Hřebabecký, M. Dejmek, M. Dračínský, M. Šála, P. Leyssen, J. Neyts, M. Kaniaková, J. Krůšek and R. Nencka, Tetrahedron, 2012, 68, 1286 CrossRef.
-
(a) N. Gauvry and F. Huet, Tetrahedron, 1999, 55, 1321 CrossRef CAS;
(b) K. J. Shin, H. R. Moon, C. George and V. E. Marquez, J. Org. Chem., 2000, 65, 2172 CrossRef CAS.
-
(a) R. G. Bhushan and R. Vince, Bioorg. Med. Chem., 2002, 10, 2325 CrossRef CAS;
(b) M. Šála, H. Hřebabecký, M. Masojídková and A. Holý, Collect. Czech. Chem. Commun., 2006, 71, 635 CrossRef.
-
(a) M. R. Harnden, P. G. Wyatt, M. R. Boyd and D. Sutton, J. Med. Chem., 1990, 33, 187 CrossRef CAS;
(b) V. Nair, F. Zhang, X. Ma and E. Bonsu, Nucleosides, Nucleotides Nucleic Acids, 2009, 28, 408 CrossRef CAS.
- L. Čechová, P. Jansa, M. Šála, M. Dračínský, A. Holý and Z. Janeba, Tetrahedron, 2011, 67, 866 CrossRef.
-
(a) X. Lin and M. J. Robins, Org. Lett., 2000, 2, 3497 CrossRef CAS;
(b) M. Šála, A. M. De Palma, H. Hřebabecký, M. Dejmek, M. Dračínsky, P. Leyssen, J. Neyts, H. Mertlíková-Kaiserová and R. Nencka, Bioorg. Med. Chem. Lett., 2011, 21, 4271 CrossRef.
-
(a) S. M. Daluge and M. T. Martin, US 5917041, Chem. Abstr., 1995, 124, 56577 Search PubMed;
(b) A. Rossi, E. Vecchio, R. Pizzocaro and A. Bedeschi, EP 1857458, Chem. Abstr., 2006, 147, 522524 Search PubMed;
(c) S. M. Daluge, M. T. Martin, B. R. Sickles and D. A. Livingston, Nucleosides, Nucleotides Nucleic Acids, 2000, 19, 297 CrossRef CAS.
- H. Niu, C. Xia, G. Qu, S. Wu, Y. Jiang, X. Jin and H. Guo, Chem.–Asian J., 2012, 7, 45 CrossRef CAS.
- S. K. Chakraborty and R. Engel, Synth. Commun., 1991, 21, 1039 CrossRef CAS.
- P. Franchetti, L. Cappellacci, G. Abu Sheikha, M. Grifantini, A. G. Loi, A. De Montis, M. G. Spiga and P. La Colla, Nucleosides, Nucleotides Nucleic Acids, 1995, 14, 607 CAS.
- H. Niu, C. Xia, G. Qu, Q. Zhang, Y. Jiang, R. Mao, D. Li and H. Guo, Org. Biomol. Chem., 2011, 9, 5039 CAS.
- A. M. De Palma, A. Holý, H. Hřebabecký, J. Neyts and M. Šála, WO2008131502, Chem. Abstr., 2008, 149, 513628 Search PubMed.
- N. Platzer, H. Galons, Y. Bensaïd, M. Miocque and G. Bram, Tetrahedron, 1987, 43, 2101 CrossRef CAS.
- C. Cesario, L. P. Tardibono Jr. and M. J. Miller, Tetrahedron Lett., 2010, 51, 3053 CrossRef CAS.
- A. K. Bakkestuen and L. Gundersen, Tetrahedron Lett., 2003, 44, 3359 CrossRef CAS.
- R. Liboska, M. Masojídková and I. Rosenberg, Collect. Czech. Chem. Commun., 1996, 61, 313 CrossRef CAS.
- A. T. Horhota, J. W. Szostak and L. W. McLaughlin, Org. Lett., 2006, 8, 5345 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v).
1 (v/v).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v).
b Sealed microwave reactor. Conditions A) 140 °C/2 h; B) 160 °C/2 h; C) 140 °C/1 h; D) 120 °C/1 h.
c Isolated yields. In parentheses are HPLC-determined yields.
d Complicated reaction mixture.
e Reaction time had to be prolonged to 4 h in order to reach the full conversion. Yields are diminished due to occurrence of 6-alkoxy derivatives (more significant at temperatures > 120 °C).
f Nucleophilic adduct at the position C-6 with the base.
1 (v/v).
b Sealed microwave reactor. Conditions A) 140 °C/2 h; B) 160 °C/2 h; C) 140 °C/1 h; D) 120 °C/1 h.
c Isolated yields. In parentheses are HPLC-determined yields.
d Complicated reaction mixture.
e Reaction time had to be prolonged to 4 h in order to reach the full conversion. Yields are diminished due to occurrence of 6-alkoxy derivatives (more significant at temperatures > 120 °C).
f Nucleophilic adduct at the position C-6 with the base.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) for the 2-amino-6-chloropurine derivatives. The results listed in Table 3 indicate that both polar and non-polar substrates undergo this reaction with yields ranging from good to near quantitative. The phosphonate diester substrates afforded monoester products in both cases (Entries 15 and 16).
1 v/v) for the 2-amino-6-chloropurine derivatives. The results listed in Table 3 indicate that both polar and non-polar substrates undergo this reaction with yields ranging from good to near quantitative. The phosphonate diester substrates afforded monoester products in both cases (Entries 15 and 16).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), 140 °C/1 h.
b Isolated yields of MW heated experiments. The isolated yields of conventionally heated experiments are shown in parentheses. The yields of conventionally heated experiments determined with HPLC are in brackets.
c Under these conditions, a 6-phenylamino or 6-p-methoxyphenylamino derivative is formed.
d An amine hydrochloride was employed as a substrate.
e A bipurine derivative is formed using 2.4 mmol of pyrimidine reagent.
f The yield was less than 10% (HPLC based), the product was not isolated.
g Isolated yield of phosphonate monoester.
1, v/v), 140 °C/1 h.
b Isolated yields of MW heated experiments. The isolated yields of conventionally heated experiments are shown in parentheses. The yields of conventionally heated experiments determined with HPLC are in brackets.
c Under these conditions, a 6-phenylamino or 6-p-methoxyphenylamino derivative is formed.
d An amine hydrochloride was employed as a substrate.
e A bipurine derivative is formed using 2.4 mmol of pyrimidine reagent.
f The yield was less than 10% (HPLC based), the product was not isolated.
g Isolated yield of phosphonate monoester.

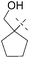

















![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min 95% A-5% B, t6
min 95% A-5% B, t6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min 100% B, t16
min 100% B, t16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min 100% C; flow 1 mL min−1. Concentration of products in reaction mixtures was determined by calibration on an external standard on the same column, analytes were identified by UV (Waters 2996). Elution was carried out with aqueous acetonitrile gradient. A = 100 mM TEAA, B = 100 mM TEAA in 50% MeCN, C = 100% MeCN. t0
min 100% C; flow 1 mL min−1. Concentration of products in reaction mixtures was determined by calibration on an external standard on the same column, analytes were identified by UV (Waters 2996). Elution was carried out with aqueous acetonitrile gradient. A = 100 mM TEAA, B = 100 mM TEAA in 50% MeCN, C = 100% MeCN. t0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min 95% A-5% B, t6
min 95% A-5% B, t6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min 100% B, t16
min 100% B, t16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min 100% C; flow 1 mL min−1.
min 100% C; flow 1 mL min−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9).
9).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and collected again. Pale red solid, m.p. 354 °C (decomp.). Found: C, 58.47; H, 4.52; N, 26.95; S, 10.52. Calc. for C15H14N6S: C, 58.38; H, 4.55; N, 27.08; S, 10.33%. 1H NMR: δ 3.18 (2H, m, 3′–CH2), 4.25 (2H, m, N–CH2), 6.80 (2H, bs, NH2), 6.99 (1H, ddd, J5′,4′ = 7.9, J5′,6′ = 6.9, J5′,7′ = 1.0, H-5′), 7.06–7.09 (2H, m, H-2′, H-6′), 7.34 (1H, dm, J7′,6′ = 8.1, H-7′), 7.56 (1H, dm, J4′,5′ = 7.8, H-4′), 7.74 (1H, s, H-8), 10.85 (1H, bs, H-1′), 11.86 (1H, bs, H-1). 13C NMR: δ 25.33 (3′-CH2), 43.51 (N-CH2), 110.37 (C-3′), 111.59 (C-7′), 118.37 (C-4′), 118.57 (C-5′), 121.23 (C-6′), 123.17 (C-2′), 127.05 (C-3′a), 128.34 (C-5), 136.35 (C-7′a), 140.65 (C-8), 147.94 (C-4), 153.08 (C-2), 174.91 (C-6). MS (ESI) m/z: 311.1 (MH+, 62%); 333.1 (MNa+, 100); HRMS (ESI) calc. for C15H14N6NaS: 333.08929; found: 333.08928.
1) and collected again. Pale red solid, m.p. 354 °C (decomp.). Found: C, 58.47; H, 4.52; N, 26.95; S, 10.52. Calc. for C15H14N6S: C, 58.38; H, 4.55; N, 27.08; S, 10.33%. 1H NMR: δ 3.18 (2H, m, 3′–CH2), 4.25 (2H, m, N–CH2), 6.80 (2H, bs, NH2), 6.99 (1H, ddd, J5′,4′ = 7.9, J5′,6′ = 6.9, J5′,7′ = 1.0, H-5′), 7.06–7.09 (2H, m, H-2′, H-6′), 7.34 (1H, dm, J7′,6′ = 8.1, H-7′), 7.56 (1H, dm, J4′,5′ = 7.8, H-4′), 7.74 (1H, s, H-8), 10.85 (1H, bs, H-1′), 11.86 (1H, bs, H-1). 13C NMR: δ 25.33 (3′-CH2), 43.51 (N-CH2), 110.37 (C-3′), 111.59 (C-7′), 118.37 (C-4′), 118.57 (C-5′), 121.23 (C-6′), 123.17 (C-2′), 127.05 (C-3′a), 128.34 (C-5), 136.35 (C-7′a), 140.65 (C-8), 147.94 (C-4), 153.08 (C-2), 174.91 (C-6). MS (ESI) m/z: 311.1 (MH+, 62%); 333.1 (MNa+, 100); HRMS (ESI) calc. for C15H14N6NaS: 333.08929; found: 333.08928.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 5 mL), 2-amino-4,6-dichloro-5-formamidopyrimidine (248 mg, 1.2 mmol) and DIPEA (348 μL, 2 mmol) were added and the reaction mixture was heated in a microwave reactor on 140 °C for 60 min (Table 3, Entry 10, Z = NH2). Cyclopropylamine was added afterwards (346 μL, 5 mmol) and the reaction mixture was heated in a microwave reactor on 140 °C for further 20 min. Flash chromatography (5–15% methanol in ethyl acetate) and crystallization from a small amount of ethyl acetate afforded product (215 mg, 75%) as slightly red crystals. Spectral characteristics match those described in literature.22c
1 v/v, 5 mL), 2-amino-4,6-dichloro-5-formamidopyrimidine (248 mg, 1.2 mmol) and DIPEA (348 μL, 2 mmol) were added and the reaction mixture was heated in a microwave reactor on 140 °C for 60 min (Table 3, Entry 10, Z = NH2). Cyclopropylamine was added afterwards (346 μL, 5 mmol) and the reaction mixture was heated in a microwave reactor on 140 °C for further 20 min. Flash chromatography (5–15% methanol in ethyl acetate) and crystallization from a small amount of ethyl acetate afforded product (215 mg, 75%) as slightly red crystals. Spectral characteristics match those described in literature.22c